Introduction
Small-interfering RNA (siRNA) therapeutics are
synthetic RNA duplexes designed to target and degrade specific mRNA
(1,2). Cationic liposomes, commonly used as
vectors to deliver siRNA, can form complexes called siRNA
lipoplexes (3,4). However, upon systemic administration,
these positively charged siRNA lipoplexes bind blood components,
including erythrocytes (5), forming
agglutinates that are sequestered by lung capillaries (6). To avoid this, poly(ethylene glycol)
modification (PEGylation) of siRNA lipoplexes is commonly applied
to improve circulating stability (5,7).
However, the presence of PEG on lipoplex surfaces also poses an
obstacle, widely known as the ‘PEG dilemma’, because PEGylation
reduce cellular uptake and endosomal escape, thereby compromising
overall gene-silencing efficacy (8,9).
To minimize interactions between siRNA lipoplexes
and blood components, their surface charge should be negative or
neutral. Negatively charged ternary siRNA lipoplexes have been
created using anionic liposomes composed of
dioleoylphosphatidylglycerol (DOPG) and
dioleoylphosphatidylethanolamine (DOPE) with Ca2+ ions
and siRNA, where Ca2+ electrostatically binds the
phosphate groups of DOPG and siRNA (10,11).
Alternatively, biodegradable anionic polymer coatings, such as
hyaluronic acid, chondroitin sulfate, and poly-L-glutamic acid, can
convert cationic siRNA lipoplexes into anionic lipoplexes (12,13).
Meanwhile, neutral or anionic siRNA lipid nanoparticles can be
generated by rapidly mixing organic solvent-dissolved phospholipid,
cholesterol, PEG-lipid, and ionizable cationic lipid, with aqueous
solution-dissolved siRNA via microfluidics (14,15).
Although these approaches prevent blood component agglutination,
they require specialized preparation equipment.
A single-step modified ethanol injection (MEI)
protocol was previously developed to prepare cationic siRNA
lipoplexes (16,17). This method involves the rapid
injection of siRNA dissolved in phosphate-buffered saline (PBS)
into a lipid-ethanol solution, resulting in efficient formation of
small, uniform siRNA lipoplexes without the need for specialized
equipment. In the present study, a two-step MEI approach is
employed to generate anionic siRNA lipoplexes. Their gene-silencing
efficiency and interaction with erythrocytes are evaluated.
Materials and methods
Materials
1,2-Dioleoyl-3-trimethylammonium-propane methyl
sulfate salt (DOTAP) was obtained from Avanti Polar Lipids Inc.
(Alabaster, AL, USA). Dimethyldioctadecylammonium bromide (DDAB;
cat.no. DC-1-18),
N-hexadecyl-N,N-dimethylhexadecan-1-aminium
bromide (cat. no. DC-1-16), and
11-((1,3-bis(dodecanoyloxy)-2-((dodecanoyloxy)methyl)propan-2-yl)amino)-N,N,N-trimethyl-11-oxoundecan-1-aminium
bromide (cat. no. TC-1-12) were purchased from Sogo Pharmaceutical
Co., Ltd. (Tokyo, Japan). DOPE and polyethylene glycol-cholesteryl
ether (PEG-Chol, average PEG molecular weight: 1,600) were
purchased from NOF Co., Ltd. (Tokyo, Japan). Cholesteryl
hemisuccinate (CHS) was purchased from Sigma-Aldrich (St. Louis,
MO, USA).
Firefly luciferase (Luc)-specific siRNA (target
sequence: hLuc+; GenBank accession no. AY535007.1),
firefly pGL3 Luc-specific siRNA (target sequence: Luc+;
GenBank accession no U47295.2), non-silencing control siRNA (Cont
siRNA), and cyanine 5 (Cy5)-conjugated Cont siRNA (Cy5-siRNA) were
synthesized by Sigma Genosys (Tokyo, Japan) as previously described
(17-19).
AlexaFluor555-labeled Allstars negative control siRNA (AF555-siRNA)
was purchased from Qiagen (Valencia, CA, USA). Hoechst 33342,
trihydrochloride trihydrate, was purchased from Thermo Fisher
Scientific (Waltham, MA, USA).
Cell culture
Human breast cancer MCF-7-Luc cells stably
expressing Luc (hLuc+) were gifted by Dr. Kenji Yamato
(University of Tsukuba, Ibaraki, Japan). Short tandem repeat (STR)
DNA profile analysis verified that the MCF-7-Luc cells utilized in
this study were identical to the MCF-7 cells registered with the
Japanese Collection of Research Bioresources Cell Bank (National
Institute of Biomedical Innovation, Ibaraki, Osaka, Japan). Human
cervical carcinoma HeLa-Luc cells (CVCL_5J41) stably expressing Luc
(Luc+) were acquired from Caliper Life Sciences
(Hopkinton, MA, USA).
MCF-7-Luc cells were maintained in Roswell Park
Memorial Institute-1640 medium (FUJIFILM Wako Pure Chemical
Industries, Ltd., Osaka, Japan) supplemented with 10%
heat-inactivated fetal bovine serum (FBS, Thermo Fisher Scientific)
and 1.2 mg/ml G418 sulfate (Santa Cruz Biotechnology, Dallas, TX,
USA) in a humidified incubator at 37˚C and 5% CO2.
HeLa-Luc cells were cultured under identical conditions in Eagle's
minimum essential medium (FUJIFILM Wako Pure Chemical Industries,
Ltd.) supplemented with 10% FBS and 100 µg/ml kanamycin.
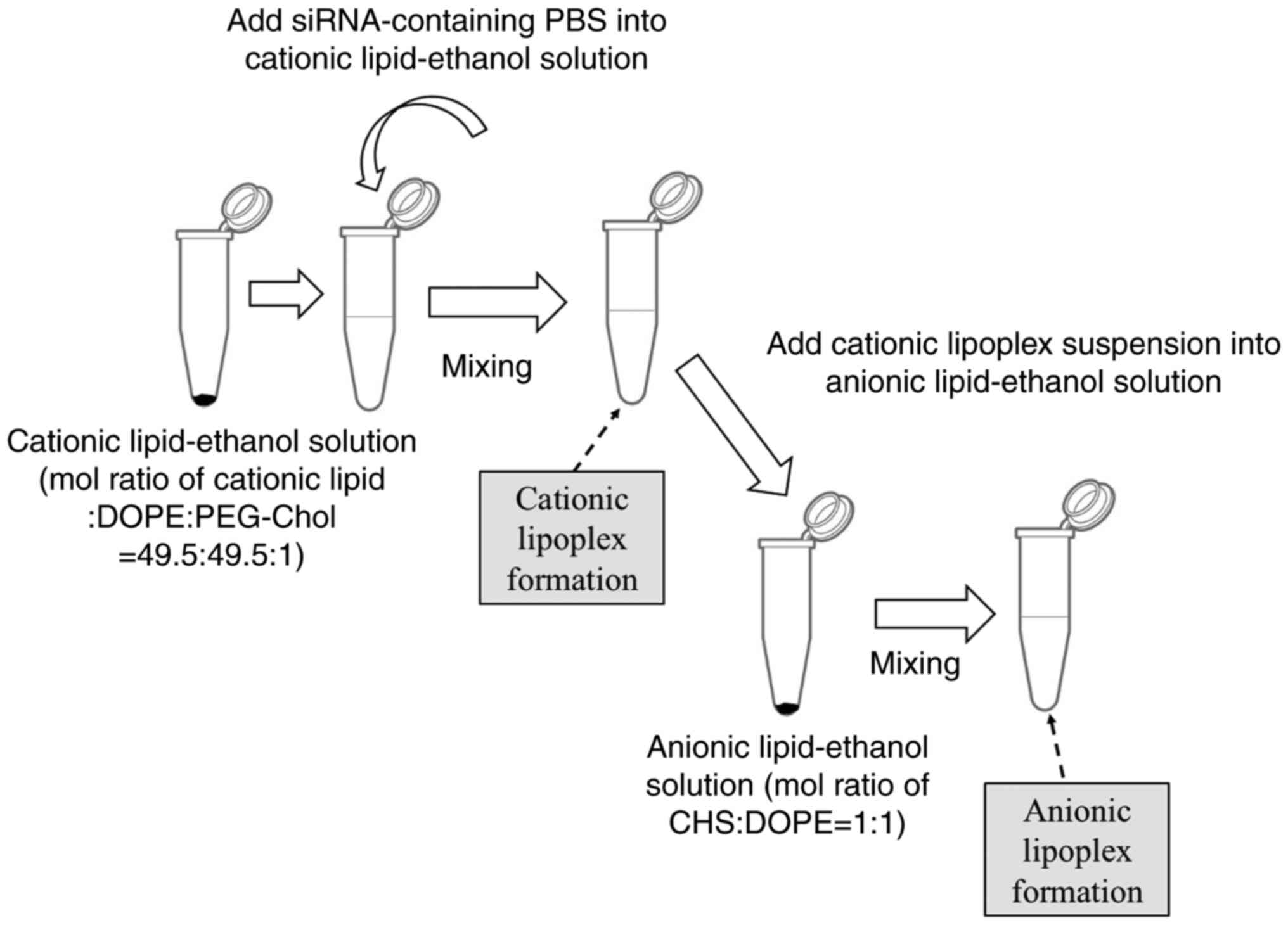
Preparation of cationic and anionic
siRNA lipoplexes using MEI method
To prepare cationic siRNA lipoplexes, a
lipid-ethanol solution was prepared by dissolving cationic lipid
(DOTAP, DDAB, DC-1-16, or TC-1-12), DOPE, and PEG-Chol in ethanol
at a molar ratio of 49.5:49.5:1 (2 mg/ml concentration for the
cationic lipid), following previously reported methods (17). For example, 2 mg TC-1-12, 1.53 mg
DOPE, and 0.08 mg PEG-Chol were dissolved in 1 ml of ethanol.
