Introduction
The β-glucans are of particular interest to
researchers because they are naturally occurring glucose polymers,
are orally active when taken as food supplements, and have a long
track record of safe use. Due to their immunomodulatory properties,
purified β-glucans have been used clinically as part of a
combination therapy for a variety of cancers (1).
Carboxymethyl-glucan (CM-G) is a water-soluble
derivative of the Saccharomyces cerevisiae cell wall
β(1–3)(1–6) glucan. It is classified as a biological response
modifier and is well known for its immunomodulatory and
hematopoiesis-enhancing activities (2–4).
Several experimental studies in vivo have shown that
β-glucans administered orally, intravenously or enterally enhance
hematopoietic regeneration without side effects (5–8).
Prostate cancer is the sixth most common cancer in
the world and represents approximately 10% of all cancers
diagnosed. In Brazil, prostate cancer is the most common type of
cancer in males (excluding non-melanoma skin cancer), and is
prevalent in all regions of the country. More than any other type
of cancer, prostate cancer is considered a disease of the elderly,
since nearly three-fourths of the cases worldwide occur at the age
of 65 or above (www.inca.gov.br) (9).
While a man with localized prostate cancer is more
likely to die from comorbidities, the probability of death from
prostate cancer exceeds other causes when clinical metastases
occur. Metastatic prostate cancer remains incurable, and it is
known that many parameters of immunity decline with age (10). At this stage, the medical goals are
to increase the short survival period of the patients and, above
all, to improve their quality of life, which often declines as the
disease progesses.
Considering the beneficial properties ascribed to
CM-G and its potential use as an adjuvant to cancer treatment, we
assessed in vivo the effects of oral CM-G administration on
the peripheral blood cells of patients with advanced prostate
cancer (PCa) and on alterations in hepatic and renal functions.
Patients and methods
This study was conducted with the approval of the
Committee on Ethical Research Involving Human Beings of the
University of Londrina and the Northern State of Paraná University
Hospital. CONEP 268 and 212/07 for this project are available under
registration no. 0214.0.268.000-07 at the National Information on
Ethics in Research Involving Human Beings, Brazil (SISNEP).
Patients at the Londrina Cancer Institute with a histological
diagnosis of stage T3 (n=15) and T4 (n=15) prostate adenocarcinoma
according to TNM staging (www.cancerstaging.org) and undergoing androgen
deprivation therapy (ADT) with goserelin acetate or leuprolide were
included in this study after giving written informed consent. The
age of the patients ranged from 52 to 84 years, with a median age
of 69 years. The ADT treatment duration ranged from 5 to 36 months.
Among the 30 patients studied, 9 underwent radiation before ADT, 8
were smokers and 8 consumed alcohol regularly. During the 28-day
treatment, patients were contacted weekly in order to to monitor
their conditions and to receive reports of any adverse effects
associated with the CM-G treatment.
Soluble β-glucan in the carboxymethylated (CM-G)
form was obtained from the cell wall of Saccharomyces
cerevisiae, according to the protocol described by Magnani
et al (11). The CM-G was
distributed into gelatin capsules containing 20 mg of CM-G with
starch as an excipient vehicle. This procedure was carried out
under medical request by Vico Farma®, which is
registered at the National Health Surveillance Agency (ANVISA) in
Brazil under no. 136420-0.
Peripheral venous blood samples were drawn for
initial values from the patients early in the morning of Day 1
before the patients ingested the first capsule of CM-G. Early every
morning, before eating, the patients ingested a 20-mg CM-G capsule
(12). After 28 days, the blood
samples were analyzed. Both samples were collected while patients
were fasting. Vaccutainer™ tubes (4.5 ml) containing
EDTA were used, and all the samples were processed immediately
after collection by the Abbott Cell Dyn 3200 flow cytometer.
The results obtained before and after CM-G treatment
for each patient were analyzed using the Wilcoxon signed-rank test
and the t-test for dependent paired samples. p≤0.05 indicated
significant differences.
