Introduction
Typically, healthy body cells grow and divide under
restricted conditions to form new cells to match the body's needs.
All types of cancer however, exhibit continual uncontrolled
division of the cell, and this may occur almost anywhere in the
human body, with the potential to spread to other regions. Several
types of cancer form solid masses of tissue (tumors); however,
cancers of the blood, such as leukemias, are an exception (1,2). The
amino acid L-asparagine, is required for the survival of both
normal and cancer cells. Normal cells can synthesize L-asparagine
in amounts sufficient for their metabolic activities, but tumor
cells depend mainly on an exogenous supply of L-asparagine
(3). L-asparaginases (L-asparagine
amidohydrolase; EC 3.5.1.1) catalyzes the breakdown of the amide
group of the sidechain of L-asparagine into aspartic acid and
ammonia (4), reducing its
concentration, which is why regulating L-asparaginases is a
cornerstone of the treatment protocols for acute lymphoblastic
leukemia (2).
For several years, L-asparaginases have been broadly
used for the treatment of lymphoid systems malignancies, childhood
acute lymphoblastic leukemia, Hodgkin's lymphoma, lymphosarcoma,
and melanosarcoma (5). Apart from
its clinical use, L-asparaginases have been used as a diagnostic
biosensor for L-asparagine, due to the large amounts of ammonia
produced by the enzymatic reaction and its direct correlation with
the level of L-asparagine in a patient's blood (6). L-asparaginases have also been
characterized successfully as inhibitors of the formation of
acrylamide in heated food (7).
L-asparaginase is widely present in several
organisms, including plants, animals and microbes, but not in
humans. Microbes are the best source for the production of enzymes,
including L-asparaginase, as they are easy to cultivate and
manipulate (5). Microbes can
produce several different types of asparaginases that differ in
their cellular location and properties; namely periplasmic
asparaginase, extracellular asparaginase, intracellular
asparaginase and glutaminase-asparaginase, which all play a role in
basic metabolism (8).
In contrast to asparaginases obtained from fungi,
the longstanding use of bacterial L-asparaginases can cause
toxicity and hypersensitivity reactions, leading to anaphylaxis.
This may suggest that fungal asparaginases are more closely related
to those present in humans, as fungi are eukaryotic microorganisms.
Thus the chances of adverse effects and immunological reactions
against fungal L-asparaginases are expected to be lower (8). Another advantage of fungal
asparaginases is that they are produced extracellularly, and thus
it is considerably easier to separate and purify (4,8). Owing
to these advantages, fungi are considered to be the most suitable
organisms for production of L-asparaginase, and therefore, the
search for novel fungal producers is very important.
The utilization of agro-industrial-based products
has become of increasing importance in bio-industries, due to the
high nutrient content and low-cost (9-11).
Wheat bran (WB) as an example, has been reported in the literature
for the useful production of value-added biomolecules by
fermentation to reduce both the process cost and the environmental
waste (12).
The outer pericarp layer of WB, left as a by-product
after wheat grain milling, forms 13-17% of the entire organism
(13). WB is rich in minerals (such
as iron, zinc, manganese, magnesium and phosphorus), Vitamin-B
complex and bioactive compounds (such as phytic acid, ferulic acid
and vitamin E) (14). A total of
34-63% of the WB is formed of soluble and insoluble dietary fibers,
a complex compound made of edible plant polysaccharides (cellulose,
hemicelluloses and pentosan polymers) attached to proteins
(15.2-16.9%), lignin and other substances (15,16),
such as lipids (5.5-5.6%) (17).
The high nutritional value of WB makes it superior to other
agro-industrial candidates for enzyme production. Therefore, it was
used in the present study as a source of nutrient and physical
support during enzyme production.
Based on the consistency, culture media is
classified into one of three types; solid, semi-solid and liquid
medium. Solid-state fermentation (SSF) is defined as any
fermentation process where the growth of microorganisms takes place
on a solid substrate in the absence of free water. With SSF it is
possible to utilize renewable and low-cost natural resources
(18). However, when plants or crop
residues with low water and high sugar and nutrient contents are
utilized for SSF, the availability of its constituents may be low
or microbial growth may be inhibited, resulting in low system
productivity. Therefore, the SSF system was been modified by
increasing the water content to increase sugar and nutrient
availability. This system of semi-SSF ensures easier growth of
fungi and a higher efficiency of the biosynthetic process (19). Identification of a new and
affordable hyper-producer of L-asparaginases with fewer adverse
effects is of the utmost priority and a considerable challenge for
biotechnologists. Hence, in the present study, WB was used for
fermentation of Rhizopus oryzae AM16 as a novel source of
L-asparaginase grown under semi-SSF conditions, and the anticancer
activity of the fungal enzyme was assessed.
Materials and methods
Chemicals
All chemicals used in the present study were of
analytical grade and were obtained from Sigma-Aldrich; Merck KGaA
unless otherwise stated. WB was utilized as a solid substrate
during the fermentation process, and was kindly provided by the
South Cairo and Giza Mills Company.
Cell lines
In the present study, four tumor cell lines, HepG2
human liver cancer cells, MCF-7 breast cancer cells, HCT colon
carcinoma cells and A549 lung carcinoma cells were obtained from
Vacsera, Co. To ensure the authenticity of the cells used, all cell
lines used were confirmed using short Tandem Repeat DNA profiling
as previously described (20).
Sampling sites
Soil samples were collected from two Governorates in
Egypt; three form the Al Gharbia governorate, including from two
sites of cultivated soil, (Al-Dalgamon, N"22.67'30˚48
E"22.37'30˚50; Tanta, N"40.77'30˚47 E"59.94'31˚00; and one sample
obtained from soil contaminated with oil, the Oil & Soap
Company, N"20.82'30˚49 E"37.58'30˚49).
Soil from a total of five sites in the Al-Behira
governorate, including three cultivated soil sites (Al-Delengat,
N"31.94'30˚49 E"49.36'30˚32; and two sites from Itay El-baroud,
N"57.49'30˚52 E"09.76'30˚40 and N"06.87'30˚52 E"41.94'30˚39), a
non-cultivated site (Shubrakhit, N"55.38'31˚01 E"34.20'30˚42) and
one site from soil contaminated with oil obtained from Al-Delengat
(N"20.82'30˚49 E"37.58'30˚49) were obtained in the present
study.
Air-borne fungi were isolated from the same sites by
opening a Petri dish containing sterilized Czapek-Dox agar plates
for ~30 min in the open air, then incubated for 7 days at
28±2˚C.
Fungal isolation and screening of
L-asparaginase activity
Fungi were isolated on modified Czapek-Dox agar
plates composed of (in gl-1) agar powder supplemented
with L-asparagine (10.0 gl-1) and 3 ml/l 2.5% phenol red
dye as an indicator (21). Plates
were incubated at 28±2˚C for 3-5 days. Developed fungal isolates
surrounded by a pink zone were selected and grown on potato
dextrose slants. Fungal colonies isolated in the previous step were
descriptively screened for L-asparaginase capability using a rapid
plate assay, as described below. A 5-mm fungal mycelial plug was
inoculated onto the modified Czapek-Dox's agar in triplicates.
All plates were incubated at 28±2˚C for 3-5 days.
Positive asparaginolytic fungi were detected on the yellow-colored
medium based on the appearance of a pink zone around the fungal
colony, indicating L-asparaginase activity due to the change in
color of the phenol red indicator. The diameter of the pink zone
was measured and used to compare fungal isolates.
Identification of fungal isolates
For determination of morphological structures,
portions of fungal growth were mounted in lactophenol cotton blue
stain on clean slides, and the prepared slide was examined under a
light microscope at x40 and x100 magnification for vegetative
mycelium; septation, diameters, conidiophores (sporangiophores) and
the reproductive structures, conidia and sporangiospores amongst
others. Fungal colonies were examined at x10 magnification. The
colony size, texture and color of the colonies were assessed. The
fungal genera and species were identified according to their
cultural properties, using morphological and microscopical
screening as described previously (22-27).
