Introduction
Osteoarthritis (OA) is an articular disorder causing
pain and increasing movement limitations due to the progressive
destruction of articular cartilage. OA is known as the most common
joint disease in developed countries, including Japan, and is
highly prevalent among the elderly, resulting in one of the leading
causes of chronic disability. Moreover, OA is recognized to
interfere with several aspects of an individual's life, including
not only physical functions but also social activities, economical
burdens and psychological well-being (1). Knee OA, one of the most common forms
of OA, is estimated to affect up to 30 million people in Japan, who
are mostly over 50 years of age (2).
Current treatments of knee OA include surgical
treatment, ranging from arthroscopy to total knee replacement, and
pharmacological treatments, with adjunctive exercise and/or
physical therapies. Since there is no curative therapy for OA, the
most pressing need for patients with OA is non-operative care that
helps to relieve symptoms and suppresses disease progression
(3). Pharmacological treatments of
OA can be divided into two groups: symptom-modifying and
disease-modifying (chondroprotective) drugs. Symptom-modifying
drugs include simple analgesics (e.g., acetaminophen) and
nonsteroidal anti-inflammatory drugs (NSAIDs). Although NSAIDs are
most widely used, the risk of peptic ulcer, renal hypertension and
hemorrhage is high in elderly patients taking NSAIDs. Moreover,
certain NSAIDs are shown to have negative effects on cartilage
metabolism and to accelerate the progression of OA in humans
(4).
Adverse effects of NSAIDs have prompted the
screening and development of novel drugs (i.e., disease-modifying
drugs) that could interfere with disease progression and protect
and regenerate cartilage in OA. Although no drug has yet been
identified in the second category, various natural compounds, such
as glucosamine and chondroitin sulfate, have been investigated as
promising candidates (5).
Hyaluronic acid (HA) is a glycosaminoglycan with a
repeating unit of D-glucuronic acid and N-acetylglucosamine. It is
a constituent of synovial fluid and cartilage matrix and
contributes to lubricating articular cartilage as well as
synthesizing proteoglycans. HA content in the synovial fluid is
reduced, and the viscoelastic properties of the fluid are
compromised in knee OA, thereby increasing susceptibility of
cartilage to injury. Thus, intra-articular administration
(viscosupplementation) of HA has been widely accepted for improving
pain and articular function in knee OA (6,7).
Regarding HA administration, several clinical trials have indicated
that the pain-relieving effect of intra-articular HA persists for
substantially longer than its half-life within the injected joint
(8,9), suggesting that HA possesses not only
symptom-modifying, but also disease-modifying activity (5,10,11).
This is further supported by the experimental findings that HA
directly stimulates articular chondrocytes to proliferate and
synthesize sulfated glycosaminoglycan and type II collagen (CII)
(12,13).
However, intra-articular HA injection causes
deleterious complications at the injection site and discomfort
associated with repeated injections (14). Moreover, an animal experiment found
that orally administered HA was detected in the circulating blood
of rats (15). These findings led
us to investigate the symptom- and/or disease-modifying effects of
oral HA in patients with knee OA. For this purpose, we evaluated a
commercially available supplementary diet containing a chicken comb
extract (CCE) and other nutrients (Kojun®). Previously,
Hatayama et al conducted a randomized, placebo-controlled
study in 29 patients with knee pain and revealed that a 2-week
ingestion of this CCE-containing diet (1,800 mg/day containing 630
mg of CCE and ∼60 mg of HA) significantly improved pain and
discomfort in the affected knee (16).
The present study was undertaken, not only to
confirm the symptom-modifying effect of the CCE-containing product
(active diet), but also to investigate its potential
disease-modifying effect in patients with knee OA. The most
established method by which to evaluate joint damage in knee OA is
the joint space width (JSW) measurement using plain X-rays.
However, since changes in JSW are small, at least one year and
preferably two years are usually necessary to accurately assess the
progression of joint damage or the effect of disease-modifying
treatment (17). Therefore, for
efficient monitoring, more sensitive and feasible methods must be
developed. Several biochemical markers (biomarkers) have been
established and have greater reliability and sensitivity than
radiographs for the early diagnosis or prognosis of OA (18). Furthermore, such biomarkers are
useful for evaluating the actions of disease-modifying drugs as
they specifically reflect changes in the metabolism of cartilage
and related tissues (19).
Although increasing numbers of OA-related biomarkers are available,
we utilized three cartilage biomarkers specific for CII metabolism,
including two CII degradation biomarkers, C-terminal cross-linked
telopeptide of CII (CTX-II) and CII C2C neopeptide (C2C), and one
CII synthesis biomarker, C-terminal propeptide of CII procollagen
(CPII), since abnormalities in CII metabolism play a key role in
the pathogenesis of OA (20).
Furthermore, it is suggested that OA develops from an imbalance in
the synthesis and degradation of cartilage CII, and that the ratio
of a CII degradation biomarker to a synthesis biomarker predicts
more precisely the progression of OA than individual biomarkers
(21,22). Thus, we also assessed the
CTX-II/CPII and C2C/CPII ratios as parameters for CII
metabolism.
Materials and methods
Study design
A prospective randomized double-blind
placebo-controlled, parallel-group comparative study was designed
to assess the actions of the active diet and placebo on both
symptoms and CII metabolism in adult subjects with knee OA. The
safety of the active diet was also evaluated. The study was
performed from June 2008 to December 2008 and involved two clinical
service organization centers in Japan. The study protocol was
approved by the local ethics committee and was conducted in
accordance with the principles of the amended Declaration of
Helsinki and ‘Ethical Guidelines for Epidemiological Research’.
Written informed consent was obtained from all participants prior
to their enrollment in the study. The entire study period consisted
of a 16-week intervention phase and a preceding 8-week screening
phase. Subjects were screened at a baseline visit by a physical
examination, a knee radiograph according to a standardized method,
a symptom questionnaire and routine laboratory tests. After the
start of intervention, medical examinations and laboratory tests
were performed at weeks 4, 8, 12 and 16 for the enrolled
subjects.
Subjects
Male and female Japanese subjects, 40–85 years of
age, diagnosed with knee OA with Kellgren/Lawrence (K/L) grades 0–3
(mainly 1–2) were enrolled. The entire subject populations
consisted of almost equal numbers of subjects undergoing concurrent
exercise therapy (ET, including resistance exercise, sit-to-stand
exercise, aerobic/walking exercise and water/pool walking exercise)
and without ET. The former subjects were referred to as
ET-receivers and the latter as ET-unreceivers, and both classes of
subjects were almost equally distributed between the active diet
and placebo groups. Subjects with bilateral diagnosed knee OA were
asked to specify their worse knee at baseline, and this knee was
evaluated throughout the study period. Exclusion criteria were: any
inflammatory condition of bone or cartilage; previous surgical
treatment of knee joint(s) or its necessity; complication(s)
necessary for hospitalization and surgical treatment; known allergy
to chicken or some constituent of the active diet; participation in
any clinical trial; pregnant women; nursing mothers or women with
child-bearing potential; treatment with intra-articular HA within 2
weeks or corticosteroids within 3 months in the target joint; use
of health foods, including constituents with knee pain-relieving
potential (e.g., glucosamine, chondroitin sulfate and HA) within 2
weeks; and the presence of any clinically significant condition
judged by the medical investigator to preclude the subject's
inclusion in the study.
Intervention and subject assignment
The active diet is manufactured in the form of a
300-mg pill. Its ingredients are: 105 mg of CCE, 10 mg of propolis
extract, 4.9 mg of xanthooligo-sugar, 5 mg of vitamin B1, 5 mg of
vitamin B6, 0.1 mg of vitamin B12, 2.5 mg of vitamin E, 2 mg of
ferric pyrophosphate, 20 mg of calcium lactate and 140 mg of
vehicle (comprising crystalline cellulose, dextrin and fatty acid
sugar esters).
Each population of 22 ET-receivers and 21
ET-unreceivers (8 males and 35 females in total) was randomly
assigned to receive 6 pills (1,800 mg) of the active diet or dummy
placebo containing only vehicle (crystalline cellulose, dextrin and
fatty acid sugar esters). The daily dose of the active diet used in
the present study was determined on the basis of the results
obtained in our preceding study (16). Adherence to the intervention was
evaluated based on the doses recorded in the study diary, and a
value <80% was considered a protocol violation.