Subsequently, 50 pmol siRNA solution was transferred to a tube
containing 100 µl of PBS (pH 7.4). The resulting solution was
rapidly added to the lipid-ethanol solution (3.1 µl for LP-DOTAP,
2.3 µl for LP-DC116, 2.5 µl for LP-DDAB, and 3.9 µl for LP-TC112)
in another tube to achieve a charge ratio (+:-) of 4:1, based on
previous reports (16,17). The charge ratio (+:-) refers to the
molar ratio of quaternary amines in the cationic lipid to the siRNA
phosphates.
To prepare anionic siRNA lipoplexes, another
lipid-ethanol solution was prepared by dissolving 2 mg CHS and 3.06
mg DOPE (molar ratio of 1:1) in 1 ml of ethanol. The lipid-ethanol
solution was then mixed with a cationic lipoplex suspension
containing 50 pmol siRNA. Volumes of 1.95 and 3.89 µl of the
lipid-ethanol solution were used to achieve charge ratios (+:-) of
1:1 and 1:2 (1CHS and 2CHS), respectively. The charge ratio (+:-)
was calculated as the molar ratio of cationic lipids to CHS.
Measurement of liposome and siRNA
lipoplex sizes
Cationic and anionic siRNA lipoplexes containing 5
µg Cont siRNA were prepared using the MEI method. Cationic and
anionic liposomes were prepared using the same amount of
lipid-ethanol solution as the siRNA lipoplexes but without siRNA.
The liposomes and siRNA lipoplexes were diluted threefold with
water. Subsequently, the particle size, polydispersity index (PDI),
and ζ-potential of the liposomes and siRNA lipoplexes were measured
using a light-scattering photometer (ELS-Z2; Otsuka Electronics
Co., Ltd., Osaka, Japan).
Evaluation of Luc knockdown efficiency
in Luc siRNA lipoplexes
Anionic and cationic siRNA lipoplexes containing 50
pmol of Cont and Luc siRNAs were diluted in culture medium
supplemented with 10% FBS to a final siRNA concentration of 50 nM.
The siRNA lipoplexes were added to MCF-7-Luc and HeLa-Luc cells in
12-well culture plates. Luc activity [counts per second (cps)] was
determined 48 h post-transfection as previously described (5). Luc activity (%) was calculated
relative to that (cps/µg protein) of untreated cells.
Cytotoxicity after transfection with
anionic and cationic lipoplexes
MCF-7-Luc and HeLa-Luc cells were seeded in 96-well
culture plates at a density of 1x104 cells/well. After
24 h of incubation at 37˚C, anionic and cationic lipoplexes
containing 50 pmol Cont siRNA were prepared using the MEI method.
The lipoplexes were diluted with culture medium containing 10% FBS
(final siRNA concentration: 50 nM) and subsequently added to the
cells (100 µl/well). Cytotoxicity was assessed 48 h
post-transfection using the Cell Counting Kit-8 (Dojindo
Laboratories, Inc., Kumamoto, Japan), as previously reported
(20).
Cellular uptake of siRNA
lipoplexes
To quantify the amount of siRNA taken up by the
cells, MCF-7-Luc cells were seeded in 12-well culture plates at a
density of 1x105 cells/well. After 24 h of incubation at
37˚C, cationic and anionic lipoplexes containing 50 pmol
AF555-siRNA in 1 ml of culture medium were added to the cells
(final siRNA concentration: 50 nM). After 24-h incubation, the
plates were washed with PBS to remove unbound lipoplexes, and the
cells were lysed with cell lysis buffer (Pierce™ Luciferase Cell
Lysis Buffer; Pierce; Thermo Fisher Scientific, Inc.), followed by
centrifugation at 15,000 g for 10 sec at 4˚C. AF555-fluorescence
intensity [relative fluorescence unit (RFU)] in the supernatant
were then measured using a fluorescence microplate reader
(GloMax® Discover system, Promega Corporation, Madison,
WI, USA) at 525 nm of excitation and 580-640 nm of emission.
Protein concentrations of the supernatants were determined with
bicinchoninic acid (BCA) reagent (Pierce™ BCA Protein Assay Kit;
Pierce; Thermo Fisher Scientific, Inc.). The fluorescence intensity
of AF555-siRNA (RFU/µg protein) in the cells was calculated by
subtracting the background fluorescence (RFU/µg protein) of
untreated cells.
To observe the localization of siRNA in the cells,
MCF-7-Luc cells were seeded in 12-well culture plates at a density
of 1x105 cells/well. After 5 or 24 h of incubation at
37˚C, anionic and cationic lipoplexes containing 50 pmol Cy5-siRNA
in 1 ml of culture medium were added to the cells (final siRNA
concentration: 50 nM). At 24 h post-transfection, the cells were
fixed with Mildform 10N (FUJIFILM Wako Pure Chemical Industries,
Ltd.) for 10 min at room temperature. Subsequently, the cells were
incubated with 10 µg/ml Hoechst 33342 for 10 min to stain the
nuclei. Cy5-siRNA and nuclei within the cells were detected using
an Eclipse TS100-F fluorescence microscope (Nikon Corporation,
Tokyo, Japan) equipped with optical filters for Cy5 (excitation
filter: 620/60 nm, dichroic mirror: 660 nm, absorption filter:
700/75 nm) and Hoechst 33342 (excitation filter: 365/10 nm,
dichroic mirror: 400 nm, absorption filter: 400 nm).
Animal experiments
Animal experiments adhered to the Guide for the Care
and Use of Laboratory Animals of the U.S. National Institutes of
Health and were approved by the Institutional Animal Care and Use
Committee of Hoshi University (Tokyo, Japan; approval number:
P24-151, Research period: from December 10, 2024, to February 28,
2025). The predetermined endpoint for experimental termination was
set as: the mice would be euthanized by cervical dislocation if the
solid tumor volume in a tumor-bearing mouse exceeded 2,000
mm3. However, this endpoint criterion was not applied to
any mice during the course of the study.
One eight-week-old female BALB/c mouse obtained from
Sankyo Labo Service Corporation, Inc. (Tokyo, Japan) was maintained
in a temperature-controlled room (24˚C) at 55% relative humidity
under a 12/12-h light/dark cycle (08:00 to 20:00) with ad
libitum access to food and water.
Hemolysis and agglutination assay
Study on erythrocyte characteristics based on donor
sex shows that female donors have erythrocytes with greater
resilience to storage damage and hemolysis (21). Therefore, erythrocytes from female
mice were used. Total duration of the study was 1 day. Blood (0.2
ml) was collected from the jugular vein of one female BALB/c mouse
under anesthesia induced by isoflurane inhalation (4% gas-air
mixture for induction and 2% gas-air mixture for maintenance;
FUJIFILM Wako Pure Chemical Corporation). Immediately after the
blood collection, the mice were euthanized by cervical dislocation;
death was confirmed by cessation of heartbeat. Erythrocytes were
collected from the blood at 4˚C by centrifugation at 300 x g for 3
min and resuspended in PBS as a 2% (v/v) suspension of
erythrocytes.
To assess agglutination, cationic and anionic siRNA
lipoplexes containing 50 pmol Cont siRNA in 100 µl of PBS were
added to 100 µl of a 2% (v/v) erythrocyte suspension. After
incubating at 37˚C for 15 min, the sample was placed on a glass
slide, and agglutination was observed under a light microscope
(Eclipse TS100-F, Nikon Corporation).
To examine erythrocyte hemolysis induced by siRNA
lipoplexes, cationic and anionic siRNA lipoplexes containing 50
pmol Cont siRNA in 100 µl of PBS were added to 100 µl of a 2% (v/v)
erythrocyte suspension; the hemolysis proportion was calculated as
previously reported (22).
Erythrocytes suspended in water served as positive controls for
complete hemolysis (100% hemolysis).
Statistical analysis
Two groups were compared using an unpaired Student's
t-test. Multiple groups were compared using one-way analysis of
variance (ANOVA), followed by Tukey's post-hoc test. All data were
analyzed using GraphPad Prism software (version 4.0; GraphPad
Software Inc., San Diego, CA, USA), and statistical significance
was set at P<0.05.
