Introduction
Melanocytes are a type of neural crest-derived cells
that migrate to the epidermis during embryonic development
(1). Upon differentiation,
melanocytes spread throughout the skin and begin their main
physiological function, which is the production of melanin. Melanin
synthesis is a complex process that occurs within melanosomes,
specialized organelles found in melanocytes responsible for pigment
production and characterized by dendritic morphology (2,3).
Melanin plays a crucial role in protecting the skin from UV damage
(4,5). However, abnormal accumulation of
melanin can lead to skin issues such as melasma, freckles and age
spots (6). Numerous whitening
agents on the market work by regulating melanin production to
control excessive pigmentation and promote skin whitening.
One of the key regulators of melanin production is
microphthalmia-associated transcription factor (MITF), which
activates critical melanin synthesis-related genes such as
tyrosinase (TYR), TYR-related protein (TRP)-1 and TRP-2. MITF is a
common downstream target of numerous signaling pathways. The most
typical way to interfere with melanin production is through the
cyclic adenosine monophosphate (cAMP)/protein kinase A (PKA)
signaling pathway. When α-melanocyte-stimulating hormone (α-MSH)
binds to the melanocortin 1 receptor (MC1R) expressed on
melanocytes, it activates adenylyl cyclase (AC), leading to an
increase in cAMP levels. cAMP then activates PKA by binding to its
regulatory subunits. Phosphorylated (p-) PKA translocates to the
nucleus and phosphorylates cAMP response element-binding protein
(CREB), which in its phosphorylated form initiates the activation
of MITF, stimulating the transcriptional expression of key enzymes
related to melanin production such as TYR, TRP-1 and TRP-2(7).
TYR is a key enzyme in melanin production, and most
skin whitening agents function by inhibiting TYR activity to
suppress melanin production (8).
Ingredients including hydroquinone (9,10),
corticosteroids (11), ascorbic
acid (12,13), kojic acid (14,15)
and arbutin (16) have been used as
skin whitening agents to prevent or treat excessive skin
pigmentation. However, these products have drawbacks such as
carcinogenicity, instability and easy degradation (10). Therefore, it is necessary to further
explore natural active substances with low toxicity and effective
inhibition of melanin deposition.
Plant-derived extracts or compounds were used to
inhibit melanin deposition (17-19).
Essential oils are extracted from various parts of plants such as
leaves, flowers, roots and fruits through methods including steam
distillation, cold pressing, or solvent extraction. They are
composed of terpenes and other aromatic compounds (20) and possess various physiological
activities such as antibacterial, insect-repellent, insecticidal,
anti-inflammatory, antioxidant and anti-aging properties (21,22).
Research has found that the essential oil of Melaleuca
quinquenervia and its active components such as 1,8-cineole,
α-terpineol and α-pinene can reduce TYR activity and melanin
content, as well as decrease oxidative stress levels (23). Studies by Chou et al
(24) have shown that the essential
oil of Cinnamomum cassia and its main component
cinnamaldehyde exhibit favorable anti-TYR and anti-melanin
synthesis activities, along with antioxidant properties. Hsiao
et al (25) found that the
essential oil of Calocedrus formosana could completely
inhibit melanin production at a concentration of 80 µg/ml. Further
studies reported that the essential oil of Calocedrus
formosana could significantly inhibit the expression of TRP-1,
TRP-2 and MITF melanin synthesis regulatory proteins (25). Previous research has indicated that
essential oils have great value in the cosmetics industry (26,27).
Pinus tabuliformis Carrière, a member of the Pinaceae
family, is an important forest product from the Pinus genus,
with pine needles being one of the key products that can be
harvested year-round due to its fast regeneration rate. Studies
have reported that pine needle essential oil (PNEO) possesses
various physiological activities such as antioxidant (28), anticancer (29), antibacterial (30), mental health (31) and antiviral properties (32). However, there have been no reports
on the effect of PNEO from Pinus tabuliformis Carrière on
melanin synthesis.
Based on these previous studies and our preliminary
experiment, the experiment was designed as follows: A model of
α-MSH-induced B16F10 melanin-overexpressing cells was used to
investigate the effect of PNEO on melanin production and its
molecular mechanism by using microwave assisted extraction, Gas
Chromatography-Mass Spectrometry (GC-MS), reverse
transcription-quantitative PCR (RT-qPCR), western blotting (WB) and
other modern molecular biology techniques, including DOPA oxidation
method, BCA assay kit and Cell Counting Kit-8 (CCK-8). Then the
content of melanin and the activity of TYR in the cells, the levels
of melanogenesis-related genes and proteins such as MITF, TYR,
TRP-1 and MC1R, p-PKA and p-CREB were measured as well. The
findings of the present study have important implications for the
potential use of the PNEO in the cosmetics field.
Materials and methods
Reagents and equipment
B16F10 mouse melanoma cells were kindly provided by
Shandong Freda Biotechnology Co., Ltd. α-Arbutin (α-Ar; CAS no:
84380-01-8, purity ≥99%) and α- α-MSH (CAS no: 171869-93-5, purity
≥97%) were obtained from Shanghai Macklin Biochemical Technology.
DMEM high glucose culture medium was obtained from M&C Gene
Technology. Trypsin-EDTA digestion solution was obtained from
Yisheng Biotechnology. Penicillin-streptomycin solution was
obtained from HyClone; Cytiva. Fetal bovine serum (FBS) was
obtained from Wuhan Pricella Biotechnology Co., Ltd. The CCK-8 was
purchased from Biosharp Life Sciences. L-DOPA (CAS: 59-92-7, purity
≥98%) was sourced from Beijing Solarbio Science & Technology
Co., Ltd. H89 (PKA inhibitor), IBMX (cAMP activator) and Forskolin
(AC activator) were acquired from Beyotime Institute of
Biotechnology. RNA extraction reagent, chloroform substitute, RNA
dissolution solution, beta-actin (cat. no. GB11001) and
HRB-conjugated Goat Anti-Rabbit IgG H&L secondary antibodies
(cat. no. GB23303), primary antibody diluent (cat. no. G3337) were
obtained from Wuhan Servicebio Technology Co., Ltd. RIPA buffer
(purity ≥98%) was sourced from Beijing Solarbio Science &
Technology Co., Ltd.
The GC-MS system 8890-5977B was purchased by
Agilent. The LS-C0105 CO2 incubator was purchased by
NuAire Lab Equipment (https://www.nuaire.com/). The SW-CJ-2D type
ultra-clean workbench was procured by Suzhou Hengda Purification
Equipment. The HVE-50 high-pressure steam sterilizer pot was
obtained from Xinhua Medical Equipment Co., Ltd. and the CKX53
inverted microscope was purchased from Olympus Corporation.
Sample processing
The PNEO was prepared in the Natural Products
Research Center of Shandong Normal University. A total of 20 g of
pine needle powder from Pinus tabuliformis Carrière was
dissolved in distilled water, and the PNEO was extracted by using
microwave-assisted extraction technology and stored at 4˚C
(33).
PNEO analysis by GC-MS
GC-MS was used to analyze the constituent of PNEO.
The analysis employed an Agilent HP-5MS column (30 mm x 0.25 mm x
0.25 µm). The temperature program of the column was initiated at
60˚C, maintained for 1 min, ramped up at a rate of 8˚C/min to 140˚C
and held for 2 min the temperature was increased at 2˚C/min to
240˚C and held for 2 min, followed by a further ramp up at 8˚C/min
to 280˚C, where it was held for 5 min. Helium (purity, ≥99.999%)
was used as the carrier gas at a flow rate of 1.0 ml/min. The inlet
temperature was set at 260˚C, and a split injection mode with a
split ratio of 10:1 was employed, with an injection volume of 1
µl.
Mass spectrometry analysis was carried out by using
instrument utilized an Electron Impact (EI) ionization mode with a
voltage of 70 eV, a source and transfer line temperatures of 280˚C.
The detection mode operated in full scan (SCAN) mode, nitrogen
(N2) (purity, ≥99.999%) was used as the carrier gas. A
solvent delay of 3 min was implemented to optimize the analysis
process.
Cell culture and grouping
B16F10 melanoma cells were cultivated in DMEM medium
containing 10% FBS. The cells were maintained in a humidified 5%
CO2 incubator at 37˚C and were sub-cultured every 2-3
days to maintain logarithmic growth for subsequent experimentation.
Experimental groups included: i) A control group; ii) PNEO
treatment group (300 nM α-MSH + 12.5, 25, 50, 100, 200, 400, 800
µg/ml PNEO); iii) α-MSH model group (300 nM α-MSH); and iv) a
positive control group (300 nM α-MSH + 100 µg/ml α-Ar).