Evaluation of efficacy and safety
Symptomatic changes were assessed according to
scores of the Japanese Orthopaedic Association clinical trials
response criteria (JOA response criteria) for articular disorders
of the knee and visual analog scales (VAS). Questionnaires were
collected at baseline and at weeks 4, 8, 12 and 16 after the
initiation of intervention. Assessments were performed at each time
point.
In this study, five subscales of JOA response
criteria closely related to knee OA were selected for the
assessment: i) pain/walking function; ii) pain/step-up and -down
function; iii) joint flexion/stiffness; iv) swelling; and v)
aggregated total symptoms. The former 4 subscales were rated from 0
to 30, from 0 to 25, from 0 to 35 and from 0 to 10, respectively.
The maximum value indicated no symptoms or functional disability,
and a score of 0 indicated a condition resulting in extreme
difficulty to perform daily living tasks. The sum of the scores for
these four subscales represented the scores for the fifth subscale
(aggregate total symptoms).
Three subscales of 100-mm VAS were used to measure
pain in the target knee: i) pain at rest; ii) pain on moving; and
iii) pain on pressing. Each VAS subscale was scored from 0 to 100,
where 0 indicated no pain and 100 indicated intense pain. Since the
present study aimed to evaluate the intervention diet for its pain
relief and/or physical improvement capabilities, only knee joints
with a VAS score ≥40 were used as the target.
Serum and urine samples were collected at baseline
and at weeks 8, 12 and 16 during the intervention, and were stored
at −70°C until use. When the study was completed, the samples were
analyzed for CII degradation biomarkers (CTX-II and C2C) and a CII
synthesis biomarker (CPII) to assess changes in cartilage
metabolism. Urinary CTX-II (uCTX-II), serum C2C (sC2C) and serum
CPII (sCPII) were measured using Urine CartiLaPs EIA
(Immunodiagnostic Systems Ltd.), Collagen Type II ELISA (IBEX
Pharmaceuticals Inc.) and Procollagen II C Propeptide ELISA (IBEX
Pharmaceuticals Inc.). The level of uCTX-II was corrected for
urinary creatinine (Cr) measured by a standard colorimetric assay
and expressed as ng/mmol Cr. The changes from baseline of uCTX-II,
sC2C and sCPII, as well as uCTX-II/sCPII and sC2C/sCPII ratios,
were compared between the active diet and placebo groups.
Tolerability and safety were assessed throughout the
study on the basis of the incidence and severity of diet-related
adverse events (side effects) as well as abnormal changes in blood
pressure, pulse rate and laboratory tests, including hematology,
biochemical profile and urinalysis. In addition, to examine whether
a 16-week ingestion of the active diet influenced the
pharmakokinetics of HA, the serum HA (sHA) levels at baseline and
week 16 were compared in both the active diet and placebo
groups.
Statistical analysis
Values are expressed as the mean ± SD. Baseline
characteristics of the entire subject populations, ET-receivers and
ET-unreceivers were compared between the two groups by the
Student's t-test for continuous variables and by the Mann-Whitney U
test for category variables. Symptomatic scores and biomarker
levels during the intervention were compared to baseline values by
the Student's t-test (for quantitative variables) and the
Wilcoxon's signed rank test (for qualitative variables).
Comparisons between the groups were made by the Student's t-test
(for quantitative variables). P-values <0.05 were considered
significant.
Results
Baseline characteristics
Table I presents
the baseline characteristics of all subjects in the active diet and
placebo groups together with those of ET-receivers and
ET-unreceivers. Of the 43 enrolled subjects (8 men and 35 women),
21 subjects comprising 11 ET-receivers and 10 ET-unreceivers were
assigned to the active diet, and 22 subjects comprising 11 each of
the ET-receivers and ET-unreceivers were assigned to the placebo
group. The baseline characteristics included demographic
characteristics (age and male/female ratio), physiological
characteristics (height, body weight, body mass index,
systolic/diastolic blood pressures and pulse rate), JOA response
criteria score for ‘aggregate total symptoms’, three pain subscale
scores of VAS, subject distribution on the K/L grade, levels of CII
metabolism biomarkers (uCTX-II, sC2C and sCPII) and serum HA (sHA).
No significant differences were observed between the active diet
and placebo groups in most of these variables, except sCPII and
sHA.
 | Table I.Baseline characteristics of the study
populations in the active diet and placebo groupsa. |
Table I.
Baseline characteristics of the study
populations in the active diet and placebo groupsa.
| Variables | All subjects
| ET-receiversb
|
ET-unreceiversc
|
|---|
| Active diet
(n=21) | Placebo (n=22) | Active diet
(n=11) | Placebo (n=11) | Active diet
(n=10) | Placebo (n=11) |
|---|
| Age (years) | 62.4±12.5 | 63.3±9.5 | 66.9±14.3 | 65.4±11.1 | 57.5±8.2 | 61.2±7.5 |
| Male:Female (no. of
subjects) | 4:17 | 4:18 | 3:8 | 4:7 | 1:9 | 0:11 |
| Height (cm) | 155.0±8.5 | 157.1±6.2 | 155.0±8.6 | 159.5±4.7 | 154.9±8.8 | 154.7±6.8 |
| Body weight (kg) | 56.2±8.6 | 57.1±8.5 | 56.3±10.5 | 56.3±5.6 | 56.1±6.6 | 57.9±10.9 |
| Body mass index
(kg/m2) | 23.3±3.0 | 23.3±3.5 | 23.5±3.9 | 22.5±1.8 | 23.0±1.8 | 24.0±4.6 |
| Systolic blood
pressure (mmHg) | 127.8±16.6 | 132.0±17.0 | 132.9±17.1 | 133.1±18.3 | 122.2±14.7 | 131.0±16.5 |
| Diastolic blood
pressure (mmHg) | 76.0±7.8 | 76.6±11.2 | 74.3±8.3 | 77.1±11.5 | 77.9±7.1 | 76.1±11.4 |
| Pulse rate
(beats/min) | 69.3±8.2 | 73.0±12.7 | 66.5±9.4 | 74.1±15.5 | 72.3±5.6 | 72.0±10.0 |
| JOA response
criteria score for aggregated total symptoms | 87.6±7.0 | 88.6±6.9 | 87.3±6.5 | 85.9±7.0 | 88.0±7.9 | 91.4±6.0 |
| 100-mm VAS
scores | | | | | | |
| Pain at rest
(mm) | 41.7±21.0 | 39.1±20.0 | 42.1±15.1 | 37.1±17.5 | 41.2±26.9 | 41.0±23.0 |
| Pain on moving
(mm) | 65.9±13.5 | 56.4±18.8 | 64.9±12.9 | 61.1±14.8 | 67.1±14.8 | 51.6±21.7 |
| Pain on pressing
(mm) | 56.2±21.5 | 51.5±19.1 | 55.7±23.9 | 47.5±15.9 | 56.7±19.8 | 55.4±22.0 |
| Kellgren-Lawrence
grade | | | | | | |
| 0:I:II:III (no.
of subjects) | 4:11:5:1 | 7:7:8:0 | 2:6:2:1 | 2:4:5:0 | 2:5:3:0 | 5:3:3:0 |
| Urinary CTX-II
(ng/mmol Cr) | 384.7±186.7 | 353.7±133.0 | 364.2±201.7 | 359.4±119.0 | 409.8±175.0 | 348.1±151.3 |
| Serum C2C
(ng/ml) | 224.8±37.9 | 222.1±21.3 | 216.1±40.0 | 220.3±24.2 | 234.4±34.9 | 223.8±19.0 |
| Serum CPII
(ng/ml) | 1,146±404 | 1,638±624f | 1,272±510 | 1,587±811 | 1,007±179 | 1,690±393f |
| Serum HAd (ng/ml) | 52.5±42.0f | 24.2±16.9 | 50.8±28.1e | 23.6±17.9 | 54.4±55.1 | 24.7±16.8 |
The sCPII levels were significantly higher in the
subjects of the placebo group (1,638±624 ng/ml) than in those of
the active diet group (1,146±404 ng/ml, P<0.01). Similarly,
sCPII levels were significantly higher in ET-unreceivers of the
placebo group and in those of the active diet group (1,690±393 vs.