Culturing technique and the assessed
parameters
The semi SSF was performed using WB. Unless
otherwise specified, the fermentation medium of L-asparaginase
production was composed of 1 g WB, moistened with 1 ml distilled
water placed in 250 ml Erlenmeyer flasks to yield a 1:1 (w/v)
growth medium. The medium was sterilized by autoclaving for 15 min
and inoculated with 1 ml fungal spore suspension (1x106
spores), such that the moisture content was equivalent to 66.7%.
Next, inoculated batch fermentation flasks were incubated under
static conditions at 28±2˚C.
The main parameters that were expected to influence
the production process were investigated by varying only a single
factor at a time, and keeping the remaining factors constant. The
studied factors were fermentation time (2-10 days), nitrogen source
at 2.5 mg nitrogen per gram of dry WB (gds), and its concentration
(based on the nitrogen-equivalent), the WB to water ratio (1:2,
1:4, 1:6, 1:8 and 1:10, w/v) and, finally, the moistener pH, using
citrate phosphate buffer (3.0-5.4) and sodium phosphate buffer (pH
5.8-7.8).
Extraction of L-asparaginase
After incubation, enzyme extraction was performed by
adding 10 ml sodium phosphate buffer (pH 7) to the fermented
substrate to a final volume of 20 ml to attain an equivalent volume
in each of the different flasks, shaken for 30 min on a rotary
shaker at 200 rpm and filtered through a Whatman No. 1 filter paper
(Whatman plc; GE Healthcare Life Sciences). The filtrate was then
centrifuged at 1,500 x g and 4˚C for 15 min.
Quantitative assay of L-asparaginase
activity
The resultant extraction was assayed for
L-asparaginase activity by quantifying ammonia formation
spectrophotometrically using Nessler's reagent as described
previously (28). One unit (U) of
L-asparaginase is expressed as the amount of enzyme per gram of WB
dry substrate that catalyzes the formation of one µmol ammonia per
min under the assay conditions. A standard curve was prepared with
ammonium chloride.
Partial purification and protein
estimation
The crude Rhizopus oryzae AM16
L-asparaginase, obtained after fermentation at the optimum
conditions, was precipitated by adding ammonium sulfate with
constant stirring until 80% saturation was achieved, and was then
incubated overnight at 4˚C to ensure that the precipitation had
completed. The precipitate was separated by centrifugation at 2,100
x g for 30 min at 4˚C. The protein precipitate was resuspended in
0.01 M Tris-HCl buffer (pH 7.2) and dialyzed overnight against the
same buffer. The concentration of protein was estimated using a
Bradford assay (28), with BSA as
the standard. The specific activity is defined as the units of
L-asparaginase per mg of protein.
Cell viability assay
MTT assays were used to assess the two
L-asparaginase preparations (crude and dialyzed), to determine
their cytotoxic effects against four tumor cell lines: HepG2,
MCF-7, HCT and A549 carcinoma cell lines.
When the cells reached 75-90% confluence, usually 24
h after passaging, the cell suspension was prepared in complete
growth medium (RPMI) supplemented with 50 mg/ml gentamycin.
Aliquots of 100 µl cell suspension (1x105 cells/ml) were
added to each well of a 96-well tissue culture plate. The blank
wells contained complete RPMI medium in place of cell
suspension.
The cells were incubated for 24 h at 37˚C in a
humidified incubator with 5% CO2. After the formation of
a complete monolayer cell sheet in each well of the plate, the
enzyme (crude and dialyzed) was added in concentrations ranging
from 15.63-500 µg/ml. Serial two-fold dilutions of the samples were
added into a 96-well tissue culture plate using a multichannel
pipette. After 24 h, the culture supernatant was replaced with
fresh medium. Next, the cells were incubated at 37˚C with 100 µl
MTT solution (5 mg/ml) for 4 h. Subsequently, the MTT solution was
removed and 100 µl DMSO was added to each well. The absorbance was
detected at 570 nm using a microplate reader (Tecan Group, Ltd.).
The absorbance of untreated cells was considered as 100%. The
experiments were repeated three times, independently the percentage
of cell viability was calculated. The tested sample was, then,
compared using the half-maximal inhibitory concentration
(IC50) value: The concentration of an individual
compound leading to 50% cell death that was estimated from
graphical plots of viable cells vs. enzyme concentrations (28-32).
Statistical analysis
Data are presented as the mean ± standard deviation
of at least three replicates. Statistical analysis was performed
using CoStat version 6.4 (CoHort Software).
Results
Isolation and screening of
L-asparaginolytic fungi
L-asparaginase-producing fungi were isolated from
the soil and air on modified Czapek-Dox agar plates supplemented
with L-asparagine as a sole nitrogen source. Fungi were screened
for L-asparaginase potential using a rapid plate assay, and were
detected based on the presence of a pink zone around the fungal
colony. Isolates that showed the largest diameter pink zones were
selected (data not shown). The developed pink color on the
yellow-colored medium indicates L-asparaginase activity, as
NH3 is liberated, and this changes the phenol red color
to pink (21). The highest positive
asparaginolytic fungi were chosen and identified. Based on the
growth characteristics and morphological features, 4 isolates, with
the highest qualitative L-asparaginase in the plate assays were
obtained: Aspergillus flavus, Penicillium expansum,
Rhizopus oryzae AM16 and Trichoderma viride.
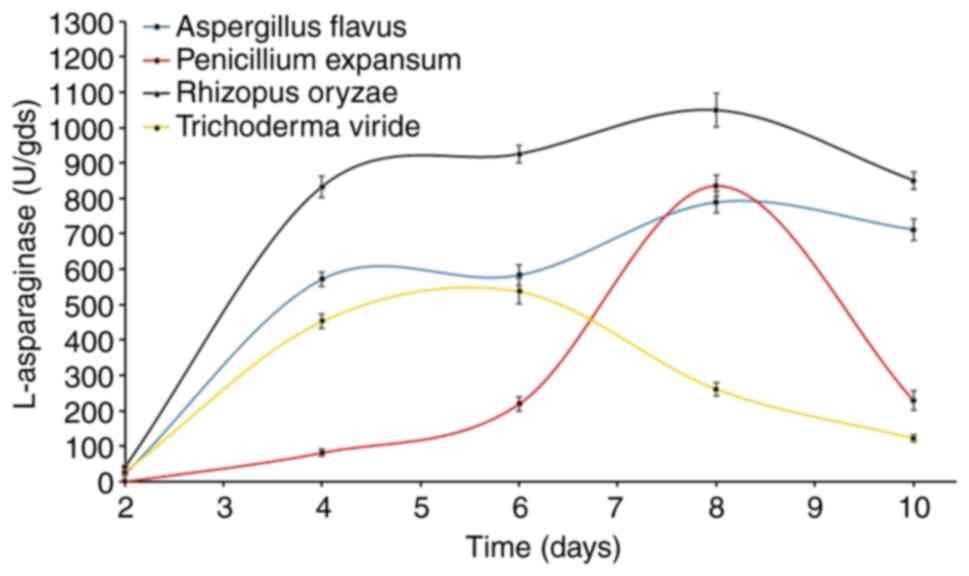
Time vs. L-asparaginase
production
The time profile of L-asparaginase biosynthesis of
the four fungi was explored for extended time periods of up to 10
days on the WB-based medium (Fig.
1). The time course of enzyme production differs between fungal
species depending on the cultivation conditions and the
physiological properties of each specific species.
Another purpose of this current test was to select
the most active asparaginolytic fungus for further investigation.
The results showed that L-asparaginase started to accumulate after
two days of fermentation, and there were detectable levels of
L-asparaginase being produced by three of the four fungi, and for
Penicilliumexpansum, its asparaginolytic activity appeared after
the 4th day. However, maximum L-asparaginase biosynthesis was
observed after 10 (Aspergillus flavus), 8 (Penicillium
expansum), 8 (R. oryzae AM16) and 6 (Trichoderma
viride) days. Notably, R. oryzae AM16 was superior to
all other tested fungi, recording 1,048.6 U, followed by
P.expansum (836.5 U), A. flavus (789.4 U) and finally
T. viride (537.8 U). Based on these data R. oryzae
AM16 was chosen for further study of enzyme production.