CCK-8 cell viability assessment
The cell viability was determined by using the CCK-8
method. B16F10 cells were seeded in a 96-well plate at a density of
5x104 cells/ml. Following a 24-h incubation period,
cells were categorized based on Section 2.2.2 and cultured for an
additional 24 or 48 h. A 10% CCK-8 working solution was prepared in
DMEM medium, replacing the original medium with 100 µl of the
working solution per well. Subsequently, cells were further
incubated at 37˚C for 1 h before measuring the optical density (OD)
at 450 nm using a microplate reader. Cell viability was calculated
as follows: (A1-A)/(A0-A) x100%.
A1 represents the OD value of the group treated with
drugs, including cells, CCK-8 solution and drug solution; A denotes
the OD value of the control group with medium and CCK-8 solution
but no cells; and A0 signifies the OD value of the group
containing cells, medium and CCK-8 solution.
BCA protein assay kit for protein
concentration determination
The protein concentration was determined using the
BCA Protein Assay Kit following the manufacturer's protocol.
Assay of melanin content
Melanin content was quantified using the NaOH lysis
method. B16F10 cells were seeded in 6-well plates at a density of
1.25x105 cells/ml per well, with 2 ml of cell suspension
added to each well and then incubated for 24 h. Subsequently,
abandoning the original culture medium, 2 ml of fresh medium was
added to each well according to the aforementioned grouping.
Following an additional 48-h static incubation, cells were washed
twice with PBS, harvested by scraping, and centrifuged at 13,201 x
g for 5 min at 4˚C. The resulting supernatant was discarded, and
300 µl of 1 M NaOH solution, containing 10% DMSO, was added to each
centrifuge tube. After thorough mixing and incubation at 80˚C to
solubilize the melanin, vigorous vortex followed. B16F10 lysate
containing melanin was then dispensed into a 96-well plate (100 µl
per well, with 3 replicates per group), and the absorbance at 405
nm was measured for each well using a microplate reader to
determine the relative melanin content. Relative melanin content
was calculated as follows: A1/A0 x 100%.
A1 represents the OD value of the experimental group,
and A0 represents the OD value of the control group.
Assay of TYR activity
The TYR activity within B16F10 cells was assayed in
terms of DOPA oxidase activity. The plating and drug administration
processes were carried out as aforementioned. After continuous
static culture for 48 h, the cells were washed twice with PBS.
Then, 500 µl of 1% RIPA solution was added to each well, and the
plate was quickly placed in a -80˚C refrigerator for freezing.
After being taken out, it was placed at room temperature to thaw so
that the cells were ruptured. This freeze-thaw process was repeated
several times. Then the cells were collected and centrifuged at
4˚C, 9,167 x g for 5 min. The supernatant was reserved in an ice
bath. In a 96-well plate, 50 µl of the supernatant was added,
followed by 50 µl of 1 mM L-DOPA solution. After reacting at 37˚C
for 1 h, the absorbance was measured at 492 nm. The TYR activity
was preliminarily calculated according to the protein
concentration, and then the relative activity was compared.
Relative TYR activity was calculated as follows:
C1/C0 x 100% (3). C1 represents TYR value of
the experimental group, and C0 represents TYR value of
the control group.
RT-qPCR detection of melanin
biosynthesis-related gene expression
RNA Extraction Solution (cat. no. G3013) was sourced
from Wuhan Seavil Biotechnology Co., Ltd. Reverse transcription
(RT) kit (cat. no. G3337) was provided by Wuhan Saiwell
Biotechnology Co., Ltd. Total RNA from B16F10 cells treated with
PNEO at a mass concentration of 50 µg/ml and the positive control
group α-Ar for 48 h was subjected to reverse transcription on a
regular PCR instrument. The cDNA first-strand product obtained from
reverse transcription (temperature protocol: 25˚C for 5 min, then
42˚C for 30 min, final 85˚C for 5 sec) served as the template, with
various reaction components sequentially added to the tube for
amplification on a fluorescent quantitative PCR instrument. SYBR
green (obtained from Wuhan Saiwell Biotechnology Co., Ltd.) was
used as fluorophore for qPCR. Thermocycling conditions were as
follows: Stage 1, 95˚C, 30 sec pre-denaturing; Stage 2, 95˚C, 15
sec denaturation; 60˚C, 30 sec annealing/elongation; Stage 3,
65-95˚C. The expression levels of the Tyr, Trp-1, Trp-2,
Mitf and Mc1r genes were analyzed using the
2-ΔΔCq method. Primer sequences are included in Table I.
 | Table ISequences of primers used for reverse
transcription-quantitative PCR. |
Table I
Sequences of primers used for reverse
transcription-quantitative PCR.
| Gene name | Primer sequence
(5'-3') |
|---|
| Tyr | Sense:
TAACTTACTCAGCCCAGCATCC |
| | Antisense:
ATAGTGGTCCCTCAGGTGTTCC |
| Trp-1 | Sense:
TTCGTTGGAGCTGTGATTGTTG |
| | Antisense:
AGGAATAATGTTGAAAGGTGGGG |
| Trp-2 | Sense:
AGAAACAACCCTTCCACAGATGC |
| | Antisense:
AAGCTCCCAGGATTCCAATGAC |
| Mitf | Sense:
GCCCTATGGCTATGCTCACTCTT |
| | Antisense:
TGTTCATACCTGGGCACTCACTC |
| Mc1r | Sense:
CTCATTGACGTGCTCATCTGTGG |
| | Antisense:
TGCTTGTAGTAGGTGATAAAGAGGGT |
| Creb | Sense:
TGGCTAACAATGGTACGGATGG |
| | Antisense:
GTGCTGTGCGGATCTGGTATGT |
| Crtc1 | Sense:
AGAAGATCGCACTGCACAACCA |
| | Antisense:
CCACGCTGCTGCTTCCAAT |
| Prkaca | Sense:
ATCGTCCTGACCTTTGAGTATCTG |
| | Antisense:
AACCGAAGTCTGTCACCTGAATAT |
| GAPDH | Sense:
CCTCGTCCCGTAGACAAAATG |
| | Antisense:
TGAGGTCAATGAAGGGGTCGT |
Detection of melanin
biosynthesis-related protein expression
The protein expression levels associated with
melanin biosynthesis in B16F10 cells were assessed through western
blot (WB) analysis. B16F10 cells were cultured for 24 h based on
distinct groupings, exposed to PNEO at a concentration of 50 µg/ml
for 48 h, rinsed twice with PBS, desiccated, harvested, lysed, and
the resulting lysates were transferred into 1.5-ml centrifuge tubes
containing an appropriate volume of RIPA lysis buffer. Cell lysis
was carried out on ice for 30 min to ensure complete cellular
disruption. Subsequent steps included centrifugation at 4˚C,
12,000-16,000 x g for 10 min, determination of protein
concentration, denaturation, SDS-PAGE electrophoresis (10%
acrylamide and 25-30 µg protein loaded per lane), PVDF membrane
transfer, incubation at room temperature for 30 min for blocking
(5% skim milk), addition of primary antibody, and overnight
agitation at 4˚C. The membrane underwent three 5-min washes with
TBST (0.1% Tween-20), followed by incubation at room temperature
for 30 min with a secondary antibody diluted in TBST at a ratio of
1:5,000. After three additional rapid 5-min washes with TBST, the
membrane was exposed, and the original image was preserved for
subsequent data analysis. An immunoblot image analysis software
based on artificial intelligence learning, launched by Wuhan
Servicebio Technology Co., Ltd., was used.
Statistical analysis
Each experiment was replicated three times, and the
results are presented as the mean ± standard deviation (X ± SD).
Data analysis was conducted using GraphPad Prism 9.0.0 software
(Dotmatics), employing one-way analysis of variance for comparisons
among multiple groups. P#x003C;0.05 was considered to indicate a
statistically significant difference.
Results
Analysis of PNEO by GC-MS
The total ion chromatogram of the PNEO obtained by
microwave-assisted extraction is shown in Fig. S1 (33), and a summary of its components and
relative peak areas is presented in Table SI. By conducting GC-MS analysis of
the PNEO extracted using the microwave-assisted method, a total of
332 substances were identified. Peaks with matching degree
exceeding 80% were considered as candidate compounds, and after
qualitative comparison by retrieving the NIST spectral library
(https://webbook.nist.gov/chemistry/),
103 chemical compounds were identified, accounting for 47.39% of
the total composition. The relative percentage content of each
component in the PNEO was calculated using area normalization. The
results showed that the PNEO mainly contained alcohols (11.01%),
hydrocarbons (11.04%), esters (9.3%) and terpenes (2.997%). These
included Thunbergol (PubChem CID: 5363523; 3.938%), Verticillol
(PubChem CID: 5377475; 3.597%), Hentriacontane (PubChem CID: 12410;
2.189%), α-Terpinyl acetate (PubChem CID: 111037; 1.901%), Methyl
dehydroabietate (PubChem CID: 14697; 1.842%), α-cadinene (PubChem
CID: 12306048; 1.534%), Isopimara-8 (PubChem CID; 13783133)
(14),
15-dien-19-saeure-methylester (PubChem CID: 13710744; 1.517%),
Cembrene (PubChem CID: 6430770; 1.139%) and α-Terpinene (PubChem
CID: 7462; 0.825%), among others.