1,007±179 ng/ml, P<0.01). Although the two groups (active diet
and placebo) were not balanced for sCPII, this biomarker was
regarded as an evaluable parameter in this study, since no
relationship was observed between the sCPII level and the severity
of symptoms or radiographies of knee OA. Furthermore, there was no
significant difference between the two groups in the levels of two
other CII metabolism biomarkers, uCTX-II and sC2C.
The concentrations of sHA ranged widely among the
entire subjects at baseline. Of the 43 subjects, 10 had values
below the detection limit (10 ng/ml), whereas 2 allotted to the
active diet had high values (111 and 155 ng/ml). Thus, the sHA
levels for all subjects as well as for ET-receivers in the active
diet group were 2-fold higher than those in the placebo group
(P<0.01 and <0.05, respectively). However, there was no
significant change in the concentrations of sHA between baseline
and week 16 in the active diet group (data not shown), suggesting
that the ingestion of the active diet containing HA had no effect
on the sHA level.
Among the 21 subjects in the active diet group, 1
female ET-receiver had her right little toe bone fractured soon
after the initiation of intervention and was released from the
study on the 3rd day of the intervention. Another female
ET-receiver in the active diet group was also removed from the
study, since her sHA level was markedly unstable (the value changed
from 111 ng/ml at baseline to 444 ng/ml at week 16), suggesting
that she may have had some abnormalities in HA metabolism. In the
placebo group, 1 female ET-receiver experienced gastric discomfort
at 2 and 3 days after the initiation of intervention. Although this
symptom disappeared on day 4, she voluntarily resigned from the
study on the next day. Thus, 19 subjects (9 ET-receivers and 10
ET-unreceivers) in the active diet group and 21 subjects (10
ET-receivers and 11 ET-unreceivers) in the placebo group completed
the study. Adherence to active diet and placebo in this study
(assessed by pill counts) exceeded 80%.
Assessment based on the JOA response
criteria
Table II shows the
changes in the five subscale scores of the JOA response criteria
during the 16-week intervention. In the active diet group, three
subscale scores, i.e., ‘pain/walking function’, ‘pain/step-up and
-down function’ and ‘aggregate total symptoms’, were increased
compared to the baseline (indicating clinical improvement) after 8
weeks of intervention (P<0.05). In the placebo group, these
subscale scores were similarly increased; however, the degrees were
much smaller than those in the active diet group. By contrast,
scores for ‘joint flexion/stiffness’ and ‘swelling’ subscales did
not essentially change in either of the groups throughout the
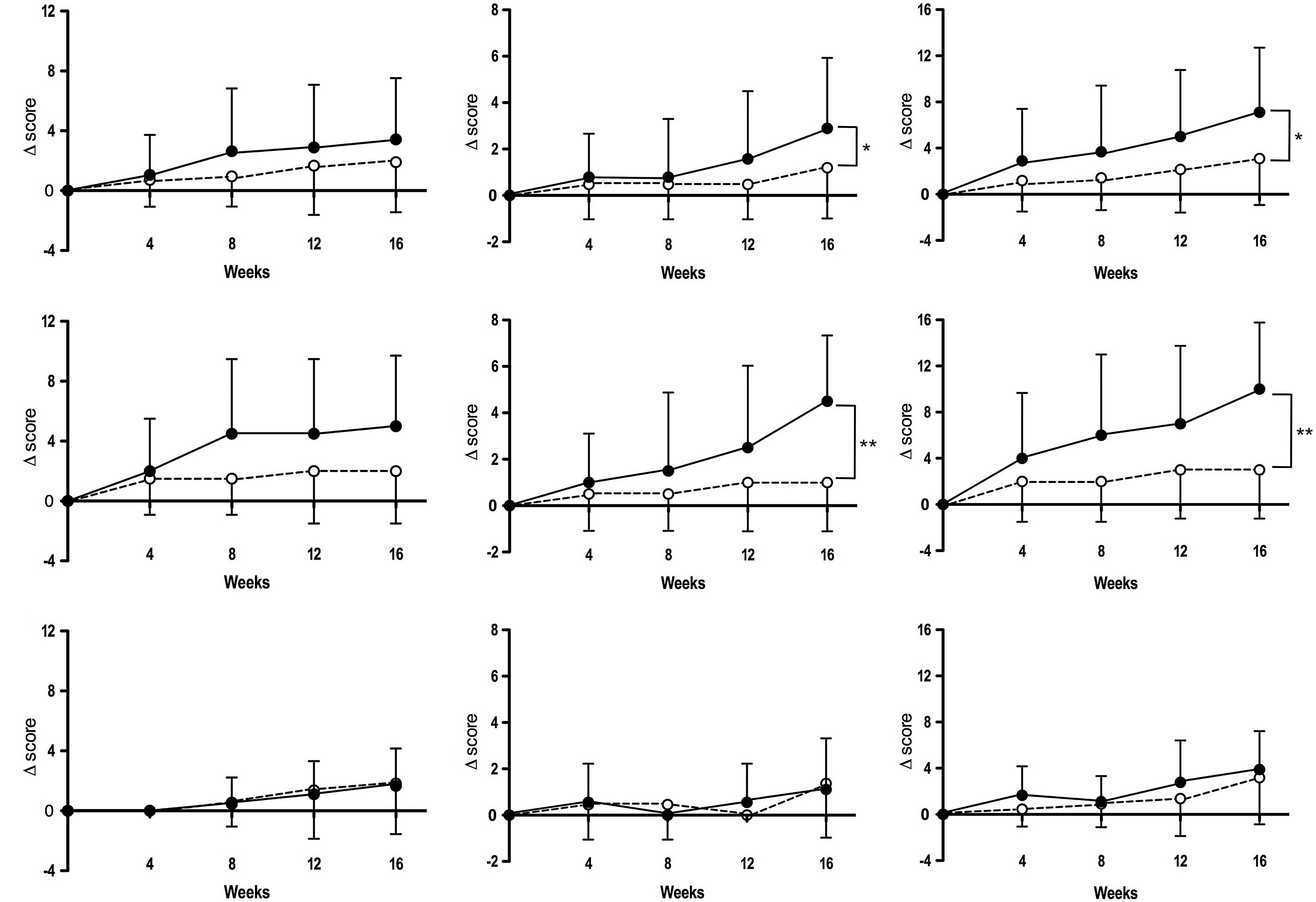
intervention period. Fig. 1 shows
the changes in subscale scores from baseline during the
intervention. Notably, ‘pain/step-up and -down function’ and
‘aggregate total symptoms’ subscale scores in the active diet group
were significantly increased compared to those in the placebo group
at week 16 (P<0.05).
 | Table II.Changes in the five subscale scores
of JOA response criteria during intervention for all subjects in
the active diet (n=19) and placebo (n=21) groups. |
Table II.