Results
Characterization of cationic and
anionic siRNA lipoplexes
To prepare cationic siRNA lipoplexes, DOTAP,
DC-1-16, DDAB, and TC-1-12 were used as cationic lipids, with DOPE
as a neutral lipid and PEG-Chol as a PEG-lipid (Fig. 1). Cationic lipoplexes were formed by
the MEI method with a 49.5:49.5:1 molar ratio of cationic lipid,
DOPE, and PEG-Chol (Table I). The
resulting LP-DOTAP, LP-DC116, LP-DDAB, and LP-TC112 lipoplexes
measured 148, 224, 200, and 92 nm in size, respectively (PDI: 0.10,
0.08, 0.11, and 0.16, respectively), and had positive ζ-potentials
(~4.6-11 mV).
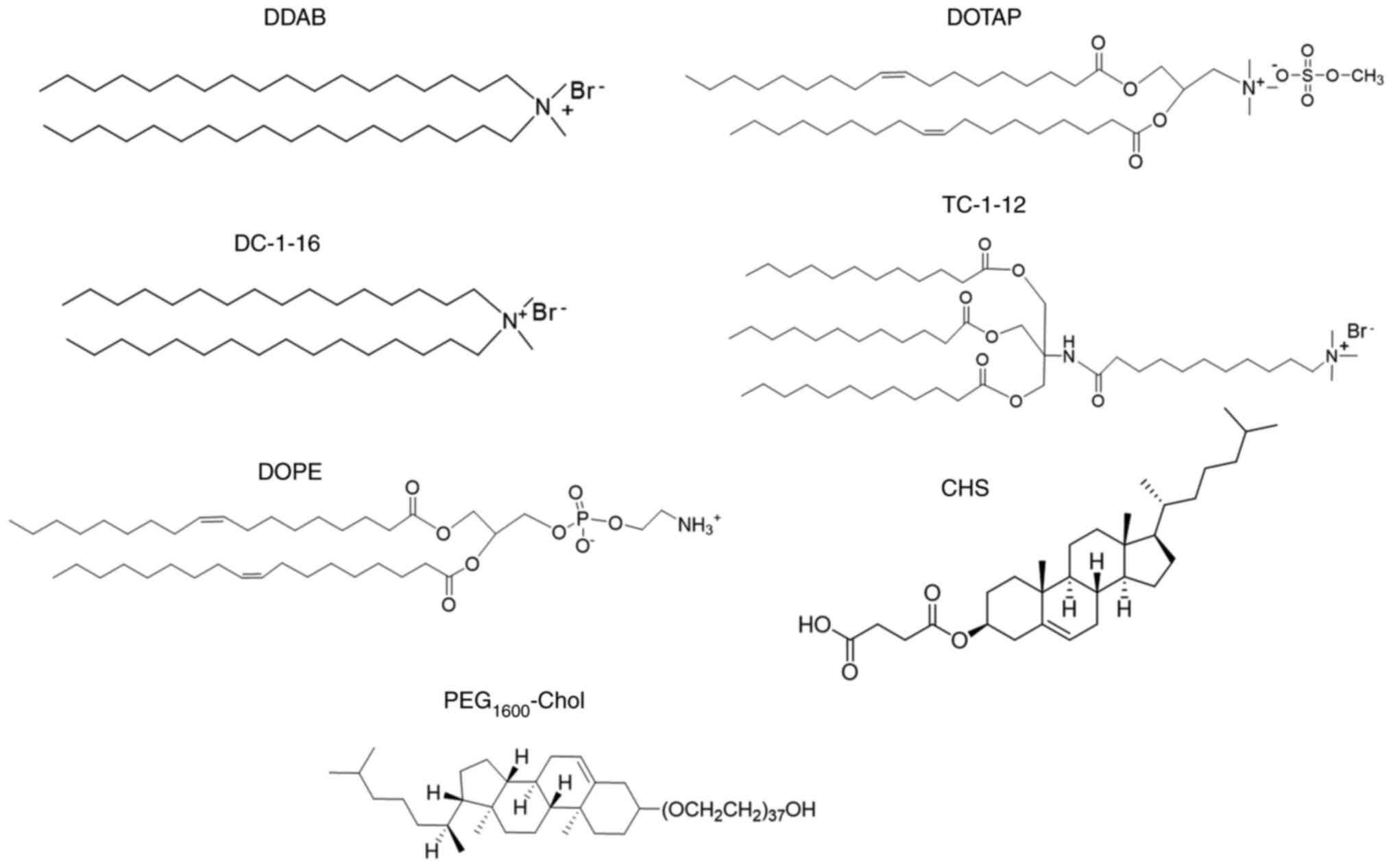 | Figure 1Structures of cationic lipids, anionic
lipid, neutral lipid and PEG-lipid. DOTAP,
1,2-dioleoyl-3-trimethylammonium-propane methyl sulfate salt; DDAB,
dimethyldioctadecylammonium bromide; DC-1-16,
N-hexadecyl-N, N-dimethylhexadecan-1-aminium
bromide; TC-1-12,
11-((1,3-bis(dodecanoyloxy)-2-((dodecanoyloxy)methyl)propan-2-yl)amino)-N,N,N-trimethyl-11-oxoundecan-1-aminium
bromide; DOPE,
1,2-dioleoyl-sn-glycero-3-phosphoethanolamine; CHS,
cholesteryl hemisuccinate; PEG-Chol, poly(ethylene glycol)
cholesteryl ether. |
 | Table ISize and ζ-potential of cationic and
anionic siRNA lipoplexes prepared using modified ethanol injection
method. |
Table I
Size and ζ-potential of cationic and
anionic siRNA lipoplexes prepared using modified ethanol injection
method.
| Lipoplex | Liposomal
formulation (molar ratio) | Sizea, nm | PDI |
ζ-potentiala, mV |
|---|
| LP-DOTAP |
DOTAP/DOPE/PEG1600-Chol
(49.5:49.5:1.0) | 147.8±1.5 | 0.10±0.01 | 10.9±3.2 |
| LP-DOTAP-1CHS |
DOTAP/DOPE/CHS/PEG1600-Chol
(24.9:49.7:24.9:0.5) | 229.2±4.3 | 0.06±0.01 | -2.2±1.8 |
| LP-DOTAP-2CHS |
DOTAP/DOPE/CHS/PEG1600-Chol
(16.6:49.8:33.2:0.3) | 206.5±1.6 | 0.12±0.02 | -26.5±1.8 |
| LP-DC116 |
DC-1-16/DOPE/PEG1600-Chol
(49.5:49.5:1.0) | 223.5±1.6 | 0.08±0.01 | 11.1±0.6 |
| LP-DC116-1CHS |
DC-1-16/DOPE/CHS/PEG1600-Chol
(24.9:49.7:24.9:0.5) | 374.4±4.0 | 0.23±0.01 | 0.3±0.7 |
| LP-DC116-2CHS |
DC-1-16/DOPE/CHS/PEG1600-Chol
(16.6:49.8:33.2:0.3) | 228.5±0.5 | 0.30±0.01 | -16.2±4.6 |
| LP-DDAB |
DDAB/DOPE/PEG1600-Chol
(49.5:49.5:1.0) | 199.9±0.9 | 0.11±0.01 | 4.6±0.0 |
| LP-DDAB-1CHS |
DDAB/DOPE/CHS/PEG1600-Chol
(24.9:49.7:24.9:0.5) | 213.6±2.3 | 0.19±0.01 | -4.8±0.1 |
| LP-DDAB-2CHS |
DDAB/DOPE/CHS/PEG1600-Chol
(16.6:49.8:33.2:0.3) | 161.8±1.5 | 0.25±0.01 | -17.8±2.8 |
| LP-TC112 |
TC-1-12/DOPE/PEG1600-Chol
(49.5:49.5:1.0) | 91.9±0.4 | 0.16±0.02 | 8.0±1.3 |
| LP-TC112-1CHS |
TC-1-12/DOPE/CHS/PEG1600-Chol
(24.9:49.7:24.9:0.5) | 156.0±1.7 | 0.10±0.01 | -12.8±0.5 |
| LP-TC112-2CHS |
TC-1-12/DOPE/CHS/PEG1600-Chol
(16.6:49.8:33.2:0.3) | 145.7±2.8 | 0.08±0.02 | -19.5±1.5 |
To prepare anionic liposomes, CHS and DOPE were
mixed at a 1:1 ratio in ethanol. Using the MEI method, LP-CHS was
obtained with a particle size of 97 nm and a ζ-potential of -63 mV
(Table SI), indicating a strong
negative charge capable of neutralizing cationic charges.
To determine the optimal amount of CHS required to
neutralize cationic liposomes, anionic liposomes were prepared
using the two-step MEI method by sequentially combining a
lipid-ethanol solution of cationic lipid, DOPE, and PEG-Chol with
PBS without siRNA, followed by the addition of a CHS-DOPE
lipid-ethanol solution. CHS was incorporated at either equimolar
(1CHS) or double the molar amount (2CHS) relative to the cationic
lipid. Adding CHS at twice the molar ratio consistently restored a
negative charge to the liposomes (Table SI).