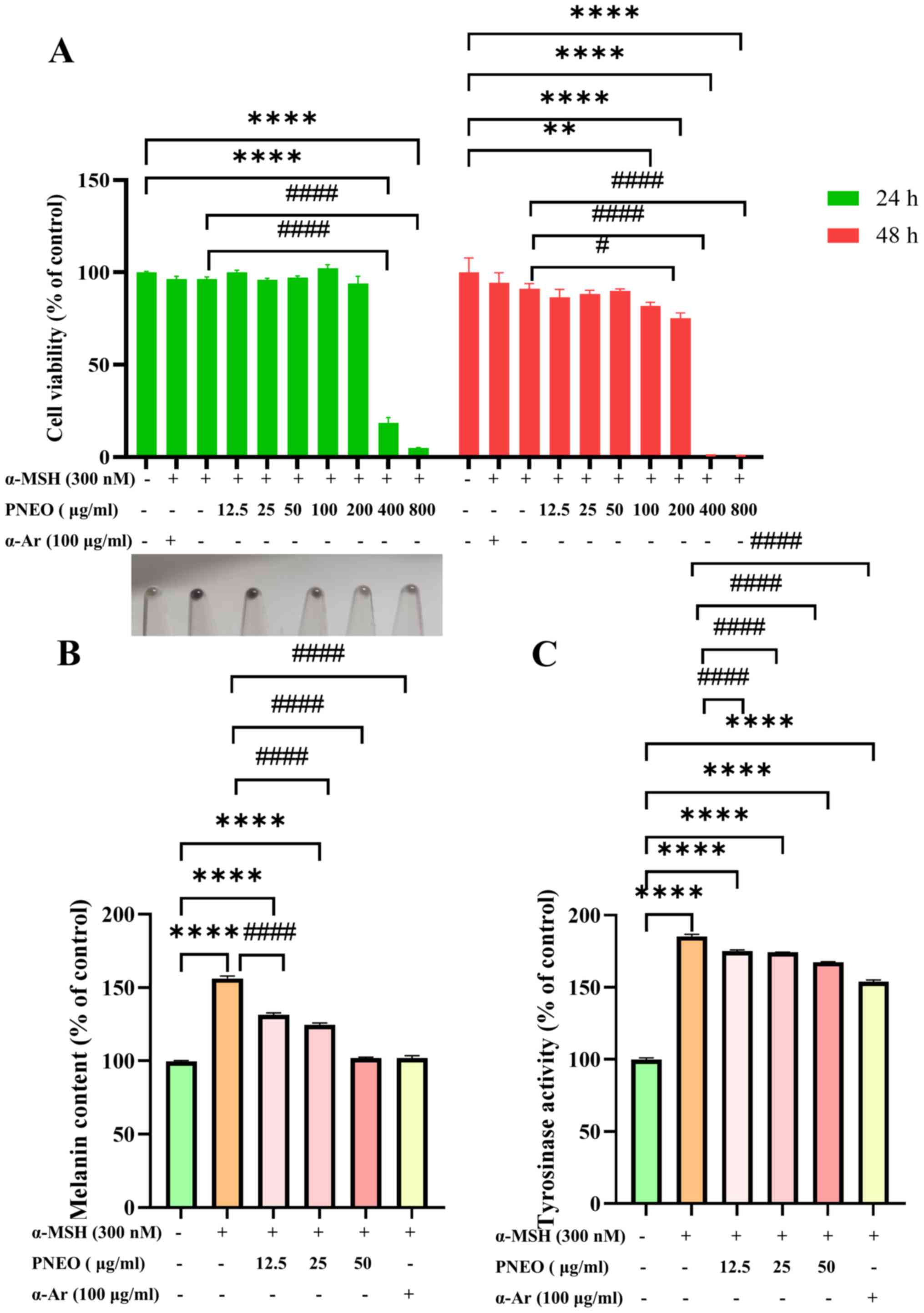
Effect of PNEO on B16F10 cell
viability
The CCK-8 results are demonstrated in Fig. 1A. Treatment of cells with different
concentrations of PNEO for 24 h showed a significant impact on cell
viability when the concentration exceeded 400 µg/ml. At
concentrations #x003C;400 µg/ml, cell morphology was normal, and
the cell viability was >85%, with no significant difference
compared with the control group (P>0.05). After 48 h of
treatment, cell viability decreased significantly with increasing
concentration of PNEO. At concentrations #x003C;50 µg/ml, PNEO did
not affect cell viability (P>0.05). However, when the PNEO
concentration was 100 µg/ml, the cell viability significantly
decreased to 81.89±3.27% (P#x003C;0.01). Subsequently, PNEO
concentrations of 12.5, 25 and 50 µg/ml were selected for further
experiments.
Effect of PNEO on melanin content in
B16F10 cells
After 48 h of intervention with 12.5, 25 and 50
µg/ml of PNEO, the melanin content in B16F10 cells was measured. As
can be observed in Fig. 1B, when
the cells were stimulated by α-MSH, the relative melanin content
was 156.25±1.70%, indicating that the high melanin expression cell
model was successfully developed. PNEO dose-dependently inhibited
melanin content, with melanin inhibition rates of 24.81±1.31%,
31.63±1.31% and 54.36±0.67% in the low, medium and high dose
groups, respectively. At 50 µg/ml, PNEO was able to achieve
inhibition effects similar to the α-Ar positive control.
Effect of PNEO on TYR activity in
B16F10 cells
The results of TYR activity measurement are
demonstrated in Fig. 1C. Compared
with the control group, the TYR activity in the α-MSH group was
185.22±1.58% (P#x003C;0.0001), indicating the success of the model.
Different mass concentrations of PNEO and the α-Ar positive control
group inhibited TYR activity in B16F10 cells, showing significant
differences compared with the α-MSH model group. In the α-Ar
positive control group, the inhibition rate of TYR in B16F10 cells
reached 31.36±17.25%, while PNEO at a mass concentration of 50
µg/ml exhibited the strongest inhibition of TYR activity, with an
inhibition rate of 17.77±8.57%. This suggests that PNEO could
effectively inhibit TYR activity in α-MSH-induced B16F10 cells.
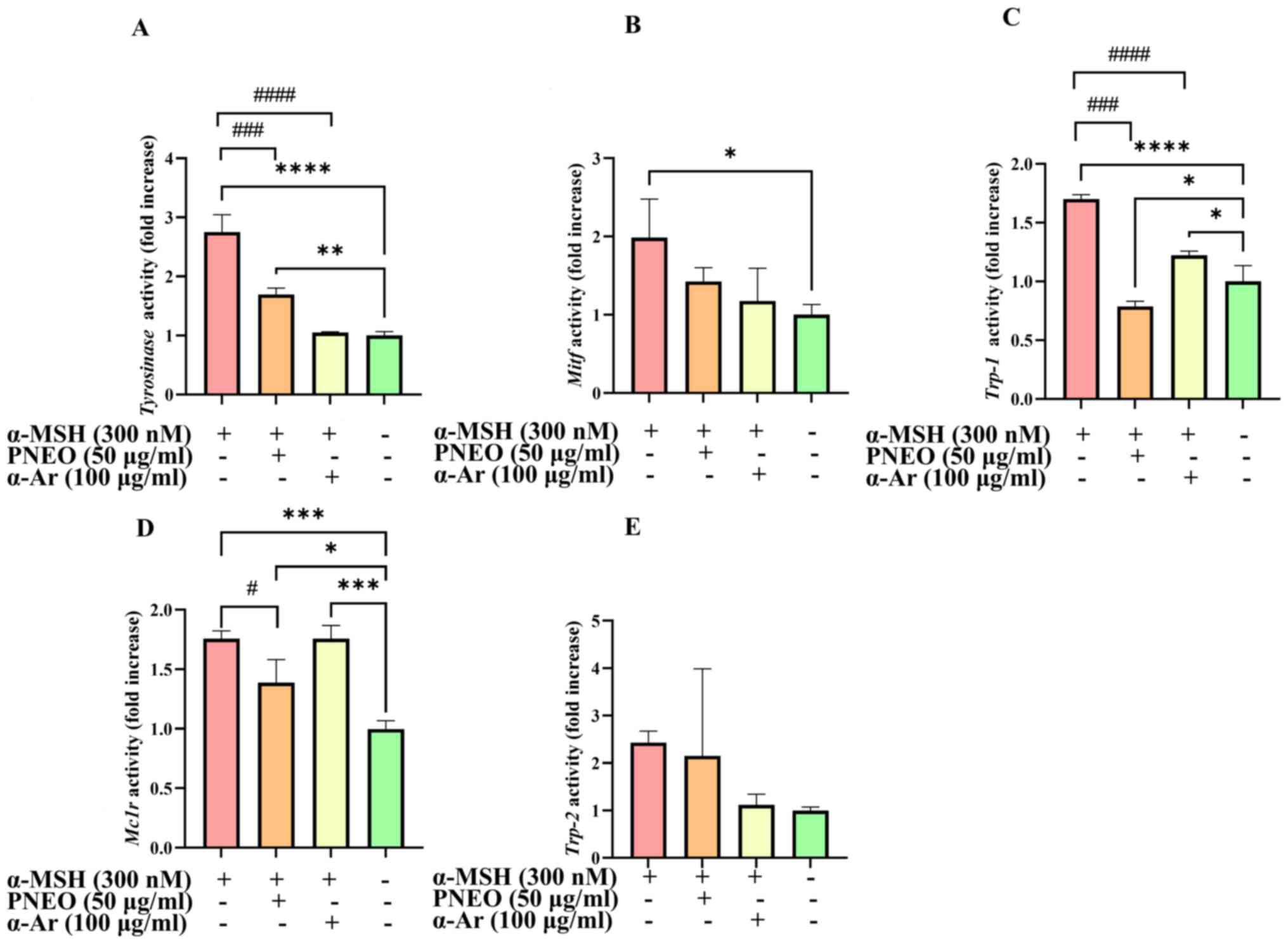
Effect of PNEO on the expression of
melanogenesis-related genes
MITF, TYR, TRP-1 and TRP-2 are important signaling
molecules in the process of melanogenesis. Based on the results of
melanin content and TYR activity, 50 µg/ml of PNEO achieved the
optimal inhibitory effect. Therefore, the concentration of PNEO was
set at 50 µg/ml in RT-qPCR and WB detection. The gene expression
levels of Tyr, Trp-1, Trp-2, Mitf and
Mc1r are revealed in Fig. 2.
