Introduction
Growth-hormone-secreting pituitary adenomas (GHomas)
account for approximately 20% of all pituitary neoplasms (1). Acromegaly results from
supraphysiological GH release from pituitary somatotroph adenomas
in 99% of cases, resulting in persistently high circulating levels
of GH, which may affect the cardiovascular system. Cardiovascular
effects, such as hypertension, cardiomyopathy and valvular heart
disease, are associated with increased morbidity and premature
mortality, and significant increases in both respiratory disorders
and malignancies have been reported (2). Retrospective cohort studies have
suggested that mortality in patients with acromegaly is at least
twice that present in the general population (3).
However, the pathogenetic mechanisms that underlie
pituitary adenomas are complex and remain enigmatic. Several
studies examining X-chromosome inactivation have shown that primary
pituitary adenomas are monoclonal (4). Heterozygous activating somatic point
mutations in the α-subunit of the stimulatory G protein have been
identified in up to 40% of human GHomas (5). The presence of these mutations
appears to correlate with a densely granulated ultrastructural
morphology of somatotroph tumors and possibly with greater GH
responsiveness to inhibition by the somatostatin analog octreotide
(6). However, GHomas very rarely
exhibit activating ras mutations in invasive or metastatic lesions
(7). Uniquely, pituitary mitotic
activity is relatively low, even in invasive adenomas. Several
growth factors, including dysregulated receptors for fibroblast
growth factors, dopamine, estrogen and nerve growth factor
(8), have been implicated,
predominantly in prolactinoma pathogenesis, but not uniformly in
acromegaly. The pathogenesis of GHoma remains to be elucidated.
It is increasingly clear that improved diagnostic
methods and a better understanding of disease mechanisms may be
derived from a careful analysis of microarray measurements
(9). Some reports have used
microarrays to study differential gene expression in GHomas
(10,11). However, with the rapid development
of human genetics and genetic research, more and more genes will be
identified and their functions will be elucidated. Therefore,
further research will likely identify new genes involved in the
pathogenesis of GHomas. To explore the possible pathogenesis of
GHomas, we used a human genome-wide bead-based fiber-optic array
(Illumina Human WG-6 v3.0), which has the characteristics of high
density, high repeatability and high sensitivity, to identify the
differentially expressed genes between GHomas and healthy pituitary
tissues. We then analyzed the differential gene expression profiles
using bioinformatic and pathway analyses to search for new
candidate genes that may be implicated in the pathogenesis of
GHomas.
Materials and methods
Tissue specimens
Five sporadic GHomas were obtained from patients at
Tiantan Hospital (Beijing, China), following transsphenoidal
surgery. The clinical and pathological characteristics of these
adenomas are listed in Table I.
Portions of the surgical specimens were immediately frozen in
liquid nitrogen and stored at −80°C. Three healthy pituitary glands
were used as controls, obtained from three adult males within 12 h
of their being involved in fatal accidents. Informed consent was
obtained from the patients and the study was approved by the local
ethics committee.
 | Table I.Clinical and pathological
characteristics of the patients. |
Table I.
Clinical and pathological
characteristics of the patients.
| Patient | Gender | Age | Clinical
features | Tumor size
(cm) |
Immunohistochemistry |
|---|
| 246 | F | 36 | Acromegaly visual
defect | 2.5×1.8×3.0 | GH2+ |
| 178 | M | 50 | Acromegaly | 2.8×1.7×3.2 | GH2+ |
| 193 | M | 20 | Acromegaly visual
loss | 2.4×2.6×3.7 | GH3+ |
| 187 | M | 56 | Acromegaly visual
defect | 1.2×2.5×0.9 | GH3+ |
| 235 | F | 27 | Acromegaly | 2.6×1.5×2.8 | GH3+ |
| 1(1) | M | 34 | Normal | - | Normal |
| 1(2) | M | 36 | Normal | - | Normal |
| 3(1) | M | 31 | Normal | - | Normal |
RNA extraction
Total RNA was extracted from healthy pituitaries
(50–100 mg) and GHomas (50–100 mg) using the Unizol reagent
protocol (Shanghai Biostar, Shanghai, China). The integrity of the
RNA in each sample was determined by denaturing agarose gel
electrophoresis, and the mRNA was quantified by
spectrophotometry.