Changes in the five subscale scores
of JOA response criteria during intervention for all subjects in
the active diet (n=19) and placebo (n=21) groups.
| Subscales | Group | Scores (mean ± SD)
|
|---|
| Baseline | 4 weeks | 8 weeks | 12 weeks | 16 weeks |
|---|
| I. Pain/walking
function | Active diet | 26.3±4.4 | 27.4±3.5 | 28.9±2.7a | 29.2±2.5b | 29.7±1.1b |
| Placebo | 26.0±4.4 | 26.7±4.3 | 26.9±3.7a | 27.6±3.4a | 27.9±3.4a |
| II. Pain/step-up
and -down function | Active diet | 19.5±1.6 | 20.3±1.1 | 20.3±2.0 | 21.1±2.1a | 22.4±2.6b |
| Placebo | 19.5±1.5 | 20.0±2.2 | 20.0±2.2 | 20.0±2.2 |
20.7±2.9* |
| III. Joint
flexion/stiffness | Active diet | 32.6±3.1 | 32.6±3.1 | 32.6±3.1 | 32.9±3.0 | 33.2±3.0 |
| Placebo | 33.3±2.9 | 33.3±2.9 | 33.3±2.9 | 33.3±2.9 | 33.3±2.9 |
| IV. Swelling | Active diet | 9.7±1.1 | 10.0±0.0 | 10.0±0.0 | 10.0±0.0 | 10.0±0.0 |
| Placebo | 10.0±0.0 | 10.0±0.0 | 10.0±0.0 | 10.0±0.0 | 10.0±0.0 |
| V. Aggregate total
symptoms | Active diet | 88.2±6.7 | 91.1±4.9a | 91.8±6.1a | 93.2±5.1b | 95.3±4.2b |
| Placebo | 88.8±7.1 | 90.0±7.4 | 90.2±7.0a | 91.0±7.0a | 91.9±7.7b |
In order to determine whether concurrent ET
influences the symptomatic response to the active diet,
ET-receivers and ET-unreceivers in both groups (active diet and
placebo) were separately analyzed. As shown in Table III, among the ET-receivers of the
active diet group, ‘pain/walking function’ and ‘aggregate total
symptoms’ scores significantly increased at weeks 8, 12 and 16
(P<0.05), and the ‘pain/step-up and -down function’ score
significantly increased at week 16 (P<0.05) compared to the
baseline. By contrast, in the ET-unreceivers of the active diet
group, these subscale scores did not essentially increase during
the intervention, although the ‘aggregate total symptoms’ scores
significantly increased at week 16 (P<0.05). In the placebo
group, only the ‘aggregate total symptoms’ subscale score slightly
increased at week 16 in ET-unreceivers (P<0.05). Consistent with
these observations, the changes in subscale scores from baseline
were significantly or almost significantly increased in the
ET-receivers of the active diet group compared to those of the
placebo group for subscale scores of ‘pain/step-up and -down
function’ (P<0.01), ‘aggregate total symptoms’ (P<0.01) and
‘pain/walking function’ (P=0.09) at week 16 (Fig. 1). However, there was no significant
difference in the changes of these three subscale scores from
baseline in ET-unreceivers of the two groups (active diet and
placebo) throughout the intervention period (Fig. 1).
 | Table III.Changes in the three subscale scores
of JOA response criteria during intervention for ET-receivers and
ET-unreceivers in the active diet and the placebo groups. |
Table III.
Changes in the three subscale scores
of JOA response criteria during intervention for ET-receivers and
ET-unreceivers in the active diet and the placebo groups.
| Subscales | Group,
subjects | Scores (mean ± SD)
|
|---|
| Baseline | 4 weeks | 8 weeks | 12 weeks | 16 weeks |
|---|
| I. Pain/walking
function | Active diet,
ET-receivers (n=10) | 24.5±5.0 | 26.5±4.1 | 29.0±3.2a | 29.0±3.2a | 29.5±1.6a |
| Placebo,
ET-receivers (n=10) | 24.5±3.7 | 26.0±3.9 | 26.0±3.9 | 26.5±4.1 | 26.5±4.1 |
| Active diet,
ET-unreceivers (n=9) | 28.3±2.5 | 28.3±2.5 | 28.9±2.2 | 29.4±1.7 | 30.0±0.0 |
| Placebo,
ET-unreceivers (n=11) | 27.3±4.7 | 27.3±4.7 | 27.7±3.4 | 28.6±2.3 | 29.1±2.0 |
| II. Pain/step-up
and-down function | Active diet,
ET-receivers (n=10) | 19.5±1.6 | 20.5±1.6 | 21.0±2.1 | 22.0±2.6 | 24.0±2.1b |
| Placebo,
ET-receivers (n=10) | 19.0±2.1 | 19.5±2.8 | 19.5±2.8 | 20.0±3.3 | 20.0±3.3 |
| Active diet,
ET-unreceivers (n=9) | 19.4±1.7 | 20.0±0.0 | 19.4±1.7 | 20.0±0.0 | 20.6±1.7 |
| Placebo,
ET-unreceivers (n=11) | 20.0±0.0 | 20.5±1.5 | 20.5±1.5 | 20.0±0.0 | 21.4±2.3 |
| V. Aggregate total
symptoms | Active diet,
ET-receivers (n=10) | 87.0±6.7 | 91.0±4.6 | 93.0±5.4a | 94.0±5.7a | 97.0±3.5b |
| Placebo,
ET-receivers (n=10) | 86.0±7.4 | 88.0±8.6 | 88.0±8.6 | 89.0±9.4 | 89.0±9.4 |
| Active diet,
ET-unreceivers (n=9) | 89.4±6.8 | 91.1±5.5 | 90.6±6.8 | 92.2±4.4 | 93.3±4.3a |
| Placebo,
ET-unreceivers (n=11) | 91.4±6.0 | 91.8±6.0 | 92.3±4.7 | 92.7±3.4 | 94.5±4.7a |
Assessment based on the pain scores of
VAS
The pain-relieving effect was assessed using the
three subscales of VAS. The number of subjects enrolled for the
assessment were 9 (5 ET-receivers and 4 ET-unreceivers) and 12 (5
ET-receivers and 7 ET-unreceivers) for ‘pain at rest’, 19 (10
ET-receivers and 9 ET-unreceivers) each for ‘pain on moving’ and 13
(7 ET-receivers and 6 ET-unreceivers) and 15 (7 ET-receivers and 8
ET-unreceivers) for ‘pain on pressing’ in the active diet and the
placebo groups, respectively.
As shown in Table
IV, the three subscale scores of VAS were reduced from baseline
in both the active diet and the placebo groups during the
intervention (4–16 weeks) (P<0.05, indicative of pain relief).
Similarly, the three subscale scores of VAS were reduced in both
the ET-receivers and ET-unreceivers of the two groups (P<0.05).
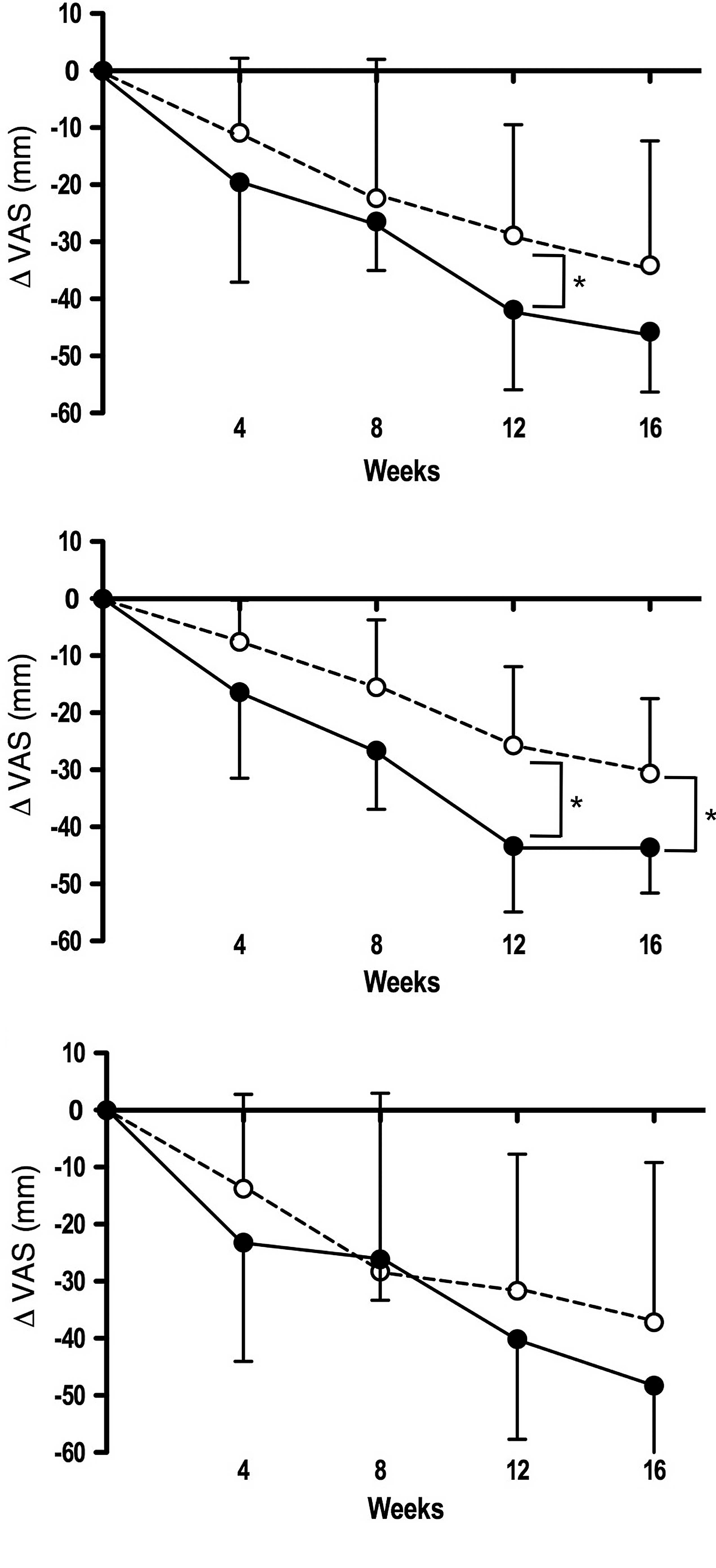
Notably, the ‘pain on pressing’ subscale scores were significantly
decreased from baseline in ET-receivers as well as in all subjects
of the active diet group compared to those of the placebo group at
weeks 12 and 16 (Fig. 2,
P<0.05).