Results
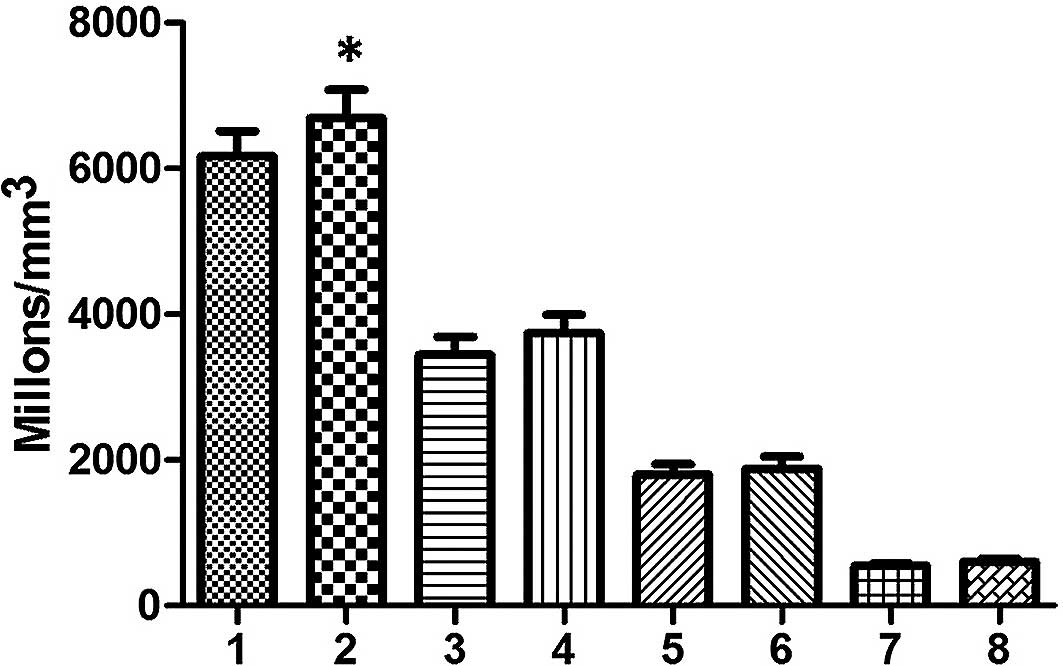
After CM-G administration, the total leukocyte
counts increased significantly (p≤0.02), averaging a 10.64%
increase. In the differential count, an increasing trend for
typical lymphocytes, monocytes and neutrophils was observed
(Fig. 1). The lifestyle habits of
patients, such as smoking and alcohol use, were not associated with
increased leukocytes.
The leukocyte counts carried out before CM-G
administration showed that no patients had leukopenia. However,
leukocyte levels closer to the minimum reference value
(3,500/mm3) were observed among patients who had
received radiation. For these patients, CM-G administration
resulted in an 11% increase in the values.
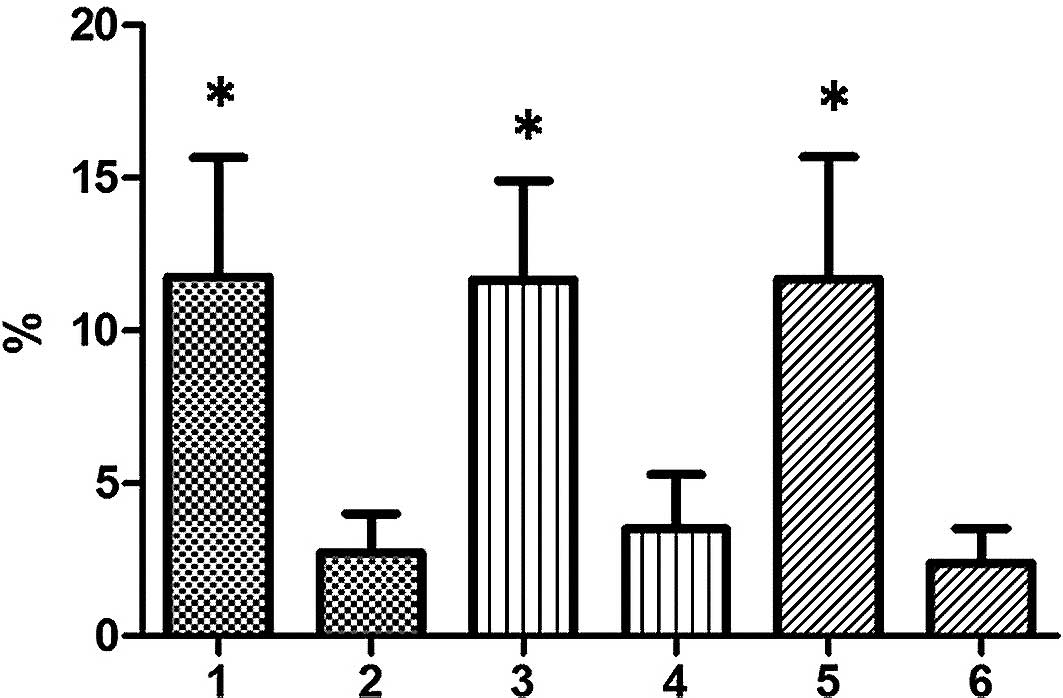
Before CM-G administration, 9 of the 30 patients
studied had red blood cell counts below the minimum reference value
(4.3/mm3), 14 patients had hemoglobin levels below
normal values (12.8 g/dl) and 10 patients had hematocrit values
below the minimum reference value (38.8%). After 28 days of CM-G
ingestion, significant increases (p≤0.001) in red blood cell,
hematocrit and hemoglobin levels were observed; this increase was
more pronounced in patients who were initially below the minimum
(Fig. 2).
There were no significant changes in VCM, HCM, CHCM
and RDW; however, the platelet counts increased significantly after
treatment with CM-G (p=0.007).
No changes in kidney and liver function were found
in associaion with CM-G administration (Table I), and no CM-G-related side effects
were observed.
 | Table I.Results of liver and kidney function
tests performed before and after carboxymethyl-glucan (CM-G)
administration for 28 days in patients with advanced prostate
cancer. |
Table I.
Results of liver and kidney function
tests performed before and after carboxymethyl-glucan (CM-G)
administration for 28 days in patients with advanced prostate
cancer.
| Before CM-G | After CM-G | Reference value
(method) |
|---|
| Liver function | | | |