After treatment with PNEO for 48 h, the expression of Tyr,
Trp-1, Mitf and Mc1r genes in B16F10 cells was
significantly inhibited compared with the α-MSH model group.
However, the expression level of Trp-2, which is related to
melanin production, showed no significant impact.
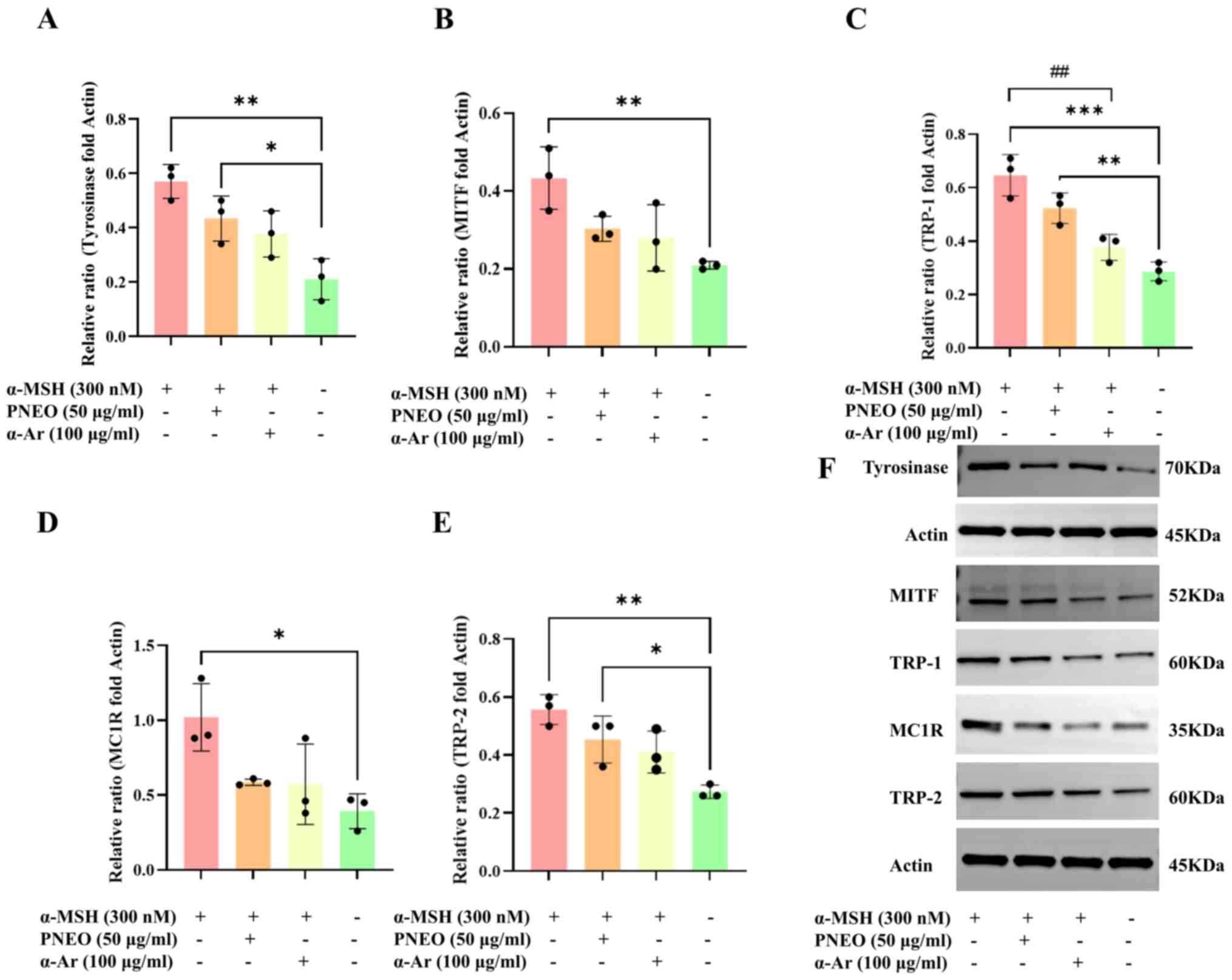
Effect of PNEO on the expression of
melanin synthesis-related proteins
Through WB experiments, the effect of PNEO on the
proteins MITF, TYR, TRP-1 and TRP-2, as well as the phosphorylation
levels of proteins in the cAMP/PKA signaling pathway was examined
to elucidate the mechanism of action by which PNEO inhibits melanin
production in B16F10 cells (Fig.
3). Treatment of B16F10 cells with 50 µg/ml PNEO for 48 h
resulted in decreased expression of TYR, TRP-1, TRP-2, MITF and
MC1R proteins.