 | Table IV.Changes in the three subscale scores
of VAS during intervention for all subjects, ET-receivers and
ET-unreceivers in the active diet and the placebo groups. |
Table IV.
Changes in the three subscale scores
of VAS during intervention for all subjects, ET-receivers and
ET-unreceivers in the active diet and the placebo groups.
| Subscales | Group,
subjects | VAS (mm) (mean ±
SD)
|
|---|
| Baseline | 4 weeks | 8 weeks | 12 weeks | 16 weeks |
|---|
| I. Pain at
rest | Active diet, all
subjects (n=9) | 55.4±8.6 | 35.6±14.6b | 23.6±11.1b | 21.1±11.2b | 12.6±6.3b |
| Placebo, all
subjects (n=12) | 54.7±8.5 | 37.9±12.0b | 29.7±18.4b | 25.2±18.4b | 22.2±21.5b |
| Active diet,
ET-receivers (n=5) | 50.2±5.1 | 38.1±18.6 | 20.7±13.1b | 22.2±13.4a | 14.4±7.7b |
| Placebo,
ET-receivers (n=5) | 52.9±7.8 | 35.0±13.5a | 30.3±9.7b | 27.6±11.0a | 20.9±11.9b |
| Active diet,
ET-unreceivers (n=4) | 61.9±7.8 | 32.4±9.2b | 27.2±8.2b | 19.7±9.6b | 10.4±4.0b |
| Placebo,
ET-unreceivers (n=7) | 56.0±9.4 | 40.0±11.4a | 29.2±23.6a | 23.5±23.0b | 23.2±27.3a |
| II. Pain on
moving | Active diet, all
subjects (n=19) | 64.3±13.1 | 42.9±14.2b | 35.6±13.8b | 31.2±15.5b | 24.2±13.2b |
| Placebo, all
subjects (n=19) | 61.8±12.4 | 45.2±15.9b | 39.7±18.0b | 36.7±15.7b | 25.3±16.2b |
| Active diet,
ET-receivers (n=10) | 62.7±11.2 | 43.6±12.0b | 31.6±13.7b | 28.8±15.9b | 25.6±13.1b |
| Placebo,
ET-receivers (n=10) | 63.8±12.6 | 43.2±19.2b | 42.9±16.0b | 37.3±11.6b | 24.9±9.4b |
| Active diet,
ET-unreceivers (n=9) | 66.1±15.3 | 42.3±17.0b | 40.1±13.2b | 34.0±15.4b | 22.6±13.9b |
| Placebo,
ET-unreceivers (n=9) | 59.7±12.7 | 47.4±12.1a | 36.1±20.5a | 36.1±20.1a | 25.8±22.1b |
| III. Pain on
pressing | Active diet, all
subjects (n=13) | 66.0±14.5 | 46.4±20.5b | 39.6±14.8b | 24.1±17.7b | 20.3±14.4b |
| Placebo, all
subjects (n=15) | 61.0±13.2 | 50.1±13.7b | 38.6±16.5b | 32.1±15.1b | 26.9±18.4b |
| Active diet,
ET-receivers (n=7) | 65.2±18.8 | 48.8±19.0a | 38.5±18.3b | 21.8±18.8b | 21.6±14.4b |
| Placebo,
ET-receivers (n=7) | 55.5±10.4 | 47.9±13.3a | 40.0±8.2a | 29.8±12.5b | 24.9±12.1b |
| Active diet,
ET-unreceivers (n=6) | 67.0±8.6 | 43.8±23.7a | 40.8±10.9b | 26.8±17.8b | 18.7±15.6b |
| Placebo,
ET-unreceivers (n=8) | 65.7±14.2 | 52.0±14.7 | 37.4±22.0a | 34.1±17.7b | 28.6±23.3b |
Assessment based on CII metabolism
To evaluate the effect on CII metabolism, urine and
serum samples were collected at baseline and three time points
(weeks 8, 12 and 16) from all subjects, except urine samples from 1
ET-unreceiver in the active diet group.
Table V shows the
changes in the levels of uCTX-II, sC2C and sCPII in the active diet
and the placebo groups during the 16-week intervention. In the
entire subject population, the uCTX-II level slightly decreased
during the intervention in both groups (indicating the suppression
of CII degradation), although the change from baseline was
significant only in the placebo group at week 16 (P<0.05).
 | Table V.Changes in the levels of CII
metabolism biomarkers urinary CTX-II, serum C2C and serum CPII
during intervention for all subjects, ET-receivers and
ET-unreceivers in the active diet and the placebo groups. |
Table V.
Changes in the levels of CII
metabolism biomarkers urinary CTX-II, serum C2C and serum CPII
during intervention for all subjects, ET-receivers and
ET-unreceivers in the active diet and the placebo groups.
| Biomarkers | Group,
subjects | Concentration (mean
± SD)
|
|---|
| Baseline | 8 weeks | 12 weeks | 16 weeks |
|---|
| Urinary CTX-II
(ng/mmol Cr) | Active diet, all
subjects (n=18)a | 397±193 | 360±163 | 361±172 | 363±172 |
| Placebo, all
subjects (n=21) | 350±135 | 338±153 | 317±118 | 292±105b |
| Active diet,
ET-receivers (n=10) | 372±211 | 349±186 | 354±207 | 355±203 |
| Placebo,
ET-receivers (n=10) | 352±123 | 349±172 | 330±136 | 296±99 |
| Active diet,
ET-unreceivers (n=8)a | 428±178 | 374±140 | 371±131 | 373±135 |
| Placebo,
ET-unreceivers (n=11) | 348±151 | 328±141 | 304±104 | 289±116 |
| Serum C2C
(ng/ml) | Active diet, all
subjects (n=19) | 224±39 | 237±25b | 271±39c | 249±47b |
| Placebo, all
subjects (n=21) | 222±22 | 236±33 | 232±31 | 231±26 |
| Active diet,
ET-receivers (n=10) | 217±42 | 236±23 | 254±27c | 234±20 |
| Placebo,
ET-receivers (n=10) | 220±26 | 246±39b | 239±29b | 236±18 |
| Active diet,
ET-unreceivers (n=9) | 231±36 | 237±27 | 290±44c | 264±63 |
| Placebo,
ET-unreceivers (n=11) | 224±19 | 226±24 | 227±34 | 226±32 |
| Serum CPII
(ng/ml) | Active diet, all
subjects (n=19) | 1,168±419 | 1,277±314 | 1,559±367c | 1,382±368 |
| Placebo, all
subjects (n=21) | 1,614±629 | 1,699±423 | 1,788±650 | 1,663±612 |
| Active diet,
ET-receivers (n=10) | 1,312±519 | 1,274±362 | 1,585±301b | 1,388±271 |
| Placebo,
ET-receivers (n=10) | 1,532±833 | 1,623±492 | 1,772±740 | 1,564±525 |
| Active diet,
ET-unreceivers (n=9) | 1,007±189 | 1,281±274b | 1,531±446c | 1,376±470b |
| Placebo,
ET-unreceivers (n=11) | 1,690±392 | 1,768±359 | 1,802±594 | 1,752±695 |
By contrast, the sC2C level was significantly
increased in the active diet group compared to the placebo group at
weeks 8, 12 and 16 (P<0.05). In the subgroup analyses, sC2C
levels were significantly increased in both ET-receivers and
ET-unreceivers of the active diet group at week 12 (P<0.01 each)
and in ET-receivers of the placebo group at weeks 8 and 12
(P<0.05 each). Furthermore, the changes in sC2C levels from
baseline were significantly increased in all subjects and
ET-unreceivers of the active diet group compared to those of the
placebo group (P<0.01 each) (data not shown).