Anionic siRNA lipoplexes were prepared using a
two-step MEI method: a lipid-ethanol solution containing cationic
lipids, DOPE, and PEG-Chol was rapidly mixed with siRNA in PBS,
followed by the addition of a CHS-DOPE ethanol solution (Fig. 2). LP-DOTAP-1CHS, LP-DC116-1CHS, and
LP-DDAB-1CHS lipoplexes had near-neutral ζ-potentials (-4.8 to 0.3
mV), and increased sizes (230, 374, and 214 nm, respectively)
compared to lipoplexes without CHS (Table I). Meanwhile, their 2CHS
counterparts exhibited strongly negative ζ-potentials (-16.2 to
-26.5 mV) and smaller sizes (207, 229, and 162 nm, respectively).
LP-TC112-1CHS and LP-TC112-2CHS lipoplexes measured 156 and 146 nm
with ζ-potentials of -12.8 and -19.5 mV, respectively.
Luc activity suppression by cationic
and anionic Luc siRNA lipoplexes
Cationic and anionic lipoplexes containing Luc siRNA
were transfected into MCF-7-Luc and HeLa-Luc cells, with Luc
activity measured 48 h post-transfection. Cationic siRNA lipoplexes
exhibited strong gene-silencing activity in both cell lines,
regardless of the cationic lipid type (Fig. 3A and B). However, for DOTAP, DC116, and DDAB
formulations, increasing CHS reduced silencing activity. Notably,
LP-DC116-2CHS and LP-DDAB-2CHS showed no silencing in MCF-7-Luc
cells. In contrast, LP-TC-112-1CHS and LP-TC112-2CHS lipoplexes did
not exhibit decreased gene silencing activity in either cell type
compared with LP-TC-112 lipoplexes.
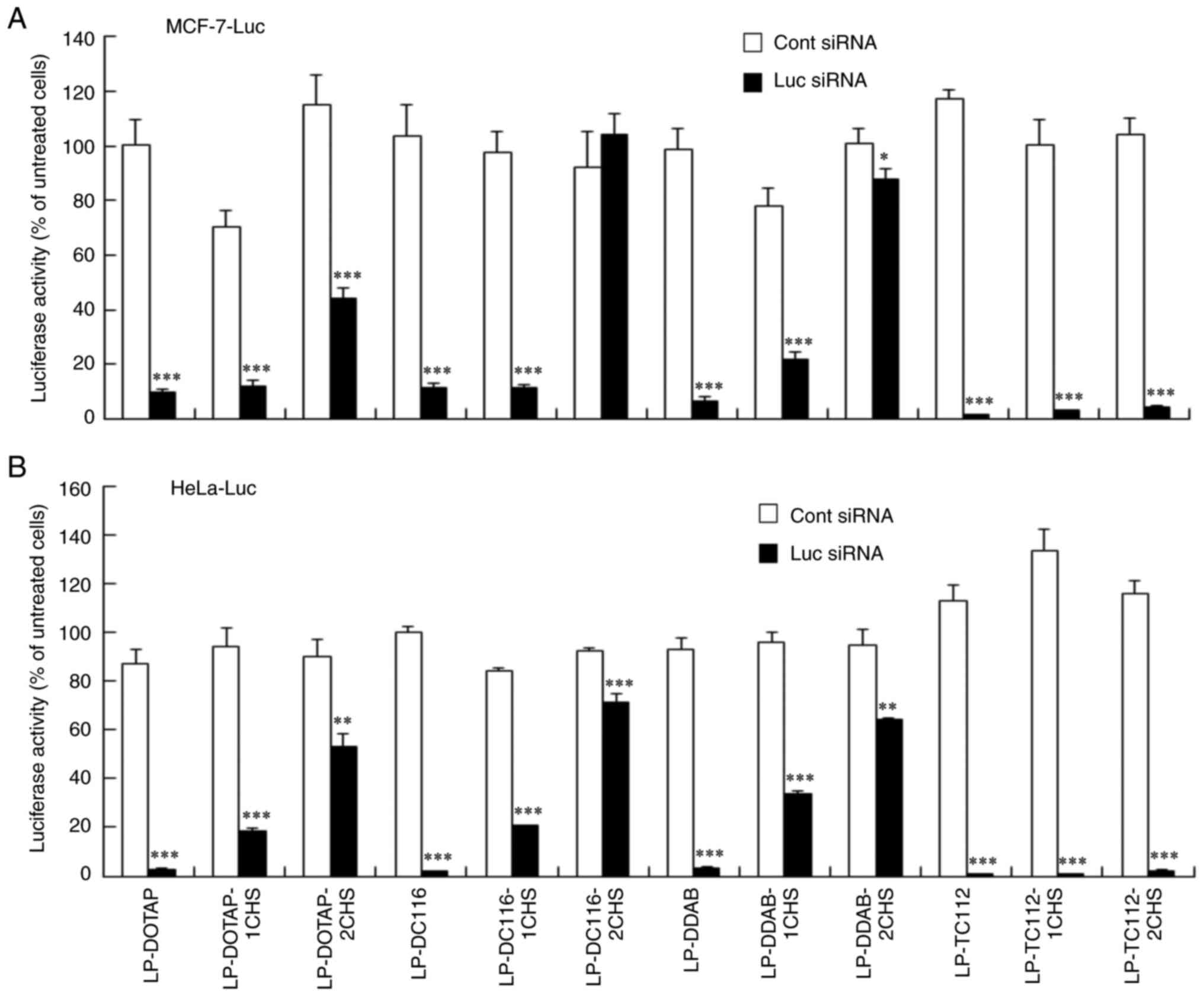 | Figure 3Luc activity suppression by cationic
and anionic siRNA lipoplexes. Cationic and anionic lipoplexes
containing Cont and Luc siRNAs were prepared using the modified
ethanol injection method. These lipoplexes were then added to (A)
MCF-7-Luc and (B) HeLa-Luc cells at a final siRNA concentration of
50 nM. Luc activity was measured 48 h post-transfection. Data are
represented as the mean + standard deviation (n=3).
*P<0.05, **P<0.01 and
***P<0.001 vs. Cont siRNA. Luc, luciferase; siRNA,
small interfering RNA; Cont, control; LP, liposome; DOTAP,
1,2-dioleoyl-3-trimethylammonium-propane methyl sulfate salt; DDAB,
dimethyldioctadecylammonium bromide; DC-1-16,
N-hexadecyl-N, N-dimethylhexadecan-1-aminium
bromide; TC-1-12,
11-((1,3-bis(dodecanoyloxy)-2-((dodecanoyloxy)methyl)propan-2-yl)amino)-N,N,N-trimethyl-11-oxoundecan-1-aminium
bromide; CHS, cholesteryl hemisuccinate. |
Cytotoxicity of cationic and anionic
siRNA lipoplexes
Cationic and anionic siRNA lipoplexes were
transfected into MCF-7-Luc and HeLa-Luc cells, with viability
measured after 48 h. Cytotoxicity of DOTAP and TC-1-12 formulations
increased in both cell types with more CHS added to LP-DOTAP and
LP-TC-1-12 lipoplexes, respectively (Fig. 4A and B). Conversely, the cytotoxicity of DC-1-16
and DDAB formulations decreased with increased CHS added to
LP-DC116 and LP-DDAB lipoplexes, respectively.