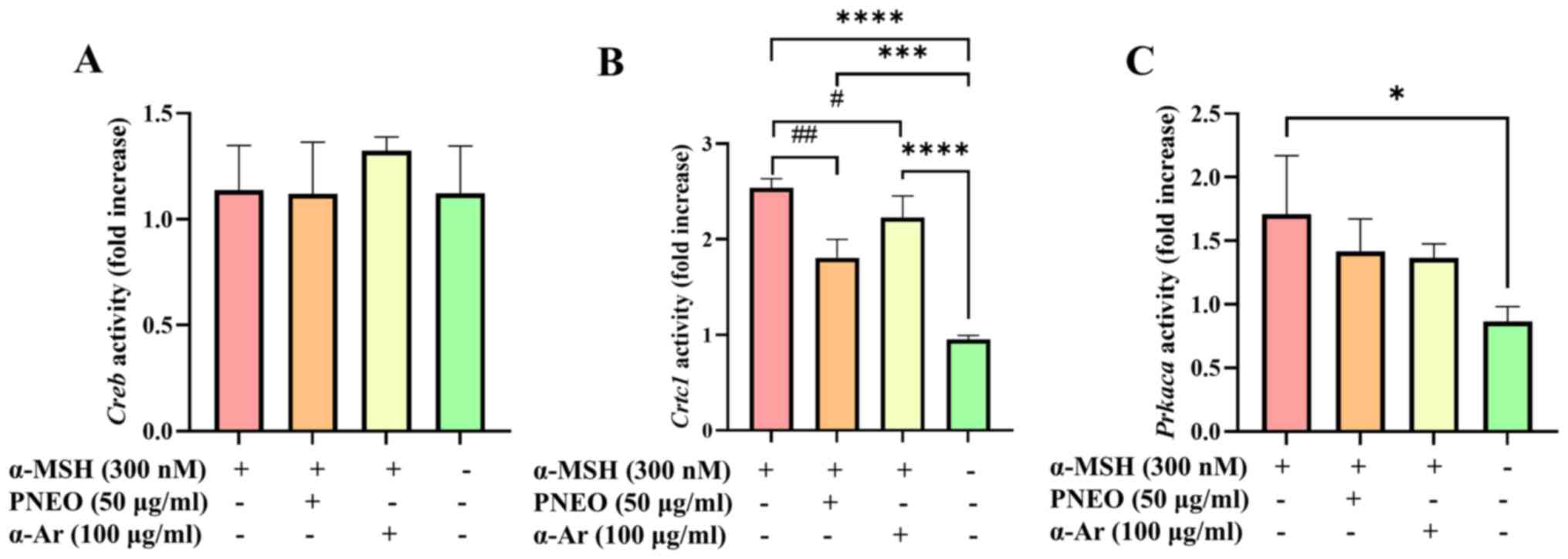
Verification of the effect of PNEO on
the cAMP/PKA signaling pathway
The CREB molecule is one of the phosphorylation
substrates of various protein kinases. The expression level of
p-CREB in the nucleus can indirectly reflect the activity of PKA in
the cytoplasm. CREB-regulated transcription coactivator 1 (CRTC1)
is dephosphorylated and enters the nucleus, forming a CREB/CRTC1
heterodimer in the nucleus, which enhances the transcription levels
on the MITF-M promoter. To verify whether PNEO acts on the cAMP/PKA
signaling pathway, the results are shown in Fig. 4. Treatment with PNEO resulted in
decreased expression of Crtc1 and Prkaca. After
treatment of B16F10 cells with PNEO for 48 h, the protein levels of
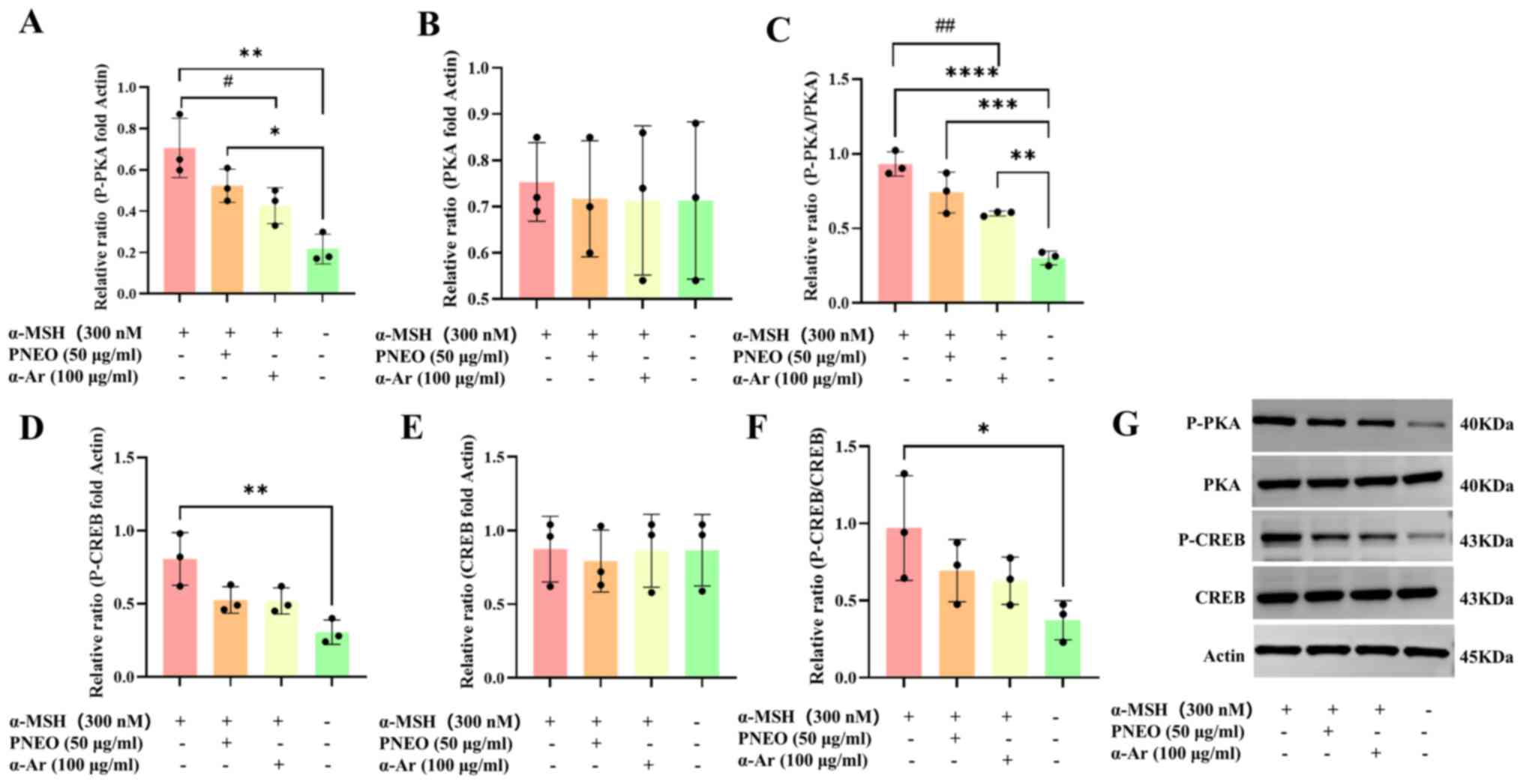
p-CREB and p-PKA were significantly inhibited (Fig. 5). It was hypothesized that the
cAMP/PKA signaling pathway is involved in the process by which PNEO
inhibits melanin production.
To further investigate the molecular mechanism
underlying the inhibitory effect of PNEO on melanin production, the
effects of the PKA inhibitor H89, the cAMP activator IBMX, and the
AC activator Forskolin on the signaling pathways related to melanin
production were studied in the context of PNEO-mediated melanin
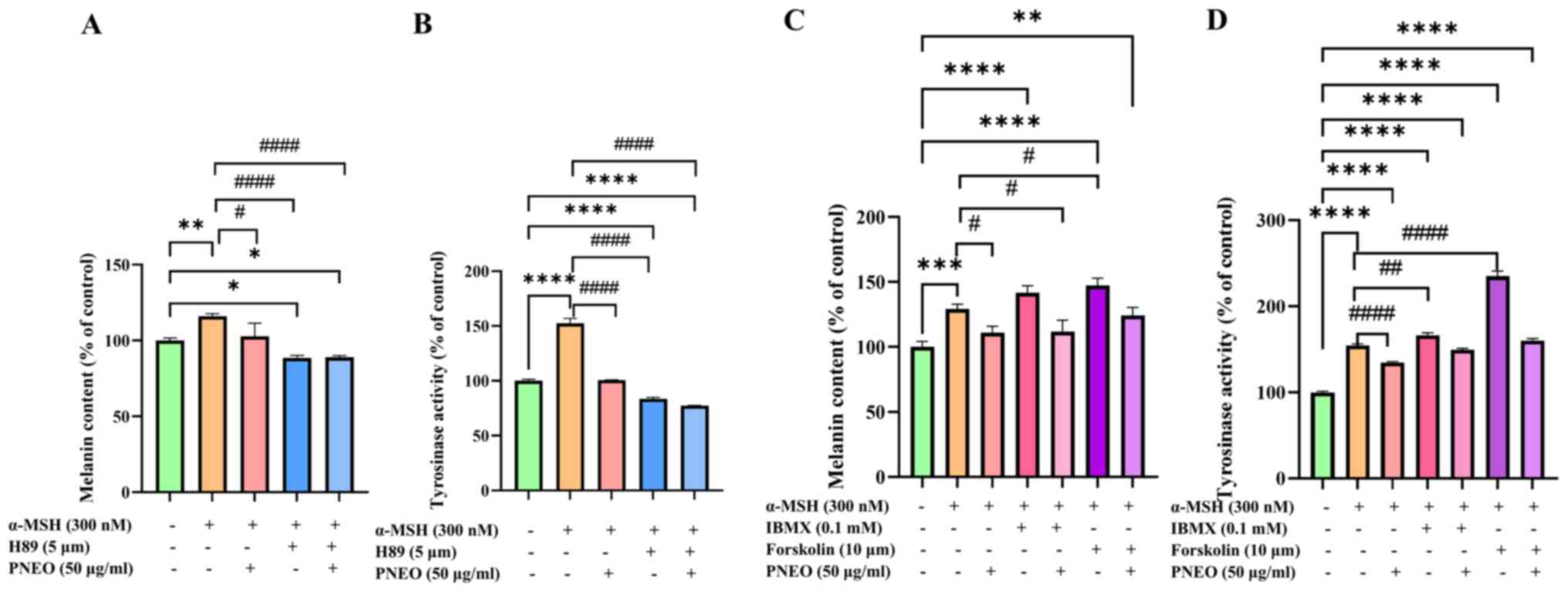
inhibition. The results, as shown in Fig. 6A, demonstrate that the addition of
PNEO or the H89 inhibitor significantly reduced melanin content,
with a synergistic effect observed between the two, resulting in a
melanin content of 88.93±1.07%. In Fig.