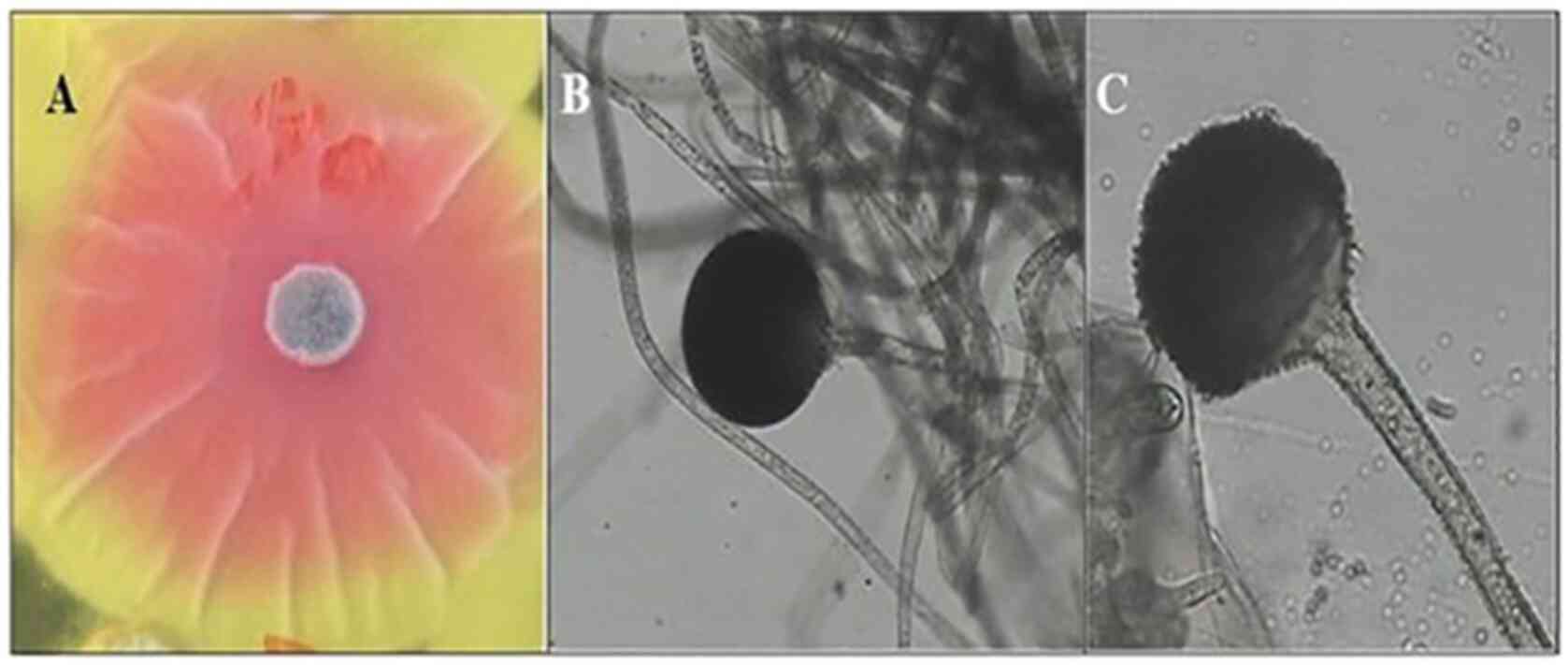
R. oryzae AM16 was selected as the most
active isolate, a representative image of the active isolate is
shown in Fig. 2. A pink zone around
the fungal colony was clearly visible (Fig. 2A). Colonies of R. oryzae AM16
appeared white initially, becoming brownish with age and grew to ~1
cm in thickness. The isolates can grow at temperatures between
7-44˚C with optimum growth at 37˚C. Sporangiospores are straight,
pale brown to brown, and grow between 210-2,500 µm in length and
5-18 µm in diameter (Fig. 2B and
C). The sporangia are globose or
sub-globose, wall spinous and black when mature, and 60-180 µm in
diameter. The columellae are globose, sub-globose. Sporangiospores
are elliptical, globose or polygonal, they are striated and grow
5-8 µm in length. The chlamydospores are abundant, globose, ranging
in 10-24 µm in diameter, elliptical and cylindrical.
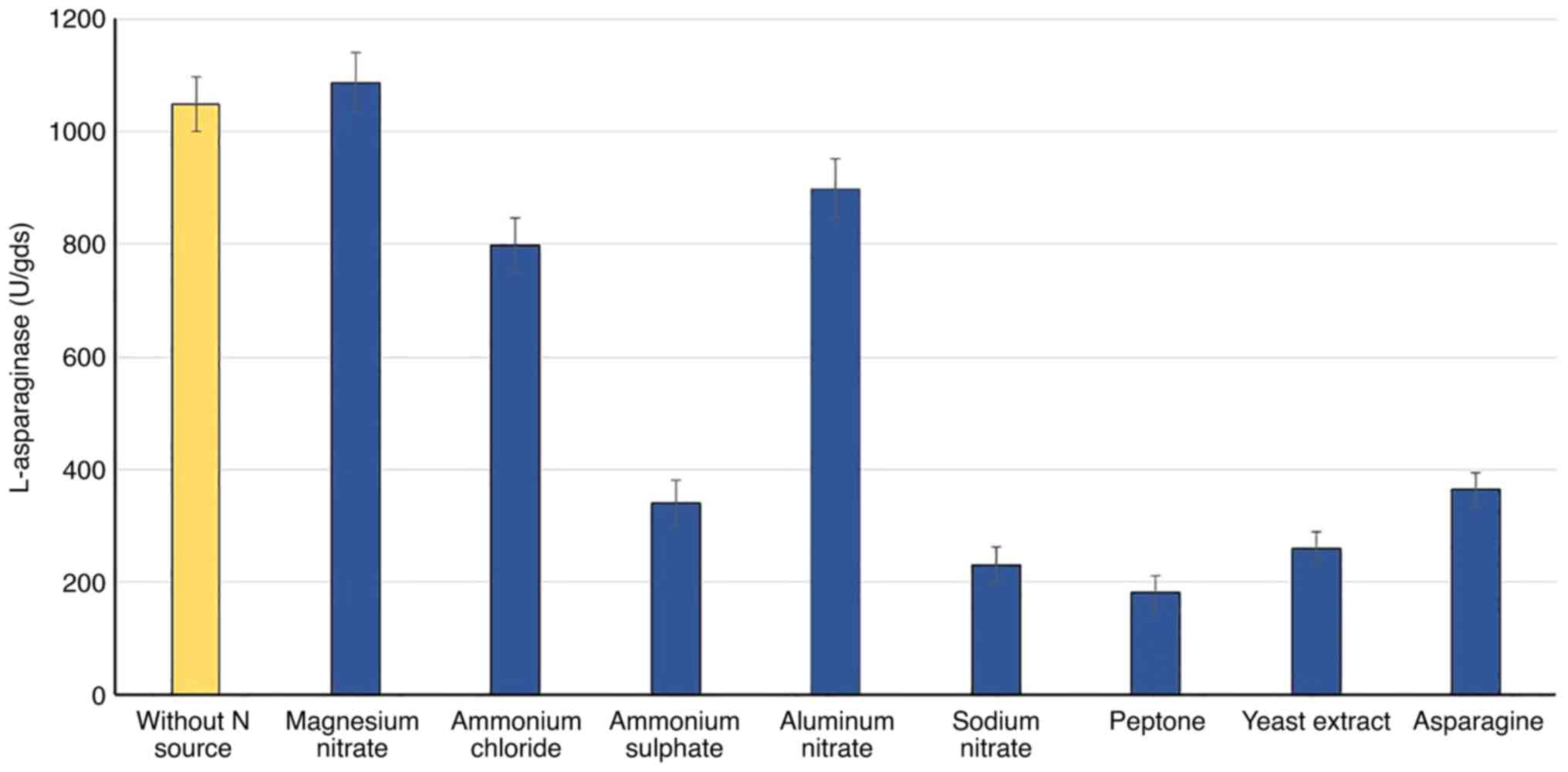
Nitrogen source
Various nitrogen sources were added separately,
based on the N-equivalent (5 mg N/gds), to WB fermented substrate
to study their effects on the accumulation of L-asparaginase. The
results in Fig. 3 show that the
enzyme biosynthesis by R. oryzae AM16 was considerably
influenced by the type of nitrogen source. Compared with the
control (without nitrogen source), all nitrogen sources were found
to suppress enzyme production, based on the lower values of
L-asparaginase production with the various N sources, apart from
magnesium nitrate. In comparison to organic nitrogen, the inorganic
nitrogen sources enhanced and stimulated the highest production of
L-asparaginase; magnesium nitrate (1,086 U) was superior, followed
by aluminum nitrate (899) and ammonium chloride (799 U).