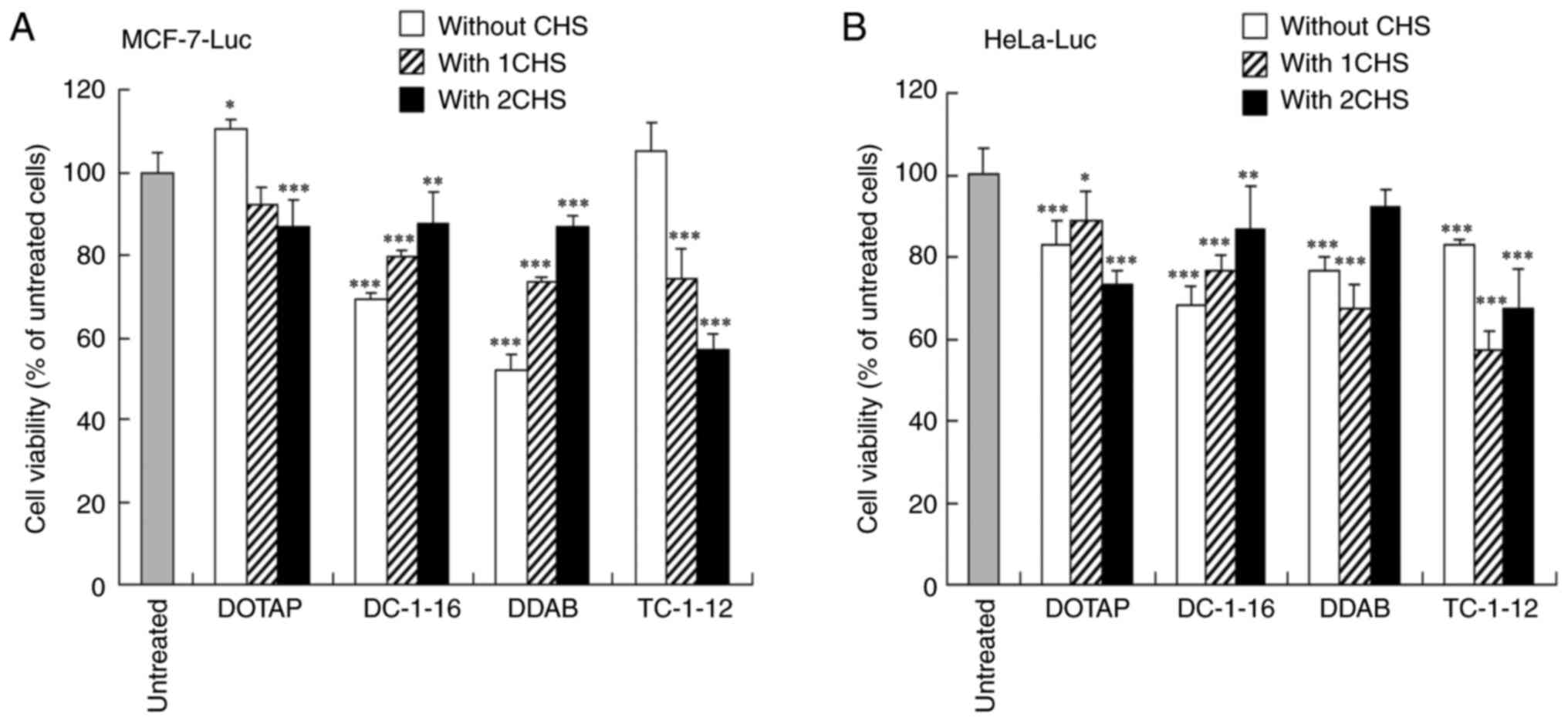 | Figure 4Cell viability after transfection
with cationic and anionic siRNA lipoplexes. Cationic and anionic
lipoplexes containing Cont siRNA were prepared by the modified
ethanol injection method and subsequently added to (A) MCF-7-Luc
and (B) HeLa-Luc cells at a final siRNA concentration of 50 nM.
Cell viability was assessed 48 h after transfection. Data are
represented as the mean + standard deviation (n=5).
*P<0.05, **P<0.01 and
***P<0.001 vs. untreated cells. Luc, luciferase;
siRNA, small interfering RNA; Cont, control; DOTAP,
1,2-dioleoyl-3-trimethylammonium-propane methyl sulfate salt; DDAB,
dimethyldioctadecylammonium bromide; DC-1-16,
N-hexadecyl-N, N-dimethylhexadecan-1-aminium
bromide; TC-1-12,
11-((1,3-bis(dodecanoyloxy)-2-((dodecanoyloxy)methyl)propan-2-yl)amino)-N,N,N-trimethyl-11-oxoundecan-1-aminium
bromide; CHS, cholesteryl hemisuccinate. |
When anionic siRNA lipoplexes with 2CHS were
transfected into the cells, the culture medium contained 7.8 µg/ml
of CHS. To examine the cytotoxicity of CHS, CHS was added into
MCF-7-Luc and HeLa-Luc cells. CHS was found to be more toxic to
MCF-7-Luc cells than to HeLa-Luc cells (Fig. S1A). Furthermore, when cationic and
anionic liposomes without siRNA were added into both cell lines,
LP-TC112-2CHS increased cytotoxicity in MCF-7-Luc cells compared to
LP-TC112 (Fig. S1B, C).
siRNA cellular uptake after
transfection with cationic and anionic lipoplexes
siRNA uptake in MCF-7-Luc cells was examined 5 or 24
h post-transfection with cationic and anionic lipoplexes. Among the
cationic lipoplexes, LP-TC112 lipoplexes exhibited the highest
siRNA uptake, showing approximately twofold higher uptake than
LP-DOTAP lipoplexes and fourfold higher uptake than both LP-DDAB
and LP-DC116 lipoplexes (Figs. 5A,
B, 6A). In contrast, all anionic lipoplexes
showed reduced siRNA uptake compared to their cationic counterparts
(Fig. 5A, B). However, LP-TC112-2CHS lipoplexes
exhibited higher siRNA uptake compared to the other anionic
lipoplexes (Figs. 6B, S2).
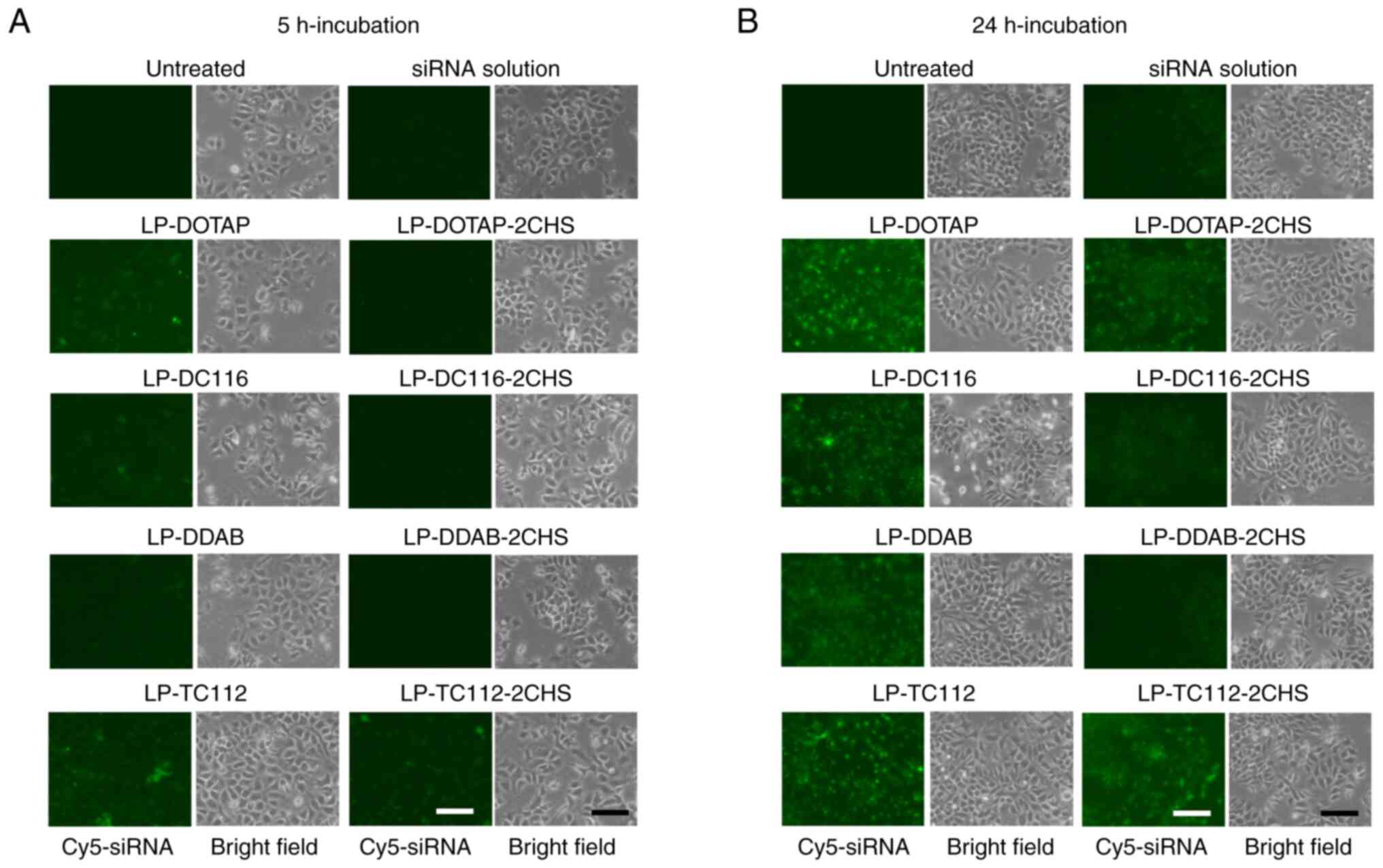 | Figure 5Cellular uptake of siRNA by MCF-7-Luc
cells after transfection with cationic and anionic lipoplexes.