6B, the results of cellular TYR activity identified that
treatment with α-MSH significantly increased TYR activity, while
the addition of PNEO or H89 inhibitors decreased TYR activity to
83.55±1.44% and 77.41±0.24%, respectively. Additionally, treatment
with PNEO in combination with IBMX or Forskolin activators resulted
in increased melanin content (Fig.
6C) and TYR activity (Fig. 6D),
with Forskolin activator showing a more significant promotion
effect, reaching a TYR activity of 235.18±4.50%. In the groups
treated with α-MSH, IBMX, or Forskolin activators in combination
with PNEO, both melanin content and TYR activity decreased. These
findings validate that PNEO regulates melanin production through
the cAMP/PKA signaling pathway.
Discussion
The present study utilized microwave-assisted
extraction to extract essential oil from Pinus tabulatus
pine needles. Microwave energy generates heat that causes plant
cell wall rupture, facilitating rapid dissolution of the extract
(34), thereby reducing extraction
time compared with traditional steam distillation and offering
advantages such as energy savings and reduced production costs
(35). Properly reducing the
extraction time with microwave-assisted extraction lowers the
likelihood of thermal degradation of the extract (36). Additionally, research indicates that
plant essential oils extracted using microwave assistance exhibit
strong antioxidant capabilities and have a longer shelf life
(37,38). In the present study, the effects of
microwave-assisted extraction of PNEO on the viability of B16F10
cells were initially tested. The results demonstrated that PNEO had
no significant toxic effects on cells at concentrations of 12.5-50
µg/ml. Using α-Ar as a positive control group, the impact of PNEO
on melanin content and TYR activity within B16F10 cells was
evaluated. The findings revealed that different concentrations of
PNEO exhibited significant inhibitory effects on melanin content
and TYR activity within the cells. At a concentration of 50 µg/ml,
PNEO achieved a similar inhibitory effect on melanin content as
α-Ar and significantly suppressed TYR activity. Studies have
reported that inhibiting TYR activity is an effective approach to
inhibiting melanin formation (39).
Previous studies reported that the anti-melanin activity of plant
essential oils was mainly attributed to terpenes such as α-pinene,
limonene, β-laurene and β-pinene (23,40,41).
Additionally, minor components in the oil demonstrate favorable
physiological activity and exhibit superior effects in inhibiting
melanin formation (42). This
suggests that the whitening potential of PNEO may be attributed to
the actions of various terpenes present in the oil.
When the skin is exposed to ultraviolet light,
keratinocytes produce α-MSH, which in turn induces the MC1R
signaling pathway in melanocytes (43,44).
The MC1R signaling pathway is crucial for melanin formation. α-MSH
activation of the MC1R pathway leads to activation of the cAMP
signaling pathway, which subsequently stimulates AC and cAMP
production downstream of α-MSH-induced MC1R activation, activating
PKA and CREB protein (45). CREB,
when phosphorylated, activates and induces the expression of
Mitf, which in turn activates the transcription of melanin
synthesis-related genes: Mitf, Trp-1, Trp-2
and Tyr. Additionally, Mitf is essential for the
development of melanocytes, melanin formation and long-term cell
survival (46). Theasinensin A in
tea significantly reduces the mRNA expression of Tyr,
Trp-1 and Trp-2. It also inhibits the increase in TYR
and MITF protein levels during α-MSH exposure, as well as inhibits
the phosphorylation of CREB and PKA, thereby reducing melanin
synthesis through the cAMP signaling pathway (47). To elucidate the mechanism by which
PNEO inhibits melanin production and to identify the signaling
pathways regulated by PNEO during the inhibition of melanin
production, treatment with PNEO reduced the expression levels of
the genes Tyr, Trp-1, Mitf, Mc1r,
Crtc1 and Prkaca in B16F10 cells, which are important
genes in the melanin production process. Among them, TRP-1 is a
75-kDa protein synthesized in the endoplasmic reticulum,
transported through the Golgi apparatus, and transferred to
melanosomes (48). TRP-1 enhances
TYR activity by forming a stable complex to increase its enzymatic
activity and is also involved in the proliferation and morphology
of melanocytes (49). Research has
shown that diacetyl-caffeic acid cyclohexyl ester can block the
nuclear entry of CRTC1 in melanocytes, inhibit the formation of the
CREB/CRTC1 heterodimer, decrease the transcription levels on the
MITF-M promoter, thus reducing melanin production. CRTC1 is
potentially a therapeutic target for pigmentary disorders including
melasma, freckles and senile lentigines (50).
Unlike previous studies, PNEO extraction was found
to have no significant impact on the expression of the Trp-2
gene. Various pathways are known to reduce melanin production, with
past research indicating that intracellular melanin synthesis is
primarily influenced by TYR activity. Inhibition of TYR activity
significantly weakens melanin synthesis in melanocytes. The
findings of this study suggest that PNEO can reduce the levels of
MITF, TYR, TRP-1, TRP-2, and MC1R proteins, as well as decrease the
phosphorylation levels of molecules related to the cAMP/PKA
signaling pathway. Phosphorylated CREB upregulates MITF levels.
Treatment with PNEO decreases the levels of the MC1R receptor,
leading to a significant reduction in the levels of downstream PKA
and CREB phosphorylation in the cAMP signaling pathway, inhibiting
MITF activation and expression. This results in the lowered levels
of proteins associated with melanin production (TYR, TRP-1, TRP-2),
ultimately suppressing melanin production through the cAMP/PKA/CREB
signaling pathway.
Similar research findings have been reported
previously. For instance, Seo et al (51) discovered in their study on the
anti-melanogenesis effects of Leathesia difformis extracts
that an increase in α-MSH levels leads to the binding of the MC1R
receptor on melanocyte cell membranes, causing an elevation in cAMP
and activation of the downstream signaling molecule PKA. This, in
turn, increases Mitf expression via CREB activation. Seaweed
extracts were found to downregulate the expression of genes related
to melanin synthesis, reducing p-CREB levels, indicating that
seaweed extracts may inhibit melanin production through the
cAMP/PKA/CREB pathway (51).
The effects of plant extracts on the mechanism of
melanin production have been previously investigated. The root
extract of Astragalus membranaceus inhibits the
downregulation of MITF, mediated by cAMP response, CREB and p38
MAPK kinase. The PKA inhibitor H89 and the p38 inhibitor SB203580
have validated that formononetin inhibits melanin synthesis and TYR
activity by regulating the PKA/CREB and p38 MAPK signaling pathways
(52).
To verify the effect of PNEO on the targets of
melanin production signaling pathways, the present study used PNEO
treatment while adding the PKA inhibitor H89. The levels of melanin
production and TYR activity were further reduced. When cAMP
activator IBMX and AC activator Forskolin were added, the levels of
melanin production and TYR activity increased. However, adding PNEO
weakened this increasing trend. It was validated that PNEO can
regulate melanin production and TYR activity through the cAMP/PKA
signaling pathway. The results of the present study provide a
certain basis for the utilization of pine needle resources.
Subsequently, the authors will analyze the effect of the screened
components on melanin production through B16F10 cell experiments
and explore the signal pathways involved in their inhibitory effect
on melanin production. In future studies, a major chemical
component in PNEO will be selected and more in-depth research shall
be carried out, which will be helpful for the exploration of the
pharmacological mechanism of the essential oil.
In conclusion, the present results indicate that
PNEO inhibits melanin production through the cAMP/PKA signaling
pathway. Mechanistically, PNEO inhibits Mitf expression in
B16F10 cells and downstream enzymes such as TYR, TRP-1 and TRP-2 by
modulating PKA and CREB protein phosphorylation to suppress melanin
synthesis (Fig. 7). This will have
important implications for the potential use of the PNEO in the
cosmetics field.