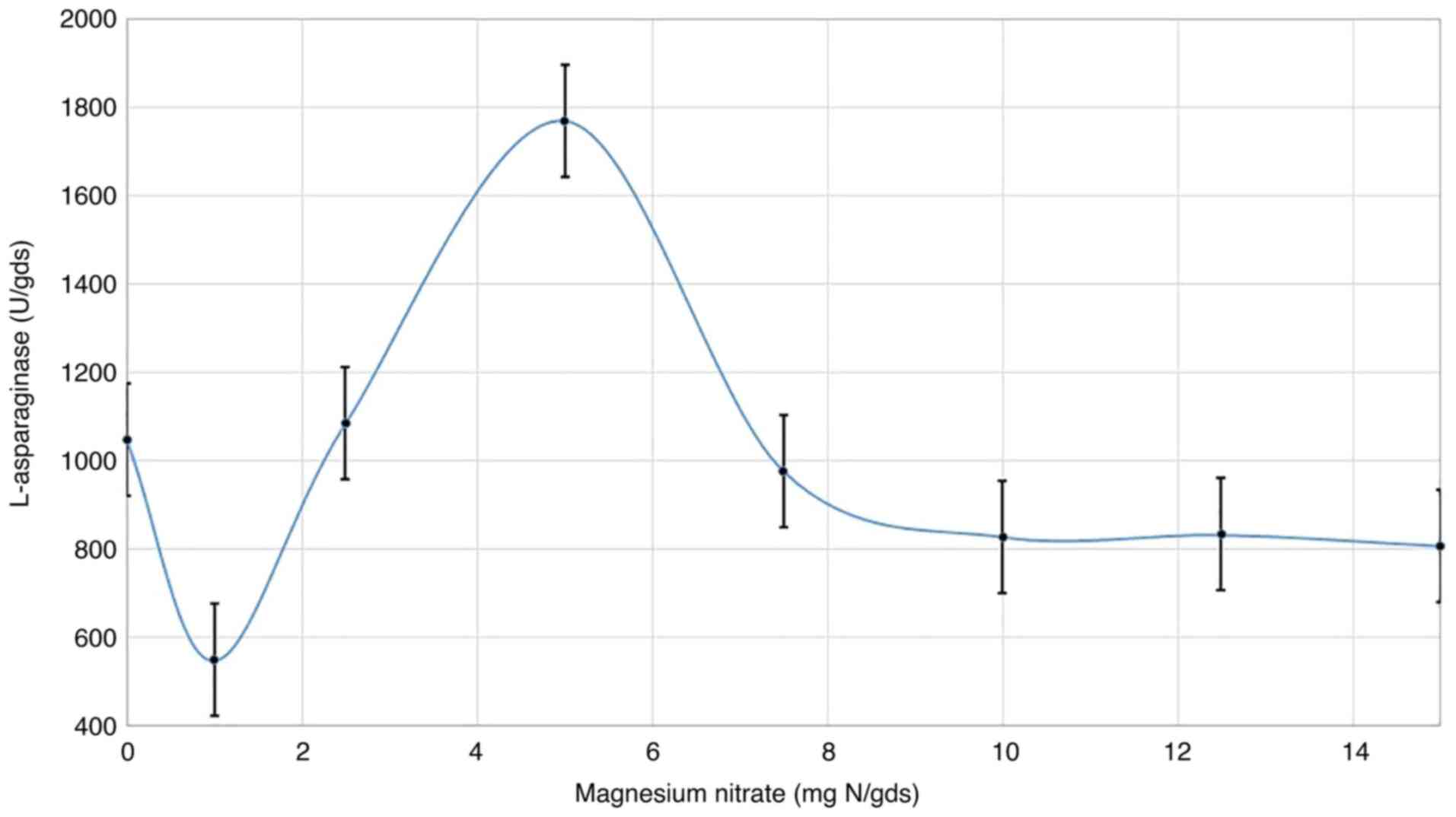
Magnesium nitrate concentration
Magnesium nitrate, as the best source of nitrogen,
was incorporated in the fermentation medium at various
concentrations. The results (Fig.
4) show that different concentrations of magnesium nitrate
notably influenced L-asparaginase synthesis. Although there was a
marked sharp decline at the initial concentration of magnesium
nitrate (1 mg N/gds) compared with the control (0 mg N/gds),
visible increases in enzyme production (1,770.8 U) were observed
with higher concentrations, up to 5 mg N/gds, before decreasing
again when the concentration was increased further. With 5 mg
N/gds, a reduction in L-asparaginase synthesis was observed.
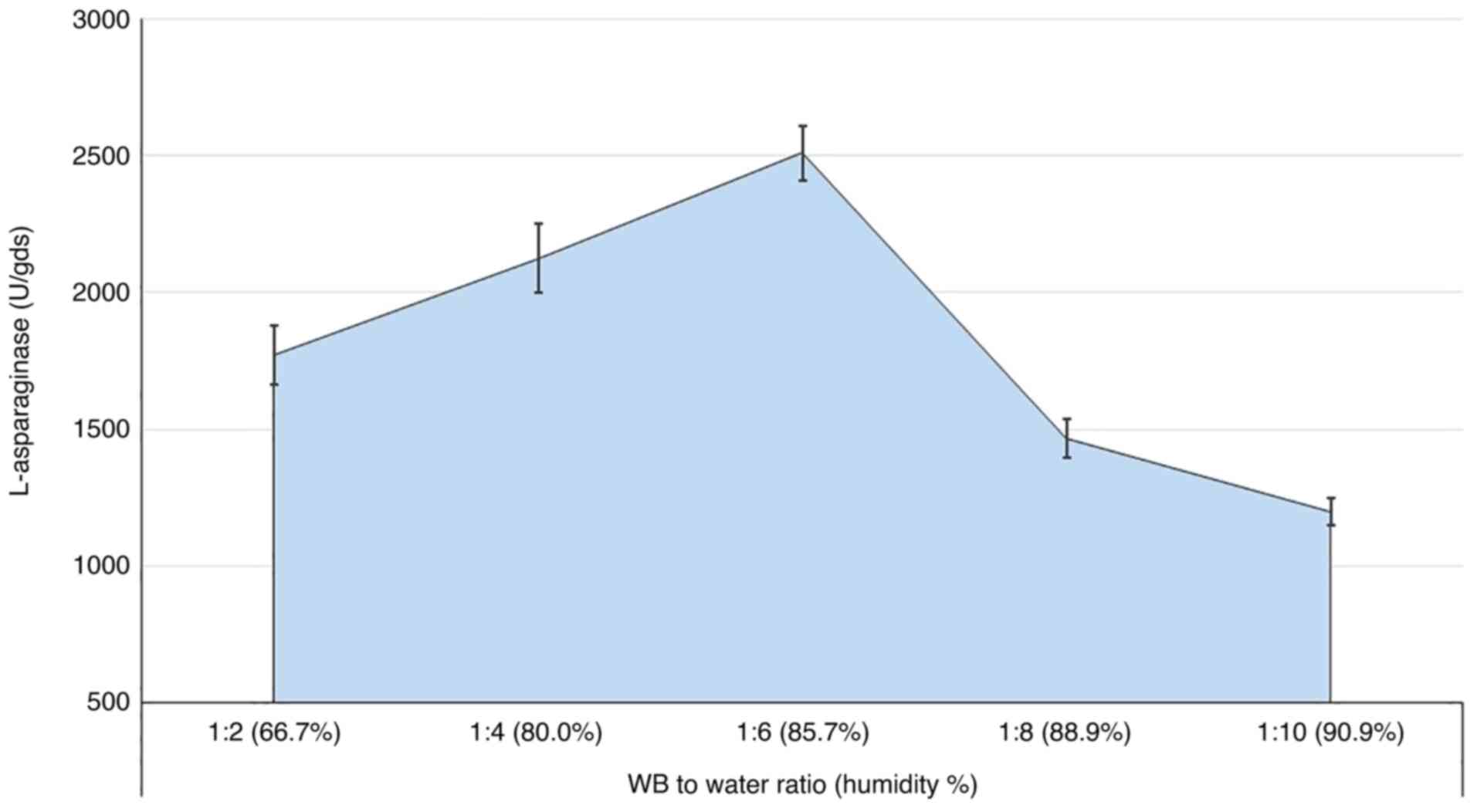
Medium humidity
The humidity of the medium is a determining factor
in the production process, even in SSF. Thus, the ratio between the
fermented substrate to the available water was explored in the
present study, with various ratios being investigated (Fig. 5). The results showed that increasing
the WB: water ratio to 1:6 (representing 85.7% moisture) increased
the secretion of the L-asparaginase levels gradually to 2,509.6 U.