RNA amplification
RNA was amplified using the TotalPrep RNA
amplification kit protocol (Ambion, Austin, TX, USA). In brief,
total RNA was used as a template, and the T7 oligonucleotide was
used for priming prior to the reverse transcription synthesis of
the first strand cDNA. DNA polymerase and RNase H were used to
simultaneously degrade the RNA and to synthesize the second strand
cDNA. cDNA purification removed the remaining RNA, primers, enzymes
and salts that would inhibit in vitro transcription. In
vitro transcription generated multiple copies of biotinylated
cRNA from the double-stranded cDNA templates, in the amplification
and labeling step. cRNA purification removed the unincorporated
NTPs, salts, enzymes and inorganic phosphates. Following
purification, the cRNA was used with direct hybridization array
kits (Illumina, San Diego, CA, USA).
Hybridization
A total of 1.5 μg of cRNA was hybridized to each
array. Sample labeling and hybridization on Illumina WG-6 v3.0
Sentrix Bead Chips (Illumina) were performed at a single Illumina
BeadStation facility according to the manufacturer’s instructions.
These chips contained over 48,000 transcripts for a total of 37,804
genes, which covered 25,158 well-characterized genes from the human
genome and 12,646 expressed sequence tags (ESTs).
Image scanning and data analysis
Illumina BeadArray Reader image analysis software
(Illumina) was used to acquire the original signal value for each
probe. In order to reduce random errors generated during the
experiment and to improve the comparability between the different
samples, the quantile normalization method was used to correct the
data. The data were then exported into Significance Analysis of
Microarrays (SAM) software to calculate the differential expression
and false discovery rate (FDR) between tumor and healthy tissues.
Filtering was performed to identify genes overexpressed or
underexpressed at least 2.0 fold and to determine q-values (similar
to the p-value, the q-value calculated the probability that the
expression differences and FDR between the two sets of data were
due to chance) of <5% in tumors compared to healthy pituitary
tissues. Further analysis as to the potential relevance of the
differentially expressed genes was performed using the Genbank
database (http://www.ncbi.nlm.nih.gov/Genbank/). The
differentially expressed genes were analyzed using pathway analysis
methods utilizing the R programming language and Bioconductor
software package. Hypergeometric tests were used to classify the
enrichment of genes in a particular pathway, and the FDR was
calculated to correct the p-values.
qRT-PCR
To verify the results of the microarray analysis,
four differentially expressed genes were randomly selected, and
their expression levels were measured in a blind manner. qRT-PCR
was performed as previously described (12) using a SYBR Green I dye detection
kit (Applied Biosystems, Foster City, CA, USA), and β-actin was
used as an internal control. The fold change of each gene was
calculated using the 2−ΔΔCt method, as previously
described (13). The expression
levels of these four genes were measured in three healthy pituitary
glands and five GHomas. The four genes analyzed were growth hormone
secretagogue receptor (GHSR; GenBank accession no.
NM_198407.1), gonadotropin-releasing hormone receptor
(GNRHR; GenBank accession no. NM_001012763.1), homeobox B2
(HOXB2; GenBank accession no. NM_002145.3) and glucagon
receptor (GCGR; GenBank accession no. NM_000160.2). The
primers for these genes were selected using Primer5.0 and
synthesized by Generay Biotech Co. (Shanghai, China), and are
listed in Table II.
 | Table II.PCR primers used for qRT-PCR. |
Table II.
PCR primers used for qRT-PCR.
| Forward primer | Reverse primer |
|---|
| GHSR | 5′
CACGGTCCTCTACAGTCTCATCG 3′ | 5′
CACGGTTTGCTTGTGGTTCTG 3′ |
| GNRHR | 5′
TCTACCAGGGAACATAGCATAAAT 3′ | 5′
TGCCAGAGTCCAGCCCTA 3′ |
| HOXB2 | 5′
CACAGAAAAATTCCGTTTGGTAG 3′ | 5′
AAACATAAAAAAGAGGAAAATAGGTC 3′ |
| GCGR | 5′
CGGAACACCAGCAACCAC 3′ | 5′
CCACAGGTAAAATAGATGGAGTTC 3′ |
|
β-actin | 5′
GACTTAGTTGCGTTACACCCTTTC 3′ | 5′
TGCTGTCACCTTCACCGTTC 3′ |
Results
Expression profile of GHomas
In the GHoma array, 353 genes and 206 ESTs were
overexpressed ≥2-fold and 565 genes and 29 ESTs were underexpressed
≥2-fold. The highest overexpressed and underexpressed genes are
listed in Table III. A total of 23
genes and 41 ESTs were exclusively expressed in the GHomas and 120
genes, and 13 ESTs were only expressed in the healthy pituitary
tissues.
 | Table III.The highest overexpressed and
underexpressed genes in GHomas. |
Table III.