Cationic and anionic lipoplexes containing Cy5-siRNA were prepared
using the modified ethanol injection method and added to MCF-7-Luc
cells at a final siRNA concentration of 50 nM. As a control,
Cy5-siRNA solution was added to the MCF-7-Luc cells. Localization
of Cy5-siRNA (green) was observed at (A) 5 and (B) 24 h
post-incubation, respectively. Scale bar, 100 µm. siRNA, small
interfering RNA; Luc, luciferase; LP, liposome; DOTAP,
1,2-dioleoyl-3-trimethylammonium-propane methyl sulfate salt; DDAB,
dimethyldioctadecylammonium bromide; DC-1-16,
N-hexadecyl-N, N-dimethylhexadecan-1-aminium
bromide; TC-1-12,
11-((1,3-bis(dodecanoyloxy)-2-((dodecanoyloxy)methyl)propan-2-yl)amino)-N,N,N-trimethyl-11-oxoundecan-1-aminium
bromide; CHS, cholesteryl hemisuccinate. |
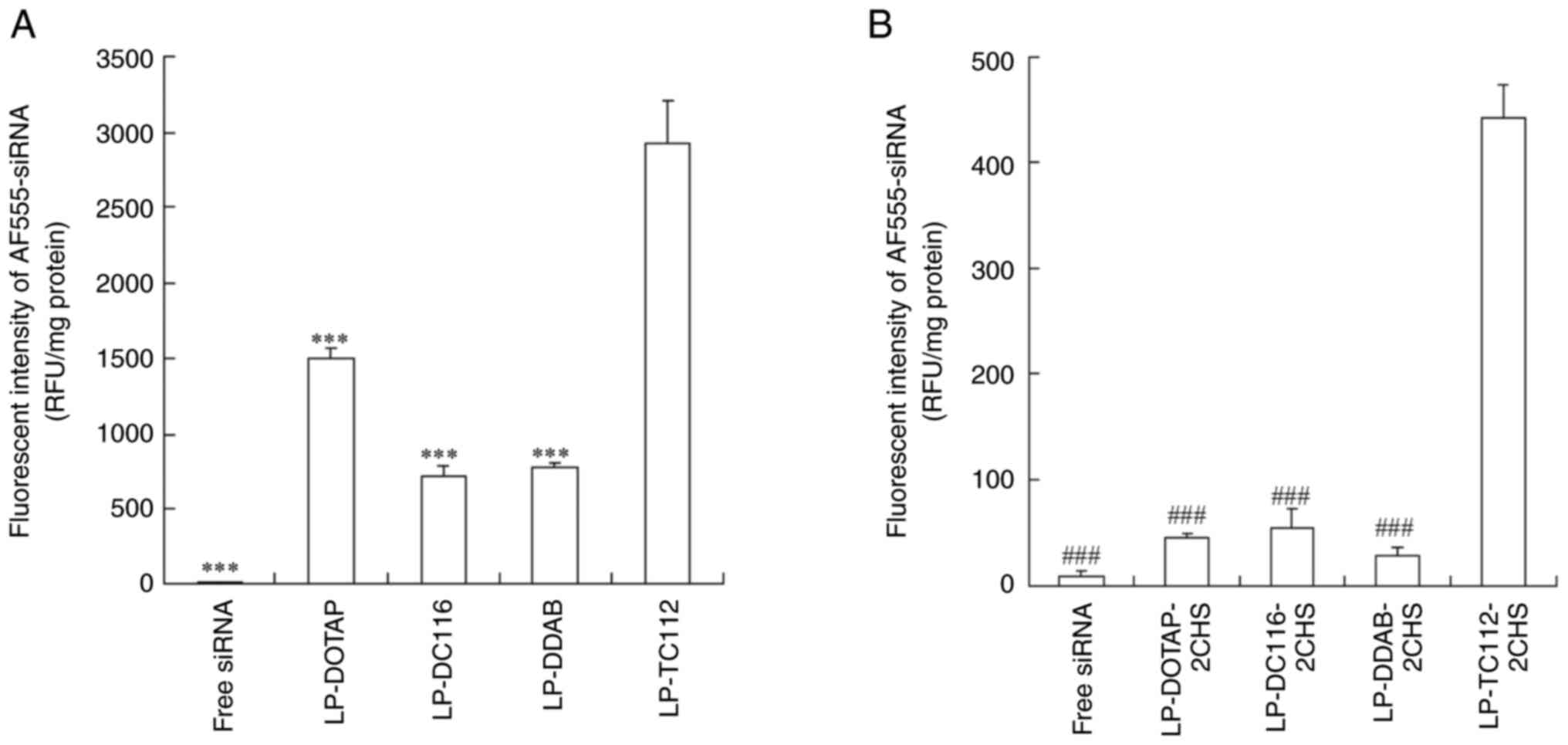 | Figure 6Cellular uptake of siRNA by MCF-7-Luc
cells after transfection with cationic and anionic lipoplexes. (A)
Cationic and (B) anionic lipoplexes containing AF555-siRNA were
prepared using the modified ethanol injection method and added to
MCF-7-Luc cells at a final siRNA concentration of 50 nM. As a
control, AF555-siRNA solution (free siRNA) was added to the
MCF-7-Luc cells. After 24 h, the cells were lysed, and the amount
of AF555-siRNA within the cells was measured using a fluorescence
plate reader. Data are represented as the mean + standard deviation
(n=3). ***P<0.001 vs. LP-TC112;
###P<0.001 vs. LP-TC112-2CHS. LP, liposome; siRNA,
small interfering RNA; DOTAP,
1,2-dioleoyl-3-trimethylammonium-propane methyl sulfate salt; DDAB,
dimethyldioctadecylammonium bromide; DC-1-16,
N-hexadecyl-N, N-dimethylhexadecan-1-aminium
bromide; TC-1-12,
11-((1,3-bis(dodecanoyloxy)-2-((dodecanoyloxy)methyl)propan-2-yl)amino)-N,N,N-trimethyl-11-oxoundecan-1-aminium
bromide; CHS, cholesteryl hemisuccinate. |
Hemolysis and erythrocyte aggregation
by cationic and anionic lipoplexes
Considering that positively charged lipoplexes can
disrupt erythrocytes through electrostatic interactions, hemolysis
and aggregation of mouse erythrocytes were investigated following
incubation with cationic and anionic siRNA lipoplexes. LP-DOTAP,
LP-DC116, and LP-DDAB lipoplexes caused marked erythrocyte
aggregation (Fig. 7A) but minimal
hemolysis (<5%; Fig. 7B).
Notably, LP-TC112 lipoplexes induced high levels of hemolysis
(65%). In contrast, LP-DOTAP-2CHS, LP-DC116-2CHS, LP-DDAB-2CHS, and
LP-TC112-2CHS lipoplexes did not cause erythrocyte aggregation or
hemolysis (<5%).
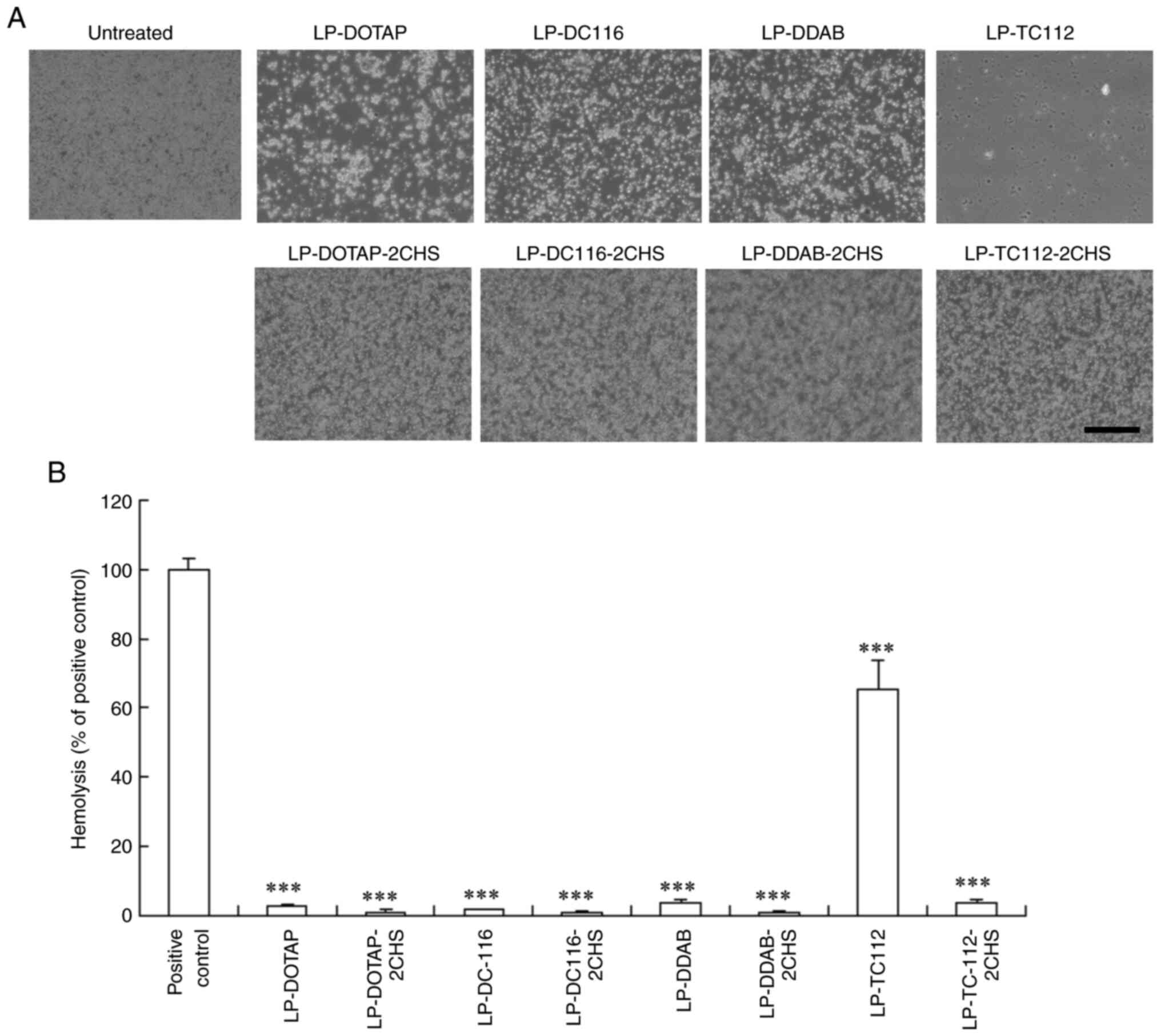 | Figure 7Hemolysis and erythrocyte
agglutination induced by cationic and anionic siRNA lipoplexes.