Higher ratios sharply reduced the enzyme levels, although a marked
level of the enzyme secretion was still observed.
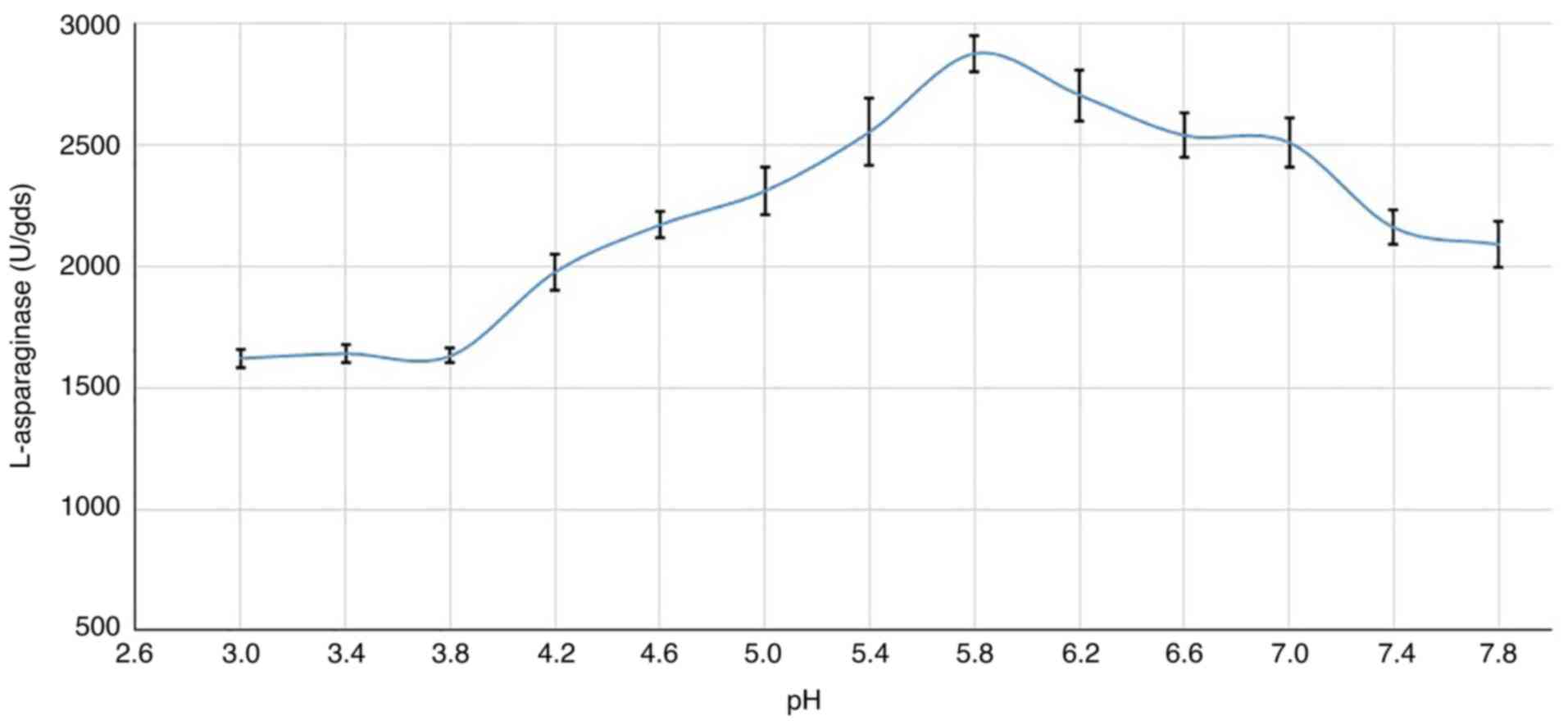
Culture pH
In the present study, maximal enzyme production was
observed at pH 5.8 (2,875.9 U) as shown in Fig. 6, and production at a pH higher or
lower than this resulted in a decrease in L-asparaginase production
by R. oryzae AM16. However, the fungus was still able to
secrete a reasonable amount of the enzyme in a wide range of pHs
(4.2-7.8).