| Transaminase
aspartate amino transferase (TGO) | 20.1±2.0 | 20.9±2.0 | 11–41 U/l (automated
kinetics) |
| Transaminase
alanine amino transferase (TGP) | 9.2±0.9 | 9.4±0.8 | 7–52 U/l (automated
kinetics) |
| Albumin | 4.10±0.75 | 4.20±0.50 | 3.35–5.62 g/dl
(capillary electrophoresis) |
| Direct
bilirubin | 0.08±0.01 | 0.07±0.02 | Up to 0.3 mg/dl
(colorimetric) |
| Indirect
bilirubin | 0.32±0.26 | 0.31±0.13 | Up to 0.7 mg/dl
(colorimetric) |
| Alkaline
phosphatase | 40.2±12.0 | 39.9±9.0 | 27–100 U/l (automated
kinetics) |
| Kidney function | | | |
| Urea | 39±10 | 38±10 | 10–52 mg/dl
(automated enzymatic) |
| Creatinine | 0.87±0.21 | 0.89±0.23 | 1.30 mg/dl (automated
kinetics) |
Discussion
CM-G stimulates retioculoendothelial system function
and modulates cellular and humoral immunity (2,13,14).
In its soluble form, β-glucan acts synergistically with in
vivo myeloid growth factors, improving hematopoietic recovery
and mobilizing progenitor cells in the peripheral blood (14). According to Rice et al
(15), orally administered soluble
derivatives of β-glucan are absorbed through the gastrointestinal
wall and pass into the circulation system, activating immune
pathways such as Dectin-1, CR-3, SIGNR1, TLR-2/6 and -4 (16).
Cancer-induced immunosuppression is a well-known
phenomenon (12), but was not
observed among the patients in this study. However, the standard
treatment for advanced PCa is hormone therapy (17) and not chemotherapy, which is
usually associated with leukopenia. By contrast, low white blood
cell counts were observed prior to CM-G treatment in patients who
had received radiation therapy. CM-G has proven effective in
stimulating hematopoiesis after repeated doses of radiation, and in
increasing granulocytes and other hematopoietic indices (18). Several experimental models have
demonstrated the ability of β-glucans and derivatives administered
by different routes to raise blood cell counts after leukopenia
caused by cancer treatments (2,6,19).