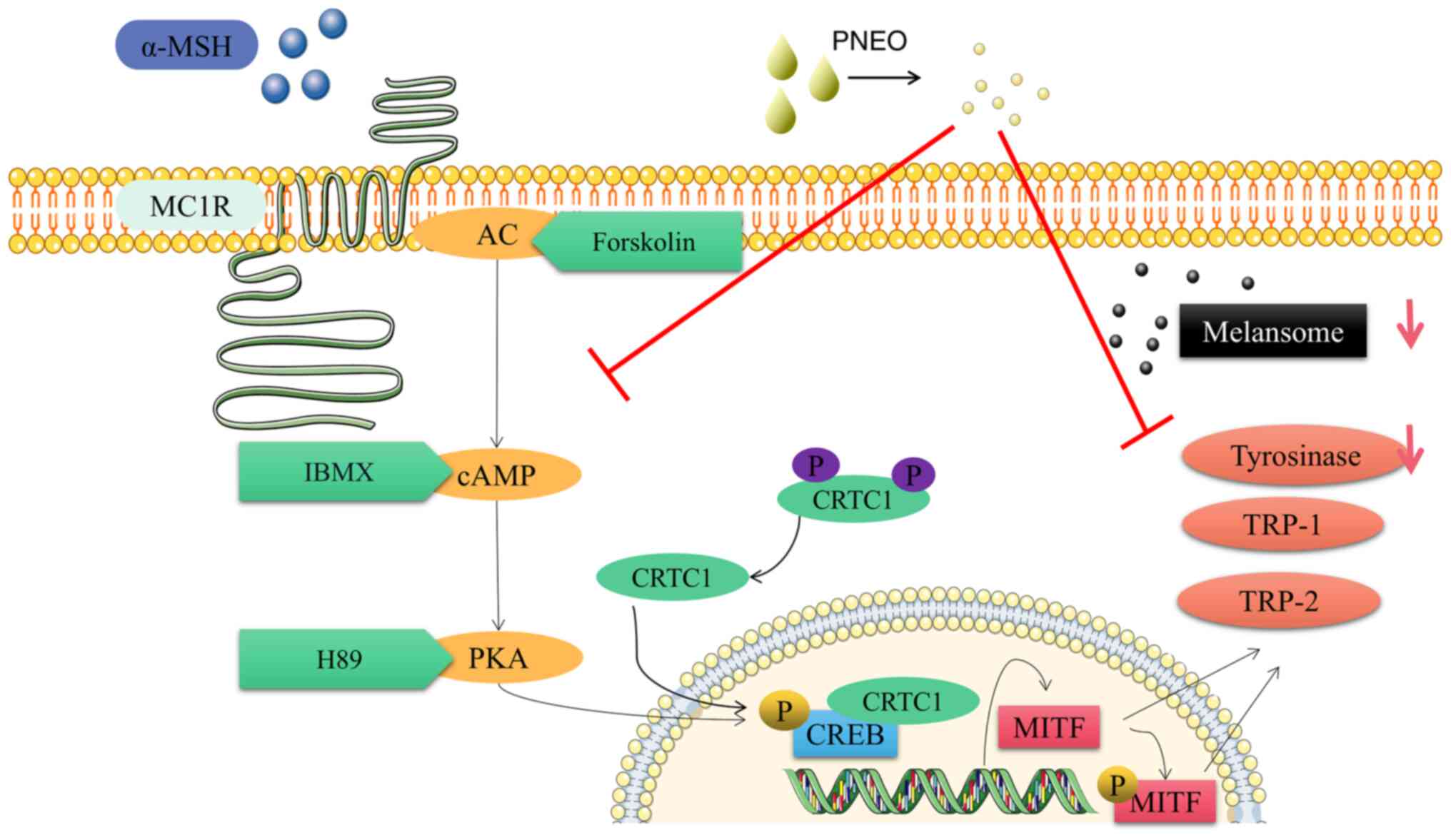 | Figure 7Regulatory mechanism of melanin
synthesis in B16F10 cells by PNEO. The red arrows indicate decrease
expression levels, and the red T bars indicate inhibition. PNEO,
pine needle essential oil; α-MSH, α-melanocyte-stimulating hormone;
PKA, protein kinase A; MITF, microphthalmia-associated
transcription factor; MC1R, melanocortin 1 receptor; CREB,
cyclic-AMP response element-binding protein; CRTC1, CREB-regulated
transcription coactivator; AC, adenylyl cyclase; TRP,
tyrosinase-related protein. |
PNEO downregulates the expression of Tyr,
Trp-1, Crtc1, Mc1r and Mitf genes, as
well as TYR, TRP-1, TRP-2, MC1R and MITF proteins in B16F10 cells.
It also diminishes the phosphorylation levels of molecules linked
to the cAMP/PKA signaling pathway, thus lowering TYR activity and
inhibiting melanin synthesis. PNEO or the H89 inhibitor notably
reduces melanin content, whereas IBMX or Forskolin activators
sustainably elevate melanin content and TYR activity. However,
co-treatment with PNEO decreases melanin content and TYR activity.
These results suggest that PNEO inhibits melanogenesis via the
cAMP/PKA signaling pathway.
Supplementary Material
Gas chromatography-mass spectrometry
total ion current diagram of pine needle essential oil extracted by
microwave-assisted extraction.
GC-MS analysis of PNEO extracted by
MAE.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
SL conducted investigation and formal analysis, and
wrote the original draft. BS and GL validated and curated data. LS
conducted software analysis and data visualization. QW developed
the methodology, acquired funding and wrote, reviewed and edited
the manuscript. YG conceptualized and supervised the study,
performed project administration, and wrote, reviewed and edited
the manuscript. All authors read and approved the final version of
the manuscript. YG and SL confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Uong A and Zon LI: Melanocytes in
development and cancer. J Cell Physiol. 222:38–41. 2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Cui YZ and Man XY: Biology of melanocytes
in mammals. Front Cell Dev Biol. 11(1309557)2023.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hou L and Pavan WJ: Transcriptional and
signaling regulation in neural crest stem cell-derived melanocyte
development: Do all roads lead to Mitf? Cell Res. 18:1163–1176.
2008.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tadokoro R, Shikaya Y and Takahashi Y:
Wide coverage of the body surface by melanocyte-mediated skin
pigmentation. Dev Biol. 449:83–89. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lin JY and Fisher DE: Melanocyte biology
and skin pigmentation. Nature. 445:843–850. 2007.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Maranduca MA, Branisteanu D, Serban DN,
Branisteanu DC, Stoleriu G, Manolache N and Serban IL: Synthesis
and physiological implications of melanic pigments. Oncol Lett.
17:4183–4187. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
D'Mello SA, Finlay GJ, Baguley BC and
Askarian-Amiri ME: Signaling pathways in melanogenesis. Int J Mol
Sci. 17(1144)2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kim HD, Choi H, Abekura F, Park JY, Yang
WS, Yang SH and Kim CH: Naturally-occurring tyrosinase inhibitors
classified by enzyme kinetics and copper chelation. Int J Mol Sci.
24(8226)2023.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Arndt KA and Fitzpatrick TB: Topical use
of hydroquinone as a depigmenting agent. JAMA. 194:965–967.
1965.PubMed/NCBI
|
|
10
|
Draelos ZD: Skin lightening preparations
and the hydroquinone controversy. Dermatol Ther. 20:308–313.
2007.PubMed/NCBI View Article : Google Scholar
|
|
11
|
McKesey J, Tovar-Garza A and Pandya AG:
Melasma treatment: An evidence-based review. Am J Clin Dermatol.
21:173–225. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kim HM, Byun KA, Oh S, Yang JY, Park HJ,
Chung MS, Son KH and Byun K: A mixture of topical forms of
polydeoxyribonucleotide, vitamin C, and niacinamide attenuated skin
pigmentation and increased skin elasticity by modulating nuclear
factor erythroid 2-like 2. Molecules. 27(1276)2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Park HJ, Byun KA, Oh S, Kim HM, Chung MS,
Son KH and Byun K: The combination of niacinamide, vitamin C, and
PDRN mitigates melanogenesis by modulating nicotinamide nucleotide
transhydrogenase. Molecules. 27(4923)2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Saeedi M, Eslamifar M and Khezri K: Kojic
acid applications in cosmetic and pharmaceutical preparations.
Biomed Pharmacother. 110:582–593. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zilles JC, Dos Santos FL,
Kulkamp-Guerreiro IC and Contri RV: Biological activities and
safety data of kojic acid and its derivatives: A review. Exp
Dermatol. 31:1500–1521. 2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wang W, Gao Y, Wang W, Zhang J, Yin J, Le
T, Xue J, Engelhardt UH and Jiang H: Kojic acid showed consistent
inhibitory activity on tyrosinase from mushroom and in cultured
B16F10 cells compared with arbutins. Antioxidants (Basel).
11(502)2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Bairagi J, Saikia PJ, Boro F and Hazarika
A: A review on the ethnopharmacology, phytochemistry and
pharmacology of Polygonum hydropiper Linn. J Pharm
Pharmacol. 74:619–645. 2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Merecz-Sadowska A, Sitarek P, Stelmach J,
Zajdel K, Kucharska E and Zajdel R: Plants as modulators of
melanogenesis: Role of extracts, pure compounds and patented
compositions in therapy of pigmentation disorders. Int J Mol Sci.