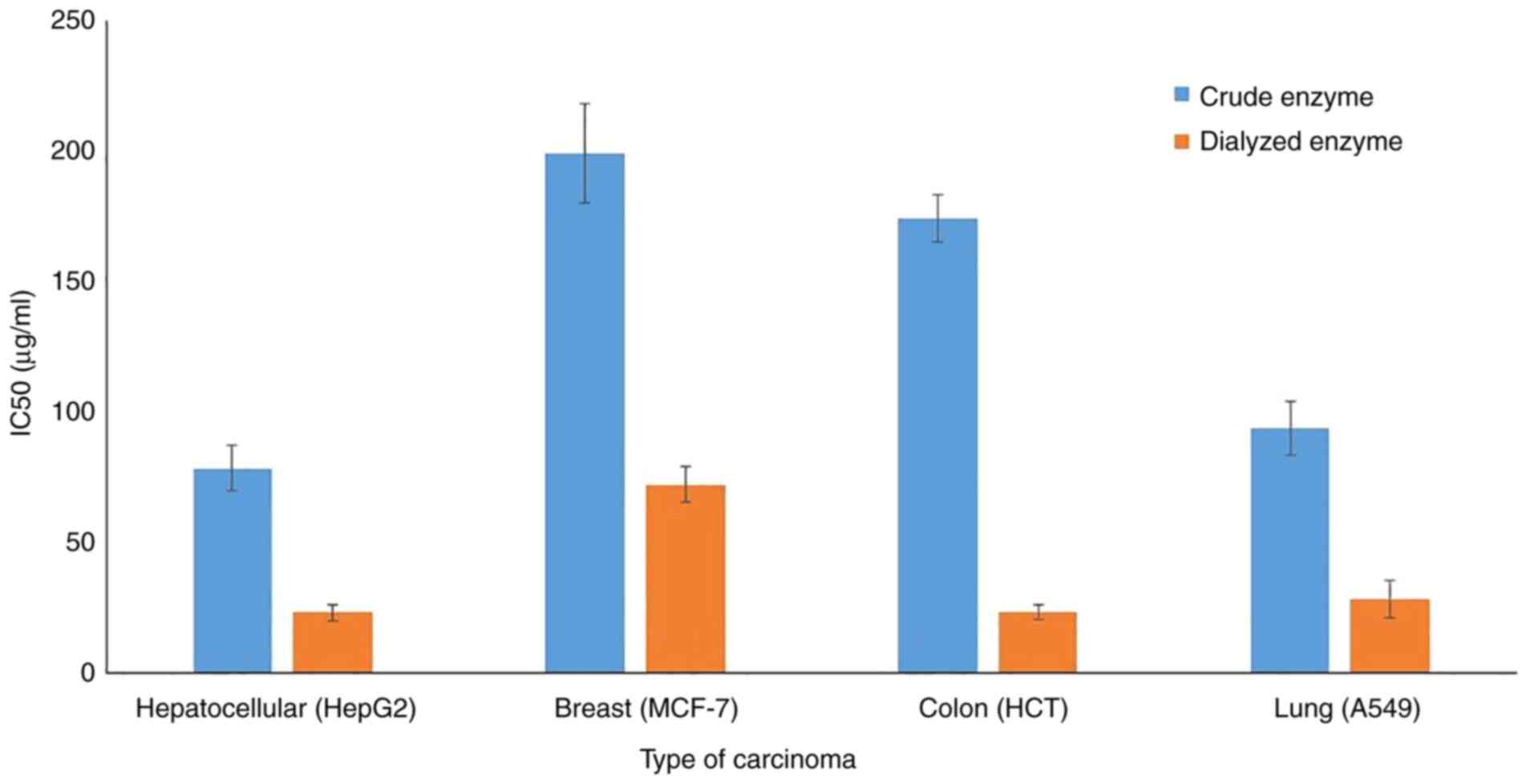
Anticancer activity
The in-vitro cytotoxic effect of crude and
partially purified L-asparaginase on the growth of four types of
human tumor cell lines were assessed. Dual incubation of carcinoma
cells with various doses of L-asparaginase in the tissue culture
medium revealed that the inhibition of human tumor cell lines was
dose-dependent. The dialyzed enzyme accomplished its inhibitory
effect at lower doses compared with the crude preparation; this
trend was true for all the tested cell lines (Fig. 7). The IC50 values of the
dialyzed enzyme were 23.4 (HepG2), 72.4 (MCF-7), 23.6 (HCT) and
28.6 (A549) µg/ml. It is hypothesized that the dialysis process
concentrated the enzyme protein without adverse impact on its
activity and performance. All cell lines were found to be sensitive
to the dialyzed protein, the sensitivity of cell lines in
descending order are HepG2 > HCT > A549 > MCF-7. However,
this order varied when using the crude protein, the HepG2 and HCT
cells were the most affected by both the crude and dialyzed
enzyme.
Discussion
Several microorganisms, such as bacteria, yeasts,
molds and filamentous fungi, have been reported to produce
L-asparaginase. Asparaginases of microbial origin have been shown
to be more stable than those from animal and plant sources. Despite
bacteria being the primary source of L-asparaginase, the enzymes
obtained from them have been shown to possess several adverse side
effects. Therefore, identifying a novel source of asparaginases is
of utmost importance for both clinical and biotechnology purposes.
Of the microbes, the most potent producers of asparaginases are
fungi (31). Thus, the aim of the
present study was to identify an L-asparaginase producing fungus
and determine the optimal fermentation conditions, as well as
studying its anticancer activity. For example, positive
asparaginolytic fungi were found to exist in marine environments
and endophytes of seaweed, belonging to the Alternaria,
Chaetomium, Cladosporium, Colletotrichum,
Curvularia, Nigrospora, Paecilomyces,
Phaeotrichoconis, Phoma and Pithomyces genera
(32). Moreover, terrestrial
endophytic fungi with positive asparaginolytic activity of the
genus Colletotrichum, Eupenicillium and
Talaromyces were also reported (32). Several asparaginolytic fungi, such
as Alternaria sp., Aspergillus nidulans, A. niger,
A. oryzae, A. tamarii, A. terreus,
Cylidrocapron obtusisporum, Mucor sp. and Fusarium
roseum have been described (4,8).
The R. oryzae AM16 investigated in the
present study was isolated from air. To the best of our knowledge,
this is the first report to describe the isolation of
asparaginolytic fungus from the air. It is also worth mentioning
that the fungus was able to secrete L-asparaginase constitutively
without the presence of an induction substrate. Although
fermentation conditions vary according to the microorganism, the
enzyme can be produced constitutively and/or after induction
(21,33).
For optimization of production conditions, the
incubation period is one of the most critical parameters in
microbial fermentation. As a general rule, the majority of the
microbial enzymes can only reach maximal production after a certain
period of incubation, which allows the culture to grow at a steady
state, therefore enzyme production is mostly based on the specific
growth rate of the microbe; thus, growth rate and enzyme synthesis
are primarily influenced by incubation time (4). Indira et al (31) reported 5 days of fungal incubation
for maximum enzyme production (35.72 U). In comparison, the current
fungal strain required a longer incubation period (8 days) to reach
peak enzyme secretion; however, its L-asparaginase yield was
increased by ~30-fold.
The L-asparaginase from R. oryzae AM18 is
classified as an extracellular enzyme. Extracellular L-asparaginase
is more advantageous than the intracellular counterpart due to the
lower production costs, higher degree of accumulation of the enzyme
in the culture broth, ease of extraction and purification, and
simpler downstream processing methods (9,34,35).
Two related families of L-asparaginase have been identified:
L-asparaginase, which is a low-affinity enzyme to the substrate,
found in the cytoplasm and secreted constitutively, and
L-asparaginase II, a high-substrate-affinity periplasmic enzyme
that is activated during anaerobiosis (34). L-asparagine was not found to induce
enzyme production. A possible explanation is that the enzyme could
be induced by its substrate and could also be secreted
constitutively (21,35). Conversely, several pieces of
literature reported L-asparagine as the most favorable source of
nitrogen (4). Other inorganic
nitrogen sources, such as ammonium sulfate by A. terreus
(31) and sodium nitrate by
Fusarium oxysporum (34),
showed increased enzyme production. Generally, the presence of
additional nitrogen sources alongside the native nitrogenous
compounds present in the fermented substrate promotes microbial
growth and, consequently, enzyme secretion, since the various kinds
of nitrogen (organic and inorganic) are metabolized to various
important molecules in the cell, including L-asparaginase (31).
In addition to the nitrogen source, the
concentration of the source also has a pronounced influence on
enzyme production. The majority of the industrially used enzymes
utilize nitrogen sources either in an organic or inorganic form, or
sometimes both. The proteinaceous part of the WB is organic,
accounting for 15.2-16.9% of the nitrogen content, consists of
18.6% glutamic acid and 7.2% aspartic acid (14), and in several instances, growth is
faster when the supply consists of both organic and inorganic
nitrogen sources (31). Supporting
the fermentation medium with additional inorganic N in the current
study boosted L-asparaginase production by R. oryzae AM16.
Additionally, the fungus showed slight differences in the
production pattern in the presence of nitrogen-free medium.
The obtained enzyme yield was higher than those
obtained in previous studies, who reported that inorganic nitrogen
sources, particularly ammonium sulfate and sodium nitrate, were
preferable for L-asparaginase production by fungi (27,34).
However, none of these studies used WB as a substrate, and using
cheap agricultural byproducts, such as WB, could be more
cost-effective. The composition of WB reflects high nutritional
quality; unfortunately, the majority of the minerals (including P,
Fe, Zn and Mg) in WB are stored in the form of phytates, forming
complexes, which drastically reduce their bioavailability (14). Thus, inorganic nitrogen sources,
especially magnesium nitrate, were found to be more favorable than
organic sources, in order to compensate for the shortage of
elements due to its association with phytates. However, earlier
work reported that magnesium nitrate supported favorable growth and
fair sporulation of Claviceps microcephala (36,37).