The increase in leukocyte counts reported in this
study supports the findings of Weitberg et al (20) regarding increases in white blood
cells after oral β-glucan administration, and those of De Felippe
et al (5), who found an
increase in leukocytes at the expense of neutrophils, lymphocytes
and monocytes on the fifth day of intravenous treatment. By
contrast, Demir et al (12)
observed no changes in leukocyte counts after 14 days of oral
β-glucan administration. Although only the increase in leukocytes
was significant in the present study, typical lymphocytes,
monocytes and neutrophils clearly showed an increasing trend with
CM-G treatment.
In the present study, no side effects associated
with the ingestion of CM-G were observed. These results are in
agreement with the findings of Demir et al (12), who observed no adverse effects of
the daily oral administration of 20 mg of soluble β-glucan from
S. cerevisiae for 14 days in 20 patients with metastatic
breast cancer. Weitberg (20)
administered 15 mg of oral β-glucan daily to 20 patients with
various types of advanced cancer for 6 months and also found no
side effects.
The absence of a significant variation in the
results of hepatic and renal function tests before and after CM-G
treatment (Table I) are in
agreement with the findings of De Felippe et al (5), who treated 11 septic patients with
100–240 mg of β-glucan from S. cerevisiae for 8 days.
Smoking is associated with increased numbers of
leukocytes (21). This association
was not observed among the smokers in this study, who showed no
difference before or after CM-G administration compared to
non-smokers.
After 28 days of treatment with CM-G, a significant
increase in red blood cell, hemoglobin and hematocrit levels was
confirmed. Previous studies have reported that β-glucan acts
directly on the myeloid progenitors, which contribute to
hematopoietic regeneration (2,22).
Increased levels of hemoglobin in cancer patients treated with oral
β-glucan have been reported by Wietberg (20). Moreover, Demir et al
(12) found no significant
differences in the hematocrit count after treatment with
β-glucan.
Platelet numbers also increased significantly in the
present study, which corresponded with the results of Weitberg
(20), but not with those
described by Demir et al (12). Differences between our results and
those reported by Demir et al (11) may be related to the duration of
β-glucan administration (28 days in the present study vs. 14 days
in that of Demir et al).
Ever closer to clinical practice, the use of
β-glucan as an adjuvant has been used effectively for decades in
Japan, improving the quality of life of cancer patients and
reducing side effects (23). The
results of this study suggest that the use of CM-G may enhance the
hematologic parameters of patients with prostate cancer, benefiting
the body weakened by the disease and its treatments.
Acknowledgements
The authors thank the National Council
for Scientific and Technological Development (CNPq-Brazil) for
financial support in the form of a doctorate scholarship to
M.M.
Reference
|
1.
|
Thompson IJ, Oyston PC and Williamson DE:
Potential of the beta-glucans to enhance innate resistance to
biological agents. Expert Rev Anti Infect Ther. 8:339–352. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Hofer M and Pospisil M: Glucan as
stimulator of hematopoiesis in normal and gamma-irradiated mice. A
survey of the authors' results. Int J lmmunopharmacol. 19:607–609.
1997.PubMed/NCBI
|
|
3.
|
Chen R and Seviour J: Medicinal importance
of fungal β-(1–3) (1–6)-glucans. Mycol Res. 3:635–652. 2007.
|
|
4.
|
Kubala L, Ruzickova J, Nickova K, et al:
The effect of (1→3)-β-D-glucans, carboxymethylglucan and
schizophyllan on human leukocytes in vitro. Carbohydrate Res.
338:2835–2840. 2003.
|
|
5.
|
De Felippe J Jr, Freire CAR and Mendes NF:
Celularidade do sangue periférico após o emprego da glucana, um
imunoestimulante de s.r.e., em pacientes sépticos e em voluntários
sem infecção. Artigos Farmácia Virtual: www.pureessence.com.br/conteudo/con_artigo_glucana.html.
Accessed May 16, 2010.
|
|
6.
|
Vetvicka V, Dvorak B, Vetvickova J, et al:
Orally administered marine (1→3)-beta-D-glucan phycarine stimulates
both humoral and cellular immunity. Int J Biol Macromol.