The highest overexpressed and
underexpressed genes in GHomas.
| Gene | Accession | Definition | Fold change |
|---|
| GCGR | NM_000160.2 | Glucagon
receptor | 158.1 |
| EST | BX089019 | BX089019
Soares_testis_NHT Homo sapiens cDNA clone IMAGp998K243513;
IMAGE:1391375, mRNA sequence | 110.3 |
| HS3ST2 | NM_006043.1 | Heparan sulfate
(glucosamine) 3-O-sulfotransferase 2 | 99.8 |
| HIGD1B | NM_016438.2 | HIG1 domain family,
member 1B | 78.5 |
| GNLY | NM_006433.2 | Granulysin | 42.7 |
| HPGD | NM_000860.3 |
Hydroxyprostaglandin dehydrogenase
15-(NAD) | −66.6 |
| GPHA2 | NM_130769.2 | Glycoprotein
hormone α2 | −66.0 |
| VASH2 | NM_024749.2 | Vasohibin 2 | −63.5 |
| GJB7 | NM_198568.1 | Gap junction
protein, β7, 25 kDa | −56.6 |
| UGT2B7 | XM_943434.1 | Predicted: Homo
sapiens UDP glucuronosyltransferase 2 family, polypeptide B7 | −49.8 |
Validation of the differentially
expressed genes by qRT-PCR
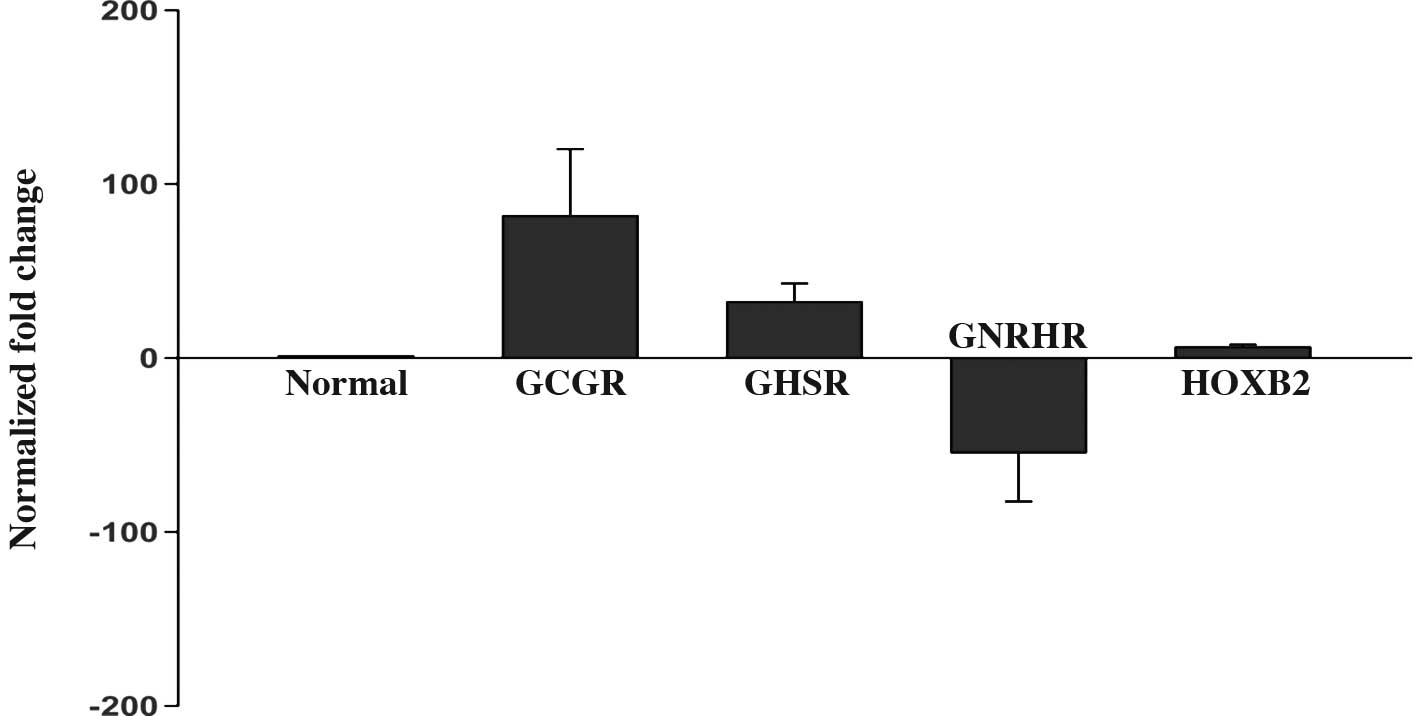
The microarray analysis showed that GCGR,
GHSR and HOXB2 mRNA were increased by 158.1-, 7.2-
and 19.3-fold, respectively, whereas the expression of GNRHR
mRNA was decreased by 31.6-fold in all GHomas compared to the
healthy pituitary tissues. qRT-PCR analysis of these four genes
confirmed the changes in mRNA levels that were observed in the
microarray analysis. The mean expression level of the four genes is
shown in Fig. 1A. Fig. 1B shows the relative expression
level of the four genes in the microarray compared to the qRT-PCR
analysis.
Pathway analysis
The KEGG pathways are compiled from multiple
literature sources and integrate individual components into a
unified pathway. As such, the KEGG pathway database was used to
characterize the enrichment of specific pathway components into
functionally regulated groups. The differentially expressed genes
from the microarray analysis were analyzed utilizing the pathway
analysis methods in the R programming language and Bioconductor
software package. A total of six KEGG pathways were significantly