Of note, the sCPII levels were significantly
increased from baseline in all subjects (week 12, P<0.01),
ET-receivers (week 12, P<0.05) and ET-unreceivers (weeks 8 and
12, P<0.05) of the active diet group (indicating the enhancement
of CII synthesis), although CPII levels remained almost constant
over the 16-week intervention period in the placebo group.
To further determine whether ingestion of the active
diet affects the synthesis of CII relative to its degradation, we
evaluated the ratio of the CII degradation marker (uCTX-II or sC2C)
to the CII synthesis marker (sCPII). As shown in Table VI, compared to baseline, the
uCTX-II/sCPII ratio was reduced by 20–35% at weeks 8, 12 and 16
(P<0.05) in all subjects of the active diet group (indicating
the relative enhancement of CII synthesis). The ratio was also
reduced in ET-receivers at week 12 (−27%; P<0.05) and
ET-unreceivers at week 12 (−41%; P<0.05) and at week 16 (−32%;
P<0.05) in the active diet group. Furthermore, the sC2C/sCPII
ratio was significantly reduced in all subjects at week 12 (−12%;
P<0.05) and ET-unreceivers at week 8 (−18%; P<0.05) and week
12 (−15%; P<0.05) in the active diet group. By contrast, none of
the subjects in the placebo group showed a significant change in
either parameter during the intervention.
 | Table VI.Changes in the ratios of CII
degradation marker to synthesis marker, uCTX-II/sCPII and
sC2C/sCPII, during intervention for all subjects, ET-receivers and
ET-unreceivers in the active diet and the placebo groups. |
Table VI.
Changes in the ratios of CII
degradation marker to synthesis marker, uCTX-II/sCPII and
sC2C/sCPII, during intervention for all subjects, ET-receivers and
ET-unreceivers in the active diet and the placebo groups.
| Ratios | Group,
subjects | Concentration ratio
(mean ± SD)a
|
|---|
| Baseline | 8 weeks | 12 weeks | 16 weeks |
|---|
| uCTX-II/sCPII | Active diet, all
subjects (n=17) | 0.355±0.178 | 0.284±0.124b | 0.232±0.100c | 0.270±0.123b |
| Placebo, all
subjects (n=21) | 0.246±0.126 | 0.214±0.120 | 0.206±0.126 | 0.204±0.115 |
| Active diet,
ET-receivers (n=10) | 0.295±0.149 | 0.270±0.104 | 0.215±0.102b | 0.255±0.132 |
| Placebo,
ET-receivers (n=10) | 0.281±0.149 | 0.243±0.157 | 0.229±0.164 | 0.217±0.122 |
| Active diet,
ET-unreceivers (n=8) | 0.429±0.193 | 0.301±0.150 | 0.253±0.099b | 0.290±0.115b |
| Placebo,
ET-unreceivers (n=11) | 0.215±0.098 | 0.187±0.070 | 0.185±0.082 | 0.193±0.113 |
| sC2/sCPII | Active diet, all
subjects (n=19) | 0.204±0.047 | 0.192±0.034 | 0.180±0.036b | 0.187±0.040 |
| Placebo, all
subjects (n=21) | 0.151±0.039 | 0.147±0.041 | 0.142±0.041 | 0.154±0.050 |
| Active diet,
ET-receivers (n=10) | 0.179±0.046 | 0.194±0.036 | 0.164±0.026 | 0.173±0.031 |
| Placebo,
ET-receivers (n=10) | 0.165±0.048 | 0.162±0.046 | 0.150±0.045 | 0.163±0.044 |
| Active diet,
ET-unreceivers (n=9) | 0.232±0.030 | 0.190±0.035b | 0.198±0.037b | 0.203±0.044 |
| Placebo,
ET-unreceivers (n=11) | 0.137±0.026 | 0.133±0.033 | 0.136±0.038 | 0.146±0.055 |
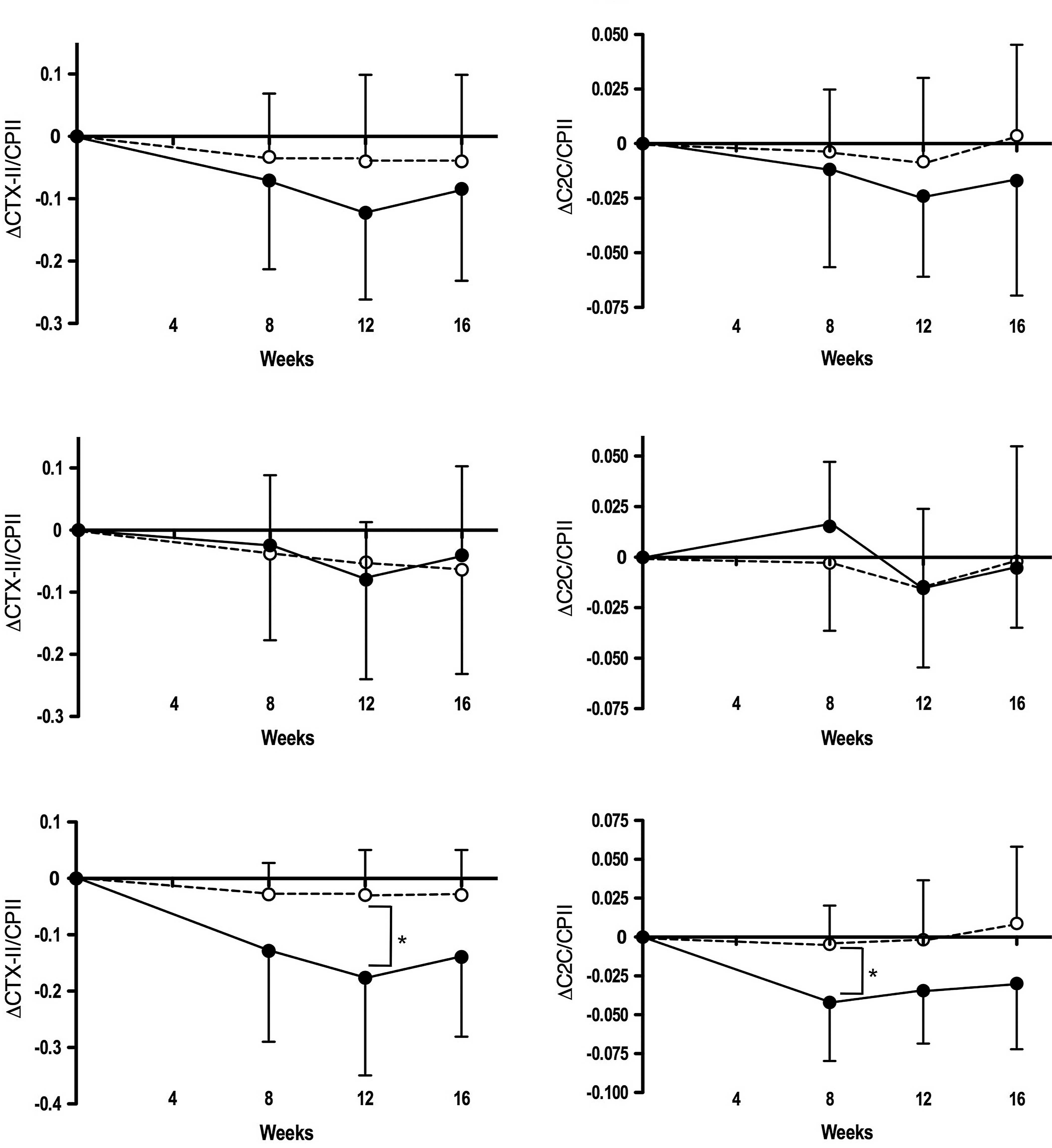
Fig. 3 illustrates
the decrease from baseline in the uCTX-II/CPII and C2C/CPII ratios
in the active diet and the placebo groups during the intervention
period. Both parameters were substantially decreased in all
subjects, particularly in ET-unreceivers of the active diet group
compared to those in the placebo group; the changes were
significantly larger in ET-unreceivers of the active diet group
than in those of the placebo group for the uCTX-II/CPII ratio at
week 12 (P<0.05) and the C2C/CPII ratio at week 8
(P<0.05).