40:291–298. 2007.
|
|
7.
|
Baran J, Allendorf DJ, Hong F and Gordon
RD: Oral β-glucan adjuvant therapy converts nonprotective Th2
response to protective Th1 cell mediated immune response in mammary
tumor-bearing mice. Folia Histochem Cytobiol. 45:107–114. 2007.
|
|
8.
|
Liu J, Gunn L, Hanses R and Yan J:
Combined yeast-derived β-glucan with anti-tumor monoclonal antibody
for cancer immunotherapy. Exp Mol Pathol. 86:208–214. 2009.
|
|
9.
|
Jemal A, Siegel R and Ward E: Cancer
Statistics. Cancer J Clin. 58:71–96. 2008.
|
|
10.
|
Pawelec G, Lustgarten J, Ruby C and
Gravekamp C: Impact of aging on cancer immunity and immunotherapy.
Cancer Immunol Immunother. 58:1907–1908. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Magnani M, Calliari CM, de Macedo FC Jr,
et al: Optimized methodology for extraction of
(1–3)(1–6)-β-D-glucan from Saccharomyces cerevisiae and in
vitro evaluation of the cytotoxicity and genotoxicity of the
corresponding carboxymethyl derivative. Carbohydrate Polym.
78:658–665. 2009.
|
|
12.
|
Demir G, Klein HO, Mandel-Molinas N and
Tuzuner N: β-glucan induces proliferation and activation of
monocytes in peripheral blood of patients with advanced breast
cancer. Int Immunopharm. 7:113–116. 2007.
|
|
13.
|
Darpossolo FPB, Quintana LR, Magnani M, et
al: Evaluation of potential immunostimulant of the
Carboxymethyl-glucan from Saccharomyces cerevisiae in
poultry (Gallus domesticus). Semina. 31:231–240. 2010.
|
|
14.
|
Patchen ML, Vaudrain T, Correira H, et al:
In vitro and in vivo hematopoietic activities of Betafectin
PGG-glucan. Exp Hematol. 26:1247–1254. 1998.PubMed/NCBI
|
|
15.
|
Rice PJ, Adams EL, Ozment-Skelton T, et
al: Oral delivery and gastrointestinal absorption of soluble
glucans stimulate increased resistance to infectious challenge. J
Pharmacol Exp Ther. 314:1079–1086. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Brown GD and Gordon S: Immune recognition
of fungal. Cell Microbiol. 7:471–479. 2005.PubMed/NCBI
|
|
17.
|
Fleming MT, Sonpavde G, Kondagunta GV, et
al: Systemic therapy and novel agents for metastatic castration
resistant prostate cancer. Update Cancer Ther. 3:133–145. 2009.
View Article : Google Scholar
|
|
18.
|
Pospisil M, Sandula J, Pipalova I, et al:
Hemopoiesis stimulating and radioprotective effects of
carboxymethylglucan. Physiol Res. 40:377–380. 1991.PubMed/NCBI
|
|
19.
|
Harada T, Kawaminami H, Miura NN, et al:
Mechanism of enhanced hematopoietic response by soluble β-glucan
SCG in cyclophosphamide-treated mice. Microbiol Immunol.
50:687–700. 2006.
|
|
20.
|
Weitberg A: A phase I/II trial of
beta-(1,3)/(1,6) D-glucan in the treatment of patients with
advanced malignancies receiving chemotherapy. J Exp Clin Cancer
Res. 27:402008. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Lebailly P, Vigreux C, Lechevrel C, et al:
DNA damage in mononuclear leucocytes of farmers measured using the
alkaline comet assay: modifications of DNA damage levels after a
one day field spraying period with selected pesticides. Cancer
Epidemiol Biomarkers Prev. 7:929–940. 1998.
|
|
22.
|
Turnbull JL, Patchen ML and Scadden DT:
The polysaccharide, PGG-glucan, enhances human myelopoiesis by
direct action independent of and additive to early-acting
cytokines. Acta Haematol. 102:66–71. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Hamuro J: Anticancer immunotherapy with
perorally effective lentinan. Gan To Kagako Royo. 32:1209–1215.
2005.PubMed/NCBI
|