The submerged fermentation technique has been widely
used, and is well established for L-asparaginase production,
although it carries with it a few disadvantages. Owing to
cost-effectiveness, simplicity of the procedure, lower possibility
of contamination, ease of recovery of the final bioproducts and
lower volume of waste-water generation, SSF has emerged as the
preferred technique for bioproduction, particularly when utilizing
agro-industrial waste and byproducts (4). The optimum ratio of WB: water (1:6),
obtained in the current study, was considered a type of SSF, but
with a higher amount of water, in which the free water content had
been increased in order to facilitate nutrient availability and
fermentation control. Thus, it was termed semi SSF. Similarly,
freshly crushed sorghum was used to produce biodiesel under semi
SSF of sweet sorghum, and this proved to be a more efficient method
(giving yields 9-11%) than SSF (16). According to Pandey et al
(18), the initial moisture content
of the solid substrate plays an important role, dictating the
growth of the organism and enzyme production. A certain quantity of
water is essential for generation and synthesis of new cells. Very
high moisture levels compacts the substrates and prevents oxygen
penetration in the SSF processes, whereas very low moisture content
inhibits growth, enzyme activity and accessibility of nutrients
(38). Since the low free liquid
content binds to and lowers nutrient availability, it restricts
fermentation control. However, this also depends on the kind of
microorganism being assessed; in the case of fungi, a wider
moisture range (20-70%) supports improved growth and metabolic
activities, but for bacteria, only a higher moisture content of the
solid matrix can yield better performance (15-17,38,39).
Another determinant factor during the fermentation
process is the pH of the culture. The initial pH level plays a key
role in successful L-asparaginase production. It is well known that
growth and metabolism along with enzyme production are regulated by
the pH of the culture, and every organism has its own optimal pH.
Any modification in this pH could result in a negative impact on
the enzymatic yield; therefore, pH was considered a significant
issue affecting L-asparaginase biosynthesis (4,39).
Contrarily, the majority of previous studies reported that
L-asparaginase production tends to be maximized under neutral
(31) or alkaline (39) pH conditions and minimum production
was observed at lower pHs. The lower pH condition for the maximum
secretion of the current R. oryzae AM16 L-asparaginase
suggests the presence of a new variant of the enzyme with unique
properties.
Summarizing the fermented substrate; in the SSF
process, the selection of appropriate substrate should be based on
nutrient value, availability and cost. The solid substrate not only
provides nutrients for growth and metabolic activities, but also
provides anchorage for microbe growth. WB is considered as the
universal agro-industrial substrate for biomolecule production, it
has a complete nutritious medium for microorganisms and physically,
remains loose even under various conditions of moisture, providing
a large surface area (39-41).
Determining the unique biochemical structure of WB
showed the presence of high nutritional value of both carbon and
nitrogen, in addition to vitamins, mineral salts and other complex
components (11,14). Of the carbon present, various
soluble sugars such as glucose, xylose, arabinose and galactose
were detected, which assist in the initiation of growth and
replication of microorganisms, WB has a low lignin content, and
relatively more protein content when compared with other
agro-industrial substrates (12-14).
The balanced biochemical composition of WB makes it a determinant
factor for the favored use in microbial growth and enzyme
production. This conclusion could be applied in the present
investigation, with hypersecretion of L-asparaginase of 2,875.9 U
by R. oryzae AM16.
IC50 is commonly used as a measure of
antagonist drug potency in pharmacological research, according to
the FDA, the IC50 represents the concentration of a drug
that is required for 50% inhibition in-vitro. The lower the
IC50, the higher the effectiveness of the drug. Recent
medical research on cancer cells has made L-asparaginases and
related enzymes potentially key therapeutic tools in the treatment
of tumors, suggesting that these protein molecules will most likely
be exploited in future clinical applications. L-asparaginase acts
on extracellular L-asparagine, reducing its concentration.
L-asparagine is a non-essential amino acid, which affects the
viability of the cells when its concentration is reduced below the
threshold level. Unlike tumor cells, normal cells have the ability
to synthesize intracellular L-asparagine using asparagine
synthetase. However, the amount of L-asparagine required by a
specific cell type will vary, and this explains the differences in
the IC50 values observed amongst the four cell types.
Moreover, the difference between crude and partially purified
proteins on the same cell line may be attributed to differences in
the presence of minerals and other components present in each
preparation.
Unlike tumor cells, in the case of deficiency,
normal cells have the ability to synthesize intracellular
L-asparagine, using asparagine synthetase. Neoplastic cells, on the
other hand, lack this enzyme and thus require an abundant exogenous
supply of L-asparagine for proper growth. In the absence of such a
supply of L-asparagine, the levels are rapidly depleted, leading to
the death of the neoplastic cells. The depletion of L-asparagine by
L-asparaginase from the plasma results in the inhibition of DNA,
RNA and protein synthesis, resulting in induction of apoptosis.
Therefore, the therapeutic action of L-asparaginase is mediated by
depletion of such amino acids from lymphatic tumor cells, resulting
in starvation and ultimately death of the cells (4,6,8).
In conclusion, the current study identified a new
L-asparaginase with promising anticancer activity on various cancer
cell lines. The modest sequential optimization trials for
L-asparaginase production by R. oryzae AM16 on semi SSF
succeeded in inducing the hyperproduction of the enzyme, with a
maximum production of 2,875.9 U/gds after 8 days of incubation with
85.7% moisture, in the presence of magnesium nitrate (5 mg N/gds)
at pH 5.8. The anticancer activity of the dialyzed enzyme against
certain types of cancer cells (HepG2, MCF-7, HCT and A549) was
confirmed at low doses.
Acknowledgements
The authors acknowledge Princess Nourah bint
Abdulrahman University Researchers Supporting Project (no.
PNURSP2022R5), Princess Nourah bint Abdulrahman University, Riyadh,
Saudi Arabia.
Funding
Funding: The present study was funded by the Princess Nourah
bint Abdulrahman University Researchers Supporting Project (no.
PNURSP2022R5), Princess Nourah bint Abdulrahman University, Riyadh,
Saudi Arabia.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
AAIM, MMEM and WIAS conceptualized and designed the
study, and performed the experiments. SIO, SAG, MAA, AAA, HAF and
RAE analyzed and interpreted the data. SAG, AAA, AAIM, MMEM and
WIAS drafted the manuscript. All authors revised the manuscript as
well as read and approved the final version. AAIM, MMEM and WIAS
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cooper GM: The development and causes of
cancer. In: The Cell: A Molecular Approach. 2nd edition. Sinauer
Associates, Sunderland, MA, 2000.
|
|
2
|
Ramya LN, Doble M, Rekha VPB and
Pulicherla KK: L-Asparaginase as potent anti-leukemic agent and its
significance of having reduced glutaminase side activity for better
treatment of acute lymphoblastic leukaemia. Appl Biochem
Biotechnol. 167:2144–2159. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kebeish R, El-Sayed A, Fahmy H and
Abdel-Ghany A: Molecular cloning, biochemical characterization, and
antitumor properties of a novel L-asparaginase from
Synechococcuselongatus PCC6803. Biochemistry (Mosc). 81:1173–1181.