Safety and tolerability
Nine subjects (43%) of the active diet group (n=21)
and 12 subjects (55%) of the placebo group (n=22) were reported to
have experienced one or more adverse events during the intervention
period. The total number of adverse events reported were 45 in the
active diet group and 32 in the placebo group, and there was no
significant difference in the frequency between the two groups.
Major adverse events and their frequencies in the active diet and
the placebo groups were: cold symptoms (12 vs. 3); pain (6 vs. 6);
myalgia (6 vs. 5); gastric discomfort (6 vs. 3); diarrhea (3 vs.
4); and cramp (2 vs. 2). None of these adverse events were severe
and all were considered to be unrelated to the study diet.
Furthermore, none of the physical measurement
parameters (body weight and body mass index), physiological
examinations (systolic/diastolic blood pressures and pulse rate)
and laboratory tests (hematology and blood chemistry) were
significantly changed from baseline in either of the groups.
Discussion
In this study, we performed a randomized
double-blind placebo-controlled clinical trial utilizing a
supplementary diet containing HA-rich CCE to subjects with mild
knee OA (mainly 1–2 of K/L grades). First, we assessed the
potential of the oral administration of HA in the management of
knee OA, since little information is available on its effect,
although the intra-articular administration of HA has almost been
established in knee OA. Second, in addition to the
symptom-modifying (improving) effect of the oral administration of
HA, we examined its potential for a structure-modifying
(chondroprotective) effect. Third, we investigated the contribution
of ET to the symptomatic and/or chondroprotective effects of the
oral administration of HA. For this purpose, subgroup analyses were
performed between ET-receivers and ET-unreceivers in the two
different groups (active diet and placebo).
The present study revealed that the CCE-containing
diet, used as an active diet, has the potential to relieve pain and
disability in subjects with knee OA based on an assessment using
the JOA response criteria. In the entire subject population, the
subscale scores of ‘pain/walking function’, ‘pain/step-up and -down
function’ and ‘aggregate total symptoms’ were increased (improved)
to a greater extent from baseline in the active diet group compared
to the placebo group during the intervention (Table II). Moreover, the ‘pain/step-up and
-down function’ and ‘aggregate total symptoms’ subscale scores were
significantly increased from baseline in the active diet group at
week 16 compared to the placebo group (Fig. 1). By contrast, neither the active
diet nor the placebo affected the remaining two subscale scores,
‘joint flexion/stiffness’ and ‘swelling’ (Table II). The former likely suggests that
the active diet confers no effect on ‘joint flexion/stiffness’
suffered by the subjects enrolled, and the latter can be explained
by the fact that none of the subjects exhibited swelling in the
affected knee.
VAS assessment indicated that pain scores for the
three subscales were significantly reduced (improved) from baseline
during the intervention in both the active diet and the placebo
groups (Table IV). Such a placebo
effect has been reported in clinical studies on OA conducted for
relatively short periods (∼8 weeks) (23). However, the ‘pain on pressing’
subscale was significantly decreased from baseline in the active
diet group compared to the placebo group (Fig. 2). These results were essentially
concordant with those obtained by Hatayama et al (16), who previously indicated that the
2-week intake of the same CCE-containing diet (1,800 mg/day) was
effective in relieving knee pain in subjects with OA.
Consistent with our observation, oral administration
of HA [a product of purified HA (Hyabest®(J), 240
mg/day) (23) and a natural
extract of chicken combs with a high HA content
(Hyal-Joint®, ∼50 mg/day) (24)] has been reported to relieve pain
and other discomforts in knee OA, 4–8 weeks after the intervention.
Together, these observations indicate that oral supplementation of
an HA-containing diet has potential as a symptom-modifying agent in
knee OA.
It was important to examine whether the concurrent
ET affects the symptom-improving effect of the active diet in
patients with knee OA. In this study, the subgroup analyses between
tET-receivers and ET-unreceivers indicated that three of five
subscale scores of the JOA response criteria, as well as one of the
three subscale scores of VAS, were significantly improved in the
ET-receivers of the active diet group at one or more time points
during the intervention. In accordance with this, several
randomized placebo-controlled trials have suggested that ET reduces
pain and improves physical function in patients with OA (25). In fact, Roos and Dahlberg found
that moderate exercise improves not only joint symptoms and
function, but also the knee cartilage glucosaminoglycan content in
patients with knee OA (26). Thus,
oral supplementation of HA is expected to be more effective in
relieving disability and pain in knee OA when combined with ET.
Accumulating evidence indicates that biomarkers for
cartilage metabolism, particularly CII metabolism, can be used in
OA not only for screening patients with a risk of progressive joint
destruction, but also for monitoring structure-modifying agents or
therapies (19). Using CII
degradation biomarkers such as CTX-II, C1, 2C and C2C, the actions
of chondroprotective agents, i.e., glucosamine (27,28)
and chondroitin sulfate (29),
have been evaluated. More recently, CII synthesis biomarkers, such
as CPII (PIICP), have been used alone or in combination with CII
degradation biomarkers (e.g., CTX-II and C2C) for monitoring the
disease state and progression of knee OA. In the present study, to
evaluate the effect of the active diet on cartilage CII metabolism,
we utilized two CII degradation biomarkers (CTX-II and C2C) and one
CII synthesis biomarker (CPII).
At first we assumed that, if the active diet
exhibited a chondroprotective action, the levels of both uCTX-II
and sC2C would be reduced, whereas the level of sCPII would be
increased. In accordance with our assumption, the active diet
caused a significant increase in the level of sCPII (Table V). However, the uCTX-II levels were
not essentially decreased in the active diet group. Furthermore,
the sC2C levels were significantly increased in the active diet
group. Although the mechanism for the discrepancy between changes
in the levels of uCTX-II and sC2C during the intervention remains
to be elucidated, it could be explained by the fact that C2C and
CTX-II originate from different domains of CII (the telopeptide and
triple helix regions, respectively), and they are believed to
reflect the distinct breakdown events of CII by different enzymatic
pathways (30). We also evaluated
the action of the active diet on CII metabolism using the ratio of
the CII degradation biomarker to the CII synthesis biomarker. Both
the uCTX-II/CPII and C2C/CPII ratios were decreased during the
intervention in all subjects, particularly in the ET-unreceivers of
the active diet group (Table VI
and Fig. 3). Thus, the combination
of the two biomarkers (CII degradation and synthesis biomarkers)
seems to be more effective than measuring a single biomarker in
monitoring the action of chondroprotective agents on CII metabolism
in OA. In this context, Cahue et al (22) demonstrated that the ratio of CII
breakdown to synthesis can be used to predict the progression of
knee OA. Together, these observations likely indicate that
intervention with the active diet containing HA may have a
chondroprotective action on knee OA by relatively enhancing CII
synthesis, particularly in ET-unreceivers. However, this
possibility should be cautiously confirmed in future, since in the
present study the baseline levels of sCPII were significantly lower
in the active diet group than in the placebo group.
In general, oral administration of HA may have an
advantage over intra-articular HA injection, as it can circumvent
potential complications at the injection site and the discomfort
associated with repeated injections (14). The present study provided evidence
for the safety and symptom-relieving effect of the active diet as a
promising candidate for oral HA supplementation. Moreover, this
study indicated that the active diet is likely to improve the
balance of CII degradation/synthesis, thereby conferring potential
chondroprotection against OA. Based on these results, it is
suggested that the CCE-containing diet could be a non-surgical
management for knee OA via the actions of not only relieving pain
and functional disability, but also by protecting articular
cartilage. However, to further elucidate the beneficial effect of
the CCE-containing diet on knee OA, the actions of propolis
extracts and other constituents contained in the active diet should
be determined.