enriched among the genes associated with GHomas (p<0.05), as
shown in Table IV. Of the
significant signaling pathways, two were selected for further
analysis: the wingless-type (Wnt) signaling pathway and
extracellular matrix (ECM)-receptor interactions.
 | Table IV.Six highly significant (p<0.05)
pathways in GHomas. |
Table IV.
Six highly significant (p<0.05)
pathways in GHomas.
| Pathway ID | Pathway title | Gene | p-value |
|---|
| hsa04310 | Wnt signaling
pathway | CCND3,
LEF1, WNT5B, WNT5A, WIF1, FZD9,
FZD2, FZD3, SFRP2, TBL1XR1,
NFAT5, VANGL2, FRAT1, FZD7,
PRICKLE1, SMAD3 | 0.004 |
| hsa04115 | p53 signaling
pathway | TNFRSF10B,
GADD45G, CCNE1, CCND3, CCNB2,
PMAP1, IGFBP3, RPRM, CDKN1A (p21,
CIP1) | 0.012 |
| hsa05217 | Basal cell
carcinoma | LEF1,
WNT5B, WNT5A, FZD9, FZD2, FZD3,
FZD7 | 0.014 |
| hsa05218 | Melanoma | PIK3CD,
FGF23, E2F3, PDGFRA, FGF17,
PDGFC, FGF2, PIK3R3, CDKN1A | 0.016 |
| hsa05210 | Colorectal
cancer | PIK3CD,
LEF1, PDGFRA, FZD9, FZD2, FOS,
FZD3, PIK3R3, FZD7 | 0.016 |
| hsa04512 | ECM-receptor
interaction | LAMA2,
ITGA2, COL11A1, COL6A1, COL6A2,
COL4A6, SDC4, COL11A2 | 0.018 |
Discussion
These experiments had two goals: First, we sought to
identify the differentially expressed genes in GHomas as compared
to healthy pituitary tissues, with the aim of identifing novel
differentially expressed genes that may be important candidates in
the pathogenesis of this poorly understood tumor. Second, we used
pathway analysis to explore the possible relationship between the
pathogenesis of GHomas and groups of genes functioning together in
related pathways.
Analysis of differentially expressed
genes
The NCBI database Genbank (http://www.ncbi.nlm.nih.gov/Genbank/) was used to
analyze the differentially expressed genes identified in GHomas
compared to healthy pituitaries, as well as the genes only
expressed in the healthy pituitary tissues or the tumor tissues. We
determined that the new genes HIGD1B, HOXB2,
ANGPT2, HPGD and BTG2 may be important
candidates in the pathogenesis of GHomas. These are discussed
below.