23(14787)2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Merecz-Sadowska A, Sitarek P, Kowalczyk T,
Zajdel K, Kucharska E and Zajdel R: The modulation of melanogenesis
in B16 cells upon treatment with plant extracts and isolated plant
compounds. Molecules. 27(4360)2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Bakkali F, Averbeck S, Averbeck D and
Idaomar M: Biological effects of essential oils-a review. Food Chem
Toxicol. 46:446–475. 2008.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Saab AM, Gambari R, Sacchetti G, Guerrini
A, Lampronti I, Tacchini M, El Samrani A, Medawar S, Makhlouf H,
Tannoury M, et al: Phytochemical and pharmacological properties of
essential oils from Cedrus species. Nat Prod Res. 32:1415–1427.
2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Al-Khayri JM, Banadka A, Nandhini M,
Nagella P, Al-Mssallem MQ and Alessa FM: Essential oil from
Coriandrum sativum: A review on its phytochemistry and
biological activity. Molecules. 28(696)2023.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Chao WW, Su CC, Peng HY and Chou ST:
Melaleuca quinquenervia essential oil inhibits
α-melanocyte-stimulating hormone-induced melanin production and
oxidative stress in B16 melanoma cells. Phytomedicine. 34:191–201.
2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Chou ST, Chang WL, Chang CT, Hsu SL, Lin
YC and Shih Y: Cinnamomum cassia essential oil inhibits
α-MSH-induced melanin production and oxidative stress in murine B16
melanoma cells. Int J Mol Sci. 14:19186–191201. 2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hsiao WW, Kumar KJS, Lee HJ, Tsao NW and
Wang SY: Anti-Melanogenic activity of Calocedrus formosana
wood essential oil and its chemical composition analysis. Plants
(Basel). 11(62)2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ailli A, Handaq N, Touijer H, Gourich AA,
Drioiche A, Zibouh K, Eddamsyry B, El Makhoukhi F, Mouradi A, Bin
Jardan YA, et al: Phytochemistry and biological activities of
essential oils from six aromatic medicinal plants with cosmetic
properties. Antibiotics (Basel). 12(721)2023.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Sharmeen JB, Mahomoodally FM, Zengin G and
Maggi F: Essential oils as natural sources of fragrance compounds
for cosmetics and cosmeceuticals. Molecules. 26(666)2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhang S, Xie H, Huang J, Chen Q, Li X,
Chen X, Liang J and Wang L: Ultrasound-assisted extraction of
polyphenols from pine needles (Pinus elliottii):
Comprehensive insights from RSM optimization, antioxidant activity,
UHPLC-Q-exactive orbitrap MS/MS analysis and kinetic model.
Ultrason Sonochem. 102(106742)2024.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Qiu B, Jiang W, Qiu W, Mu W, Qin Y, Zhu Y,
Zhang J, Wang Q, Liu D and Qu Z: Pine needle oil induces G2/M
arrest of HepG2 cells by activating the ATM pathway. Exp Ther Med.
15:1975–1981. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Khoury M, El Beyrouthy M, Ouaini N, Iriti
M, Eparvier V and Stien D: Chemical composition and antimicrobial
activity of the essential oil of Juniperus excelsa M.Bieb.
growing wild in Lebanon. Chem Biodivers. 11:825–830.
2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Lizarraga-Valderrama LR: Effects of
essential oils on central nervous system: Focus on mental health.
Phytother Res. 35:657–679. 2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ha TKQ, Lee BW, Nguyen NH, Cho HM,
Venkatesan T, Doan TP, Kim E and Oh WK: Antiviral activities of
compounds isolated from Pinus densiflora (pine tree) against
the influenza A virus. Biomolecules. 10(711)2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lü SY, Shang BQ, Sun LY, Liu GL, Wu Q and
Geng Y: Process optimization and antioxidant activity of pine
needle essential oil extracted by microwave-assisted extraction.
Sci Technol Food Ind. 46:184–191. 2025.(In Chinese).
|
|
34
|
Bagade SB and Patil M: Recent advances in
microwave assisted extraction of bioactive compounds from complex
herbal samples: A review. Crit Rev Anal Chem. 51:138–149.
2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Masota NE, Vogg G, Heller E and Holzgrabe
U: Comparison of extraction efficiency and selectivity between
low-temperature pressurized microwave-assisted extraction and
prolonged maceration. Arch Pharm (Weinheim).
353(e2000147)2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Dahmoune F, Nayak B, Moussi K, Remini H
and Madani K: Optimization of microwave-assisted extraction of
polyphenols from Myrtus communis L. leaves. Food Chem.
166:585–595. 2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Rahim MA, Ayub H, Sehrish A, Ambreen S,
Khan FA, Itrat N, Nazir A, Shoukat A, Shoukat A, Ejaz A, et al:
Essential components from plant source oils: A review on
extraction, detection, identification, and quantification.
Molecules. 28(6881)2023.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Cardoso-Ugarte GA, Juárez-Becerra GP,
Sosa-Morales ME and López-Malo A: Microwave-assisted extraction of
essential oils from herbs. J Microw Power Electromagn Energy.
47:63–72. 2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Pillaiyar T, Manickam M and Namasivayam V:
Skin whitening agents: Medicinal chemistry perspective of
tyrosinase inhibitors. J Enzyme Inhib Med Chem. 32:403–425.
2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Mansour RB, Wasli H, Bourgou S, Khamessi
S, Ksouri R, Megdiche-Ksouri W and Cardoso SM: Insights on
Juniperus phoenicea essential oil as potential anti-proliferative,
anti-tyrosinase, and antioxidant candidate. Molecules.
28(7547)2023.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Yang J, Lee SY, Jang SK, Kim KJ and Park
MJ: Inhibition of melanogenesis by essential oils from the citrus
cultivars peels. Int J Mol Sci. 24(4207)2023.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Jimenez-Lopez C, Carpena M, Lourenço-Lopes
C, Gallardo-Gomez M, Lorenzo JM, Barba FJ, Prieto MA and
Simal-Gandara J: Bioactive compounds and quality of extra virgin
olive oil. Foods. 9(1014)2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Valverde P, Healy E, Jackson I, Rees JL
and Thody AJ: Variants of the melanocyte-stimulating hormone
receptor gene are associated with red hair and fair skin in humans.
Nat Genet. 11:328–330. 1995.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Zhang C, Chery S, Lazerson A, Altman NH,
Jackson R, Holt G, Campos M, Schally AV and Mirsaeidi M:
Anti-inflammatory effects of α-MSH through p-CREB expression in
sarcoidosis like granuloma model. Sci Rep. 10(7277)2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Ozdeslik RN, Olinski LE, Trieu MM, Oprian
DD and Oancea E: Human nonvisual opsin 3 regulates pigmentation of
epidermal melanocytes through functional interaction with
melanocortin 1 receptor. Proc Natl Acad Sci USA. 116:11508–11517.
2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Cheng MC, Lee TH, Chu YT, Syu LL, Hsu SJ,
Cheng CH, Wu J and Lee CK: Melanogenesis inhibitors from the
rhizoma of ligusticum sinense in B16-F10 melanoma cells in vitro
and zebrafish in vivo. Int J Mol Sci. 19(3994)2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Lim HY, Kim E, Park SH, Hwang KH, Kim D,
Jung YJ, Kopalli SR, Hong YD, Sung GH and Cho JY: Antimelanogenesis
effects of theasinensin A. Int J Mol Sci. 22(7453)2021.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Xu Y, Vijayasaradhi S and Houghton AN: The
cytoplasmic tail of the mouse brown locus product determines
intracellular stability and export from the endoplasmic reticulum.
J Invest Dermatol. 110:324–331. 1998.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Li CY, Gao TW, Wang G, Han ZY, Shen Z, Li
TH and Liu YF: The effect of antisense tyrosinase-related protein 1
on melanocytes and malignant melanoma cells. Br J Dermatol.
150:1081–1090. 2004.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Yun CY, Hong SD, Lee YH, Lee J, Jung DE,
Kim GH, Kim SH, Jung JK, Kim KH, Lee H, et al: Nuclear entry of
CRTC1 as druggable target of acquired pigmentary disorder.
Theranostics. 9:646–660. 2019.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Seo GY, Ha Y, Park AH, Kwon OW and Kim YJ:
Leathesia difformis extract inhibits α-MSH-induced
melanogenesis in B16F10 cells via down-regulation of CREB signaling
pathway. Int J Mol Sci. 20(536)2019.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Wu KC, Hseu YC, Shih YC, Sivakumar G, Syu
JT, Chen GL, Lu MT and Chu PC: Calycosin, a common dietary
isoflavonoid, suppresses melanogenesis through the downregulation
of PKA/CREB and p38 MAPK signaling pathways. Int J Mol Sci.
23(1358)2022.PubMed/NCBI View Article : Google Scholar
|