Acknowledgements
We wish to thank Kaori Yoshimura and
Reiko Kojima (Total Technological Consultant Co., Ltd., Tokyo,
Japan) for the excellent help in the statistical analysis of the
data and preparation of the manuscript.
References
|
1.
|
Gupta S, Hawker GA, Laporte A, Croxford R
and Coyte PC: The economic burden of disabling hip and knee
osteoarthritis (OA) from the perspective of individuals living with
this condition. Rheumatology. 44:1531–1537. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Yoshimura N, Oka H, Muraki S, et al:
Epidemiology of osteoarthritis in Japan. J Jpn Orthop Assoc (In
Japanese). 81:17–21. 2007.
|
|
3.
|
Budewalter JA, Stanish WD, Rosier RN,
Schenck RC Jr, Dennis DA and Coutts RD: The increasing need for
nonoperative treatment of patients with osteoarthritis. Clin
Orthop. 385:36–45. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Rashad S, Revell P, Hemmingway A, Low F,
Rainsford K and Walker F: Effect of non-steroidal anti-inflammatory
drugs on the course of osteoarthritis. Lancet. 2:519–522. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Clayton JJ: Nutraceuticals in the
management of osteoarthritis. Orthopedics. 30:624–629.
2007.PubMed/NCBI
|
|
6.
|
Verbruggen G: Chondroprotective drugs in
degenerative joint diseases. Rheumatology. 45:129–138. 2005.
View Article : Google Scholar
|
|
7.
|
Goldberg VM and Buckwalter JA: Hyaluronans
in the treatment of osteoarthritis of the knee: evidence for
disease-modifying activity. Osteoarthritis Cartilage. 13:216–224.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Petrella RT: Hyaluronic acid for the
treatment of knee osteoarthritis: long-term outcomes from a
naturalistic primary care experience. Am J Phys Med Rehabil.
84:278–283. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Sun SF, Hsu CW, Hwang CW, Hsu PT, Wang JL,
Tsai SL, Chou YJ, Hsu YW, Huang CM and Wang YL: Hyaluronate
improves pain, physical function and balance in the geriatric,
osteoarthritic knee: a 6-month follow-up study using clinical
tests. Osteoarthritis Cartilage. 14:696–701. 2006. View Article : Google Scholar
|
|
10.
|
Hunter DJ and Hellio Le
Graverand-Gastineau M-P: How close are we to having
structure-modifying drugs available? Rheum Dis Clin N Am.
34:789–802. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Gossec L and Dougados M: Intra-articular
treatments in osteoarthritis: from the symptomatic to the structure
modifying. Ann Rheum Dis. 63:478–482. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Patti AM, Gabriele A, Vulcano A, Ramieri
MT and Della Rocca C: Effect of hyaluronic acid on human
chondrocytes cell lines from articular cartilage. Tissue Cell.
33:294–300. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Akmal M, Siingh A, Anand A, Kesani A,
Aslam N, Goodship A and Bentley G: The effects of hyaluronic acid
on articular chondrocytes. J Bone Joint Surg Br. 87:1143–1149.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Adams ME, Lussier AJ and Peyron JG: A
risk-benefit assessment of injections of hyaluronan and its
derivatives in the treatment of knee osteoarthritis. Drug Saf.
23:115–130. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Huang SL, Ling PX and Zhang TM: Oral
absorption of hyaluronic acid and phospholipids complexes in rats.
World J Gastroenterol. 13:945–949. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Hatayama T, Nagano M, Yamaguchi N, Kumagai
S and Ohnuki K: The effect of a supplement on knee pain and
discomfort evaluated by visual analog scale (VAS): a randomized,
double-blind, placebo-controlled study (in Japanese). Kenkoshien.
10:13–17. 2008.
|
|
17.
|
Ravaud P, Giraudean B, Auleley GR, Drape
JL, Rousselin B, Paolozzi L, Chastang C and Dougados M: Variability
in knee radiographing: implication for definition of radiological
progression in medical knee osteoarthritis. Ann Rheum Dis.
57:624–629. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Vignon E, Garnero P, Delmas P, Avouac B,
Bettica P, Boers M, Ehrich E, MacKillop N, Rovati L, Serni U,
Spector T and Reginster JY: Recommendations for the registiation of
drugs used in the treatment of osteoarthritis: an update on
biochemical markers. Osteoarthritis Cartilage. 9:289–293. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Garnero P and Delmas PD: Biomarkers in
osteoarthritis. Nature Clin Pract Rheumatol. 3:346–356. 2007.
View Article : Google Scholar
|
|
20.
|
Poole AR, Ionescu M, Fitzcharles MA and
Billinghurst RC: The assessment of cartilage degradation in vivo:
development of an immunoassay for the measurement of in body fluids
of type II collagen cleared by collagenases. J Immunol Methods.
294:145–153. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Garnero P, Ayral X, Rousseau J-C,
Christgau S, Sandell LJ, Dougados M and Delmas P: Uncoupling of
type II collagen synthesis and degradation predicts progression of
joint damage in patients with knee osteoarthritis. Arthritis Rheum.
46:2613–2624. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Cahue S, Sharma L, Dunlop D, Ionescu M,
Song J, Lobanok T, King L and Poole AR: The ratio of type II
collagen breakdown to synthesis and its relationship with the
progression of knee osteoarthritis. Osteoarthritis Cartilage.
15:819–823. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Sato T and Iwaso H: An effectiveness study
of hyaluronic acid [Hyabest® (J)] in the treatment of
osteoarthritis of the knee on patients in the United States. J New
Rem Clin. 58:249–256. 2009.
|
|
24.
|
Kalman DS, Heimer M, Valdeon A, Schwartz H
and Sheldon E: Effect of a natural extract of chicken combs with a
high content of hyaluronic acid (Hyal-Joint®) on pain
relief and quality of life in subjects with knee osteoarthritis: a
pilot randomized double-blind placebo-controlled trial. Nutrition
J. 7:32008. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Fransen M, McConnell S and Bell M:
Exercise for osteoarthritis of the hip or knee. Cochrane Database
Syst Rev (CD 004286). 2003.
|
|
26.
|
Roos EM and Dahlberg L: Positive effects
of moderate exercise on glycosaminoglycan content in knee
cartilage: a four-month, randomized, controlled trial in patients
at risk of osteoarthritis. Arthritis Rheum. 52:3507–3514. 2005.
View Article : Google Scholar
|
|
27.
|
Christgau S, Henrotin Y, Tankó LB, Rovati
LC, Collette J, Bruyere O, Deroisy R and Reginster JY:
Osteoarthritic patients with high cartilage turnover show increased
responsiveness to the cartilage protecting effects of glucosamine
sulfate. Clin Exp Rheumatol. 22:36–42. 2004.
|
|
28.
|
Cibere J, Thorne A, Kopec JA, Singer J,
Canvin J, Robinson DB, Pope J, Hong P, Grant E, Lobanok T, Ionescu
M, Poole AR and Esdaile JM: Glucosamine sulfate and cartilage type
II collagen degradation in patients with knee osteoarthritis. J
Rheumatol. 32:896–902. 2005.PubMed/NCBI
|
|
29.
|
Mazières B, Hucher M, Zaïm M and Garnero
P: Effect of chondroitin sulfate in symptomatic knee
osteoarthritis: a multicentre, randomised, double-blind,
placebo-controlled study. Ann Rheum Dis. 66:639–645.
2007.PubMed/NCBI
|
|
30.
|
Bay-Jensen AC, Andersen TL, Charni-Ben
Tabassi N, Kristensen PW, Kjaersgaard-Andersen P, Sandell L,
Garnero P and Delaissé JM: Biochemical markers of type II collagen
breakdown and synthesis are positioned at specific sites in human
osteoarthritic knee cartilage. Osteoarthritis Cartilage.
16:615–623. 2008. View Article : Google Scholar : PubMed/NCBI
|