Cationic and anionic lipoplexes containing 50 pmol of Cont siRNA
were prepared using the modified ethanol injection method and
incubated with erythrocyte suspensions at 37˚C for 15 min. (A)
Erythrocyte agglutination after mixing with the siRNA lipoplexes.
Scale bar, 200 µm. (B) Hemolysis (%) calculated relative to the
absorbance of the hypotonic solution and presented as the mean +
standard deviation (n=3). Erythrocytes suspended in a hypotonic
solution (water) serve as a positive control for 100% hemolysis.
***P<0.001 vs. positive control. LP, liposome; DOTAP,
1,2-dioleoyl-3-trimethylammonium-propane methyl sulfate salt; DDAB,
dimethyldioctadecylammonium bromide; DC-1-16,
N-hexadecyl-N, N-dimethylhexadecan-1-aminium
bromide; TC-1-12,
11-((1,3-bis(dodecanoyloxy)-2-((dodecanoyloxy)methyl)propan-2-yl)amino)-N,N,N-trimethyl-11-oxoundecan-1-aminium
bromide; CHS, cholesteryl hemisuccinate. |
Discussion
This study presents a two-step MEI method to prepare
anionic siRNA lipoplexes. CHS served as the anionic lipid to
neutralize the positive charge of siRNA lipoplexes. Adding a
lipid-ethanol solution of CHS and DOPE to the cationic siRNA
lipoplex suspensions reversed the surface potential from positive
to negative. It is hypothesized that either CHS and DOPE directly
integrate into the lipid membrane of the cationic siRNA lipoplexes,
forming anionic siRNA lipoplexes, or pre-formed CHS/DOPE anionic
liposomes bind and fuse electrostatically with cationic lipoplexes
to yield anionic siRNA lipoplexes.
Neutral charged siRNA lipoplexes (LP-DOTAP-1CHS,
LP-DC116-1CHS, and LP-DDAB-1CHS) were larger than their negatively
charged counterparts (LP-DOTAP-2CHS, LP-DC116-2CHS, and
LP-DDAB-2CHS), likely due to reduced electrostatic repulsion in the
neutral charged lipoplexes. At a cationic lipid to CHS ratio of
1:2, all siRNA lipoplexes exhibited strong negative ζ-potentials
(-16 to -27 mV), regardless of the cationic lipid used, indicating
that twice the molar ratio of CHS relative to the cationic lipid is
required to form anionic siRNA lipoplexes.
LP-TC112-2CHS lipoplexes with Luc siRNA achieved
strong gene silencing in both MCF-7-Luc and HeLa-Luc cells, whereas
LP-DOTAP-2CHS lipoplexes had a moderate effect. LP-DC116-2CHS and
LP-DDAB-2CHS lipoplexes were less effective. However, LP-TC112-2CHS
lipoplexes exhibited increased cytotoxicity in MCF-7-Luc cells. CHS
is an amphipathic cholesterol ester with anticancer activity
against MCF-7 cells (23). It was
found that CHS was more toxic to MCF-7-Luc cells than to HeLa-Luc
cells. In addition, LP-TC112-2CHS without siRNA also exhibited
higher cytotoxicity than LP-TC112 without siRNA in MCF-7-Luc cells.
These suggest that the LP-TC112-2CHS lipoplexes were efficiently
delivered into MCF-7 cells, and the CHS might induce
cytotoxicity.
All anionic lipoplexes reduced siRNA uptake in
MCF-7-Luc cells compared to their cationic counterparts, indicated
that the negative charge on the surface of the lipoplexes may have
weakened their interaction with cells. However, intracellular siRNA
was detected at high levels following transfection with
LP-TC112-2CHS lipoplexes, indicating better cellular uptake and
gene silencing. The reduced gene-silencing efficacy of
LP-DOTAP-2CHS, LP-DC116-2CHS and LP-DDAB-2CHS lipoplexes is likely
due to poor cellular uptake. However, the precise mechanisms
underlying the differential internalization of these lipoplexes
remain unclear. In our study, siRNA uptake after transfection with
cationic lipoplexes was highest in the order of LP-TC112
>LP-DOTAP >LP-DDAB=LP-DC116 lipoplexes. It has been reported
that the linker structure of a cationic lipid significantly impacts
the cellular uptake of lipoplexes, thereby affecting transfection
efficiency (24,25). TC-1-12 and DOTAP have a
biodegradable ester linker, while DDAB and DC-1-16 have a
non-biodegradable linker. This suggests that the difference in
linker structure may affect the interaction between the cell
membrane and the siRNA lipoplexes. In addition, TC-1-12, a lipid
with trialkyl chains, exhibits high cell membrane fusion activity
(20). Koulov et al. reported that
vesicles with cationic trialkyl chains promote greater membrane
fusion than those with structurally analogous cationic dialkyl or
monoalkyl chains (26). Therefore,
LP-TC112-2CHS lipoplexes may enhance cellular uptake and gene
silencing due to their strong membrane fusion activity, even though
they have a negative charge. Future studies should assess how
different cationic lipids used in anionic siRNA lipoplexes impact
cellular uptake.
LP-DOTAP, LP-DC116, and LP-DDAB lipoplexes caused
erythrocyte agglutination without hemolysis, while LP-TC112
lipoplexes exhibited strong hemolytic activity due to their
fusogenic properties. However, adding 2CHS prevented agglutination
after incubation with LP-DOTAP-2CHS, LP-DC116-2CHS, or LP-DDAB-2CHS
lipoplexes, whereas LP-TC112-2CHS lipoplexes did not cause
agglutination or hemolysis. Notably, LP-TC112-2CHS maintained high
cellular uptake and gene-silencing efficiency while suppressing
erythrocyte interaction. Positively charged siRNA lipoplexes
interact with erythrocytes and form agglutination in the
bloodstream, leading to their entrapment in highly vascularized
lung capillaries. However, negatively charged lipoplexes may reduce
siRNA accumulation in the lungs by avoiding interaction with
erythrocytes in the bloodstream, resulting in improved blood
stability. Further research is warranted to assess its
biodistribution and in vivo gene-silencing effects.
Erythrocytes from female mice were utilized in this study.
Nonetheless, male erythrocytes are reportedly more susceptible to
hemolysis than female ones (21),
suggesting that differences in hemolytic potential across species
should be considered carefully.
Previous studies have described the preparation of
cationic
3β-[N-(N',N'-dimethylaminoethyl)carbamoyl]
cholesterol (DC-Chol)/DOPE and anionic CHS/DOPE liposomes
separately via thin-film hydration, followed by neutralizing
positively charged DC-Chol/DOPE-plasmid DNA lipoplexes through
incubation with CHS/DOPE liposomes (27). However, this requires specialized
equipment, including evaporators, sonicators, and extruders. In the
current study, a simple two-step MEI method was developed for
preparing anionic siRNA lipoplexes without the need for evaporation
or sonication. Overall, the results suggest that TC-1-12-based
anionic siRNA lipoplexes prepared using lipid-ethanol solutions may
be suitable for siRNA transfection.