2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Naser S, Saber W, El-Metwally M, Moustafa
M and El-Kott A: Fungal assembly of L-asparaginase using
solid-state fermentation: A review. Biocell. 44(147)2020.
|
|
5
|
Duval M, Suciu S, Ferster A, Rialland X,
Nelken B, Lutz P, Benoit Y, Robert A, Manel AM, Vilmer E, et al:
Comparison of Escherichia coli-asparaginase with
Erwinia-asparaginasein the treatment of childhood lymphoid
malignancies: Results of a randomized European Organisation for
research and treatment of Cancer-Children's Leukemia Group phase 3
Trial. Blood. 99:2734–2739. 2002.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Verma N, Kumar K, Kaur G and Anand S:
L-asparaginase: A promising chemotherapeutic agent. Crit Rev
Biotechnol. 27:45–62. 2007.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kornbrust BA, Stringer MA, Lange NE,
Hendriksen HV, Whitehurst R and Oort MV: Asparaginase-an enzyme for
acrylamide reduction in food products. In: Enzymes in Food
Technology. 2nd edition, pp59-87, 2010.
|
|
8
|
Batool T, Makky EA, Jalal M and Yusoff MM:
A comprehensive review on L-Asparaginase and its applications. Appl
Biochem Biotechnol. 178:900–923. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Vivekanandha S, Muruganantham S and
Paulraj P: Anovel role of L-asparaginase enzyme production from
fungal species. J Microbiol Biotechnol Res. 3:7–14. 2013.
|
|
10
|
Thomas L, Larroche C and Pandey A: Current
developments in solid-state fermentation. Biochem Eng J.
81:146–161. 2013.
|
|
11
|
Sreenivasulu V, Jayaveera KN and Rao PM:
Solid-state fermentation for the production of L-asparaginase by
Aspergillus sp. J Pharmacogn Phytochem. 1:21–25. 2009.
|
|
12
|
Doriya K and Kumar DS: Optimization of
solid substrate mixture and process parameters for the production
of L-asparaginase and scale-up using tray bioreactor. Biocatalysis
Agricultural Biotechnol. 13:244–250. 2018.
|
|
13
|
Apprich S, Tirpanalan O, Hell J, Reisinger
M, Böhmdorfer S, Siebenhandl-Eh S, Novalin S and Kneifel W: Wheat
bran-based biorefinery 2: Valorization of products. LWT-Food Sci
Technol. 56:222–231. 2014.
|
|
14
|
Prueckler M, Siebenhandl-Ehn S, Apprich S,
Hoeltinger S, Haas C, Schmid E and Kneifel W: Wheat bran-based
biorefinery 1: Composition of wheat bran and strategies of
functionalization. LWT-Food Sci Technol. 56:211–221. 2014.
|
|
15
|
Andersson AA, Dimberg L, Åman P and
Landberg R: Recent findings on certain bioactive components in
whole grain wheat and rye. J Cereal Sci. 59:294–311. 2014.
|
|
16
|
De Brier N, Hemdane S, Dornez E, Gomand
SV, Delcour JA and Courtin CM: Structure, chemical composition and
enzymatic activities of pearlings and bran obtained from pearled
wheat (Triticumaestivum L.) by roller milling. J Cereal Sci.
62:66–72. 2015.
|
|
17
|
Babu CR, Ketanapalli H, Beebi SK and
Kolluru VC: Wheat bran-composition and nutritional quality: A
review. Adv Biotechnol Micro. 9(555754)2018.
|
|
18
|
Pandey A, Soccol CR, Rodriguez-Leon JA and
Nigam PSN: Solid-state fermentation in biotechnology: Fundamentals
and applications. Asiatech Publishers, Inc., New Delhi, 2001.
|
|
19
|
Economou CN, Makri A, Aggelis G, Pavlou S
and Vayenas DV: Semi-solid state fermentation of sweet sorghum for
the biotechnological production of single cell oil. Bioresource
Technol. 101:1385–1388. 2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Reid Y, Storts D, Riss T, Minor L,
Markossian S, Grossman A, Brimacombe K, Arkin M, Auld D, Austin CP,
et al: Authentication of human cell lines by STR DNA profiling
analysis. In: Assay Guidance Manual. Markossian S, Grossman A,
Brimacombe K, et al (eds). Eli Lilly & Company and the
National Center for Advancing Translational Sciences, Bethesda, MD,
2004.
|
|
21
|
Gulati R, Saxena RK and Gupta R: A rapid
plate assay for screening L-asparaginase producing micro-organisms.
Lett Appl Micro. 24:23–26. 1997.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Raper KB, Austwick PK and Fennell DI: The
Genus Aspergillus. R.E. Krieger, Malabar, FL, 1977.
|
|
23
|
Pitt JI: The genus Penicillium and its
teleomorphic states Eupenicillium and Talaromyces.
Academic Press, London and New York, 1979.
|
|
24
|
Pitt JI: A Laboratory Guide to Common
Penicillium Species. Commonwealth Scientific and Industrial
Research. North Ryde, 1986.
|
|
25
|
Kitch MA and Pitt JI: A laboratory guide
to the common Aspergillus species and their Teleomorphs.
Published by Commonwealth Scientific and Industrial Research
Organization. Division of Food Processing, Sydney, 1992.
|
|
26
|
Kubicek CP and Harman GE: Trichoderma and
Gliocladium. Taylor and Francis Ltd., London and Bristol, 1998.
|
|
27
|
Domsch KH, Gams W and Anderson TH:
Compendium of Soil Fungi. Academic Press, London, 1980.
|
|
28
|
Baskar G and Renganathan S: Production of
L-asparaginase from natural substrates by Aspergillus
terreus MTCC 1782: Optimization of carbon source and operating
conditions. Int J Chem Reactor Engineering: Oct 18, 2011 (Epub
ahead of print). doi: 10.1515/1542-6580.2479.
|
|
29
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Cheng YL, Chang WL, Lee SC, Liu YG, Lin
HC, Chen CJ, Yen CY, Yu DS, Lin SZ and Harn HJ: Acetone extract of
Bupleurum scorzonerifolium inhibits proliferation of A549
human lung cancer cells via inducing apoptosis and suppressing
telomerase activity. Life Sci. 73:2383–2394. 2003.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Indira K, Jayaprabha N, Balakrishnan S,
Arulmoorthy MP and Srinivasan M: Production, purification and
characterisation of extracellular L-asparaginase from salt marsh
fungal endophytes. J Pharm Pharmac Sci. 4:663–677. 2015.
|
|
32
|
Thirunavukkarasu N, Suryanarayanan TS,
Murali TS, Ravishankar JP and Gummadi SN: L-asparaginase from
marine derived fungal endophytes of seaweeds. Mycosphere.
2:147–155. 2011.
|
|
33
|
Theantana T, Hyde KD and Lumyong S:
Asparaginase production by endophytic fungi from Thai medicinal
plants: Cytotoxicity properties. Int J Integrative Biol. 7:1–8.
2009.
|
|
34
|
Ahmad N, Pandit NP and Maheshwari SK:
L-asparaginase gene-a therapeutic approach towards drugs for cancer
cell. Int J Biosci. 2:1–11. 2012.
|
|
35
|
Shakambari G, Ashokkumar B and Varalakshmi
P: L-asparaginase-A promising biocatalyst for industrial and
clinical applications. Biocatalysis Agricultural Biotechnol.
17:213–224. 2019.
|
|
36
|
Tippani R and Sivadevuni G: Nutritional
factors effecting the production of L-asparaginase by the
Fusarium sp. Afr J Biotechnol. 11:3692–3696. 2012.
|
|
37
|
Uppuluri KB and Reddy DS: Optimization of
L-asparaginase production by isolated Aspergillusniger using sesame
cake in a column bioreactor. J Pure Appl Microbiol. 3:83–90.
2009.
|
|
38
|
Singh SB, Bais BS and Singh DR: Effect of
different carbon and nitrogen sources on the growth and sporulation
of Claviceps microcephal (Wallr.) TUL. Mycopathol Mycol
Appl. 46:373–378. 1972.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Selvaraj S and Murty VR: Semi-solid state
fermentation: A promising method for production and optimization of
tannase from Bacillus gottheilii M2S2. Res J Biotechnol. 12:39–48.
2017.
|
|
40
|
Abdel-Fattah YR and Olama ZA:
L-asparaginase production by Pseudomonas aeruginosa in solid-state
culture: Evaluation and optimization of culture conditions using
factorial designs. Process Biochemistry. 38:115–122. 2002.
|
|
41
|
Reid ID: Solid-state fermentations for
biological delignification. Enzyme Micro Technol. 11:786–803.
1989.
|