Supplementary Material
Effect of CHS on cell viability. (A)
MCF-7-Luc and HeLa-Luc cells were treated with CHS at
concentrations ranging from 1.56 to 50 μg/ml. Cell viability
was assessed 48 h after treatment. Data are represented as the mean
+ standard deviation (n=3). ***P<0.001 vs. untreated
cells. Cationic and anionic liposomes without siRNA were prepared
by the modified ethanol injection method and subsequently added to
(B) MCF-7-Luc and (C) HeLa-Luc cells. Cell viability was assessed
48 h after transfection. Data are represented as the mean +
standard deviation (n=5 for B; n=4 for C). **P<0.01
and ***P<0.001 vs. untreated cells. CHS, cholesteryl
hemisuccinate; Luc, luciferase; LP, liposome; DOTAP,
1,2-dioleoyl-3-trimethylammonium-propane methyl sulfate salt; DDAB,
dimethyldioctadecylammonium bromide; DC-1-16,
N-hexadecyl-N, N-dimethylhexadecan-1-aminium
bromide; TC-1-12,
11-((1,3-bis(dodecanoyloxy)-2-((dodecanoyloxy)methyl)propan-2-yl)amino)-
N,N,N-trimethyl-11-oxoundecan-1-aminium
bromide; CHS, cholesteryl hemisuccinate.
siRNA localization after transfection
with anionic lipoplexes. Anionic lipoplexes with Cy5-siRNA were
prepared using the modified ethanol injection method and added to
MCF-7-Luc cells at a final siRNA concentration of 50 nM. As a
control, Cy5-siRNA solution was added to the MCF-7-Luc cells. The
cells were stained with Hoechst 33342 to label the cell nuclei 24 h
after incubation. Localizations of Cy5-siRNA (green) and nuclei
(blue) were observed by fluorescence microscopy. Scale bar, 100
μm. LP, liposome; DOTAP,
1,2-dioleoyl-3-trimethylammonium-propane methyl sulfate salt; DDAB,
dimethyldioctadecylammonium bromide; DC-1-16,
N-hexadecyl-N, N-dimethylhexadecan-1-aminium
bromide; TC-1-12, 11-((1,3-bis
(dodecanoyloxy)-2-((dodecanoyloxy)methyl)propan-2-yl)amino)-
N,N,N-trimethyl-11-oxoundecan-1-aminium
bromide; CHS, cholesteryl hemisuccinate.
Size and ζ-potential of cationic and
anionic liposomes prepared using modified ethanol injection
method.
Acknowledgements
The authors thank Ms. Mina Kameishi and Mr. Keita
Kariya (Department of Molecular Pharmaceutics, Hoshi University,
Tokyo, Japan) for their assistance with the siRNA transfection
experiments.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YH conceptualized the study, developed the
methodology, conducted the investigation, curated the data,
performed the formal analysis, prepared the original draft, and
wrote, reviewed and edited the manuscript. AK and MS conducted the
study. YH and AK confirmed the authenticity of the raw data. All
the authors have read and approved the final version of this
manuscript.
Ethics approval and consent to
participate
This study was approved by the Institutional Animal
Care and Use Committee of Hoshi University (approval no.
P24-151).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kim DH, Behlke MA, Rose SD, Chang MS, Choi
S and Rossi JJ: Synthetic dsRNA Dicer substrates enhance RNAi
potency and efficacy. Nat Biotechnol. 23:222–226. 2005.PubMed/NCBI View
Article : Google Scholar
|
|
2
|
Kubowicz P, Żelaszczyk D and Pękala E:
RNAi in clinical studies. Curr Med Chem. 20:1801–1816.
2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Xue HY, Guo P, Wen WC and Wong HL:
Lipid-based nanocarriers for RNA delivery. Curr Pharm Des.
21:3140–3147. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zhang S, Zhi D and Huang L: Lipid-based
vectors for siRNA delivery. J Drug Target. 20:724–735.
2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hattori Y, Tamaki K, Sakasai S, Ozaki KI
and Onishi H: Effects of PEG anchors in pegylated siRNA lipoplexes
on in vitro gene-silencing effects and siRNA biodistribution in
mice. Mol Med Rep. 22:4183–4196. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hattori Y, Tamaki K, Ozaki KI, Kawano K
and Onishi H: Optimized combination of cationic lipids and neutral
helper lipids in cationic liposomes for siRNA delivery into the
lung by intravenous injection of siRNA lipoplexes. J Drug Deliv Sci
Technol. 52:1042–1050. 2019.
|
|
7
|
Lechanteur A, Furst T, Evrard B, Delvenne
P, Hubert P and Piel G: PEGylation of lipoplexes: The right balance
between cytotoxicity and siRNA effectiveness. Eur J Pharm Sci.
93:493–503. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Hatakeyama H, Akita H and Harashima H: The
polyethyleneglycol dilemma: Advantage and disadvantage of
PEGylation of liposomes for systemic genes and nucleic acids
delivery to tumors. Biol Pharm Bull. 36:892–899. 2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Fang Y, Xue J, Gao S, Lu A, Yang D, Jiang
H, He Y and Shi K: Cleavable PEGylation: A strategy for overcoming
the ‘PEG dilemma’ in efficient drug delivery. Drug Deliv. 24
(Supp1):S22–S32. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kapoor M and Burgess DJ: Physicochemical
characterization of anionic lipid-based ternary siRNA complexes.
Biochim Biophys Acta. 1818:1603–1612. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Han X, Lu Y, Xu Z, Chu Y, Ma X, Wu H, Zou
B and Zhou G: Anionic liposomes prepared without organic solvents
for effective siRNA delivery. IET Nanobiotechnol. 17:269–280.
2023.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hattori Y, Nakamura A, Arai S, Nishigaki
M, Ohkura H, Kawano K, Maitani Y and Yonemochi E: In vivo siRNA
delivery system for targeting to the liver by poly-l-glutamic
acid-coated lipoplex. Results Pharm Sci. 4:1–7. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ribeiro MCS, de Miranda MC, Cunha PDS,
Andrade GF, Fulgêncio GO, Gomes DA, Fialho SL, Pittella F,
Charrueau C, Escriou V and Silva-Cunha A: Neuroprotective effect of
siRNA entrapped in hyaluronic acid-coated lipoplexes by
intravitreal administration. Pharmaceutics. 13(845)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Maeki M, Okada Y, Uno S, Niwa A, Ishida A,
Tani H and Tokeshi M: Production of siRNA-loaded lipid
nanoparticles using a microfluidic device. J Vis Exp.
22(181)2022.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Maeki M, Uno S, Niwa A, Okada Y and
Tokeshi M: Microfluidic technologies and devices for lipid
nanoparticle-based RNA delivery. J Control Release. 344:80–96.
2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Hattori Y, Saito H, Nakamura K, Yamanaka
A, Tang M and Ozaki KI: In vitro and in vivo transfections using
siRNA lipoplexes prepared by mixing siRNAs with a lipid-ethanol
solution. J Drug Deliv Sci Technol. 75(103635)2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hattori Y, Tang M, Suzuki H, Hattori A,
Endo S, Ishii A, Aoki A, Ezaki M and Sakai H: Optimization of
transfection into cultured cells with siRNA lipoplexes prepared
using a modified ethanol injection method. J Drug Deliv Sci
Technol. 99(106000)2024.
|
|
18
|
Hattori Y, Tang M, Aoki A, Ezaki M, Sakai
H and Ozaki KI: Effect of the combination of cationic lipid and
phospholipid on gene-knockdown using siRNA lipoplexes in breast
tumor cells and mouse lungs. Mol Med Rep. 28(180)2023.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hattori Y, Kikuchi T, Ozaki KI and Onishi
H: Evaluation of in vitro and in vivo therapeutic antitumor
efficacy of transduction of polo-like kinase 1 and heat shock
transcription factor 1 small interfering RNA. Exp Ther Med.
14:4300–4306. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hattori Y and Shimizu R: Effective mRNA
transfection of tumor cells using cationic triacyl lipid-based mRNA
lipoplexes. Biomed Rep. 22(25)2025.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Alshalani A, AlSudais H, Binhassan S and
Juffermans NP: Sex discrepancies in blood donation: Implications
for red blood cell characteristics and transfusion efficacy.
Transfus Apher Sci. 63(104016)2024.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hattori Y, Shinkawa M, Kurihara A and
Shimizu R: Optimization of PEGylation for cationic triacyl
lipid-based siRNA lipoplexes prepared using the modified ethanol
injection method for tumor therapy. J Liposome Res. 35:300–311.
2025.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Djuric Z, Heilbrun LK, Lababidi S,
Everett-Bauer CK and Fariss MW: Growth inhibition of MCF-7 and
MCF-10A human breast cells by alpha-tocopheryl hemisuccinate,
cholesteryl hemisuccinate and their ether analogs. Cancer Lett.
111:133–139. 1997.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Rajesh M, Sen J, Srujan M, Mukherjee K,
Sreedhar B and Chaudhuri A: Dramatic influence of the orientation
of linker between hydrophilic and hydrophobic lipid moiety in
liposomal gene delivery. J Am Chem Soc. 129:11408–11420.
2007.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hattori Y, Hagiwara A, Ding W and Maitani
Y: NaCl improves siRNA delivery mediated by nanoparticles of
hydroxyethylated cationic cholesterol with amido-linker. Bioorg Med
Chem Lett. 18:5228–5232. 2008.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Koulov AV, Vares L, Jain M and Smith BD:
Cationic triple-chain amphiphiles facilitate vesicle fusion
compared to double-chain or single-chain analogues. Biochim Biophys
Acta. 1564:459–465. 2002.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chen Y, Sun J, Lu Y, Tao C, Huang J, Zhang
H, Yu Y, Zou H, Gao J and Zhong Y: Complexes containing cationic
and anionic pH-sensitive liposomes: Comparative study of factors
influencing plasmid DNA gene delivery to tumors. Int J
Nanomedicine. 8:1573–1593. 2013.PubMed/NCBI View Article : Google Scholar
|