HIGD1B (HIG1 domain family, member
1B)
In our study, the expression level of the
HIGD1B mRNA was significantly up-regulated and only
expressed in GHomas. However, the function of this protein is
currently unknown. Denko et al (14) reported that in cultured cells, HIG1
and HIG2 expression is induced by hypoxia and by glucose
deprivation. In addition, tumor xenografts derived from human
cervical cancer cells display increased expression of HIG1 and HIG2
when they are deprived of oxygen. The role of HIGD1B in the
pathogenesis of GHomas is not clear, and the specific mechanisms of
action of this protein require further study.
HOXB2 (homeobox B2)
Homeobox genes are a large superfamily whose members
function in establishing and maintaining cell fate and cell
identity throughout embryonic development (15). Regarding the relationship between
Homeobox genes and cancer, numerous studies have been undertaken to
examine the differences in Homeobox expression between healthy and
neoplastic tissues. Several investigators have explored the
hypothesis that Homeobox genes expressed during embryogenesis, but
down-regulated during adulthood, are re-expressed in neoplasias –
the so-called ‘oncology recapitulates ontology’ hypothesis
(16). In our study, the
expression level of the HOXB2 mRNA was significantly
up-regulated (19.3-fold), and this was validated by qRT-PCR
analysis. However, its role in pituitary tumors is unknown and
requires further study.
ANGPT2 (angiopoietin 2)
Angiopoietins are members of a novel family of
angiogenic factors and participate in the formation of blood
vessels. Of the four currently known family members, the best
characterized are angiopoietin (ANG)-1 and -2, both of which
function as ligands for the endotheliumspecific tyrosine kinase
receptor TIE-2. ANG-1 plays an important role in maintaining vessel
integrity. ANG-2, which is thought to be an endogenous antagonist
of the action of ANG-1, competes for binding to the TIE-2 receptor.
This blocks ANG-1-induced TIE-2 autophosphorylation during
vasculogenesis, and subsequently leads to the loosening of
cell-matrix and cell-cell contacts, allowing access to angiogenic
inducers (17).
Nag et al (18) reported that the localization of
both ANG-1 and ANG-2 occurs in scattered periodic acid-Schiff
(PAS)-positive adenohypophysial cells rather than in endothelial
cells. Accumulated evidence also indicates that the production of
ANG-2 is implicated in tumor progression (19). Our study showed that ANG-2
mRNA was overexpressed by up to 5.6-fold in GHomas. We therefore
believe that ANG-2 may play an important role in promoting the
angiogenesis, progression and invasion of these tumors.
HPGD (15-hydroxyprostaglandin
dehydrogenase; 15-PGDH)
15-PGDH is a prostaglandin degrading enzyme that
catalyses the oxidization of the 15(S)-hydroxyl group of PGE2 to
yield an inactive 15-keto PGE2. PGE2 is related to carcinogenesis
through immunosuppression, the inhibition of apoptosis, an increase
in the metastatic potential of epithelial cells and the promotion
of angiogenesis (20). Genetic
deletion of 15-PGDH in mice leads to increased tissue levels of
PGE2 (21).
Previous studies identified tumor suppressor
activity for 15-PGDH in colon, lung, bladder and breast carcinomas,
and suggested that epigenetic silencing of this enzyme occurs by
DNA methylation and histone modification (22). The expression of 5-PGDH in benign
tumors has yet to be reported. Our study showed that the expression
of the 15-PGDH mRNA in GHomas was significantly
down-regulated (-66.6-fold). We therefore speculate that this gene
also functions as a tumor suppressor gene in GHomas, and plays a
key role in the tumorigenesis and progression of these tumors.
However, the specific mechanisms affected by 5-PGDH in these tumors
require further study.
BTG2 (BTG family, member 2)
BTG2 is an antiproliferative tumor suppressor
protein, as its overexpression leads to the blockade of cells at
the G1 phase of the cell cycle (23). It has also been demonstrated that
BTG2 expression is induced by cytotoxic and genotoxic stress
through a p53-dependent mechanism (24). Farioli-Vecchioli et al
(25) reported that the
down-regulation of BTG2 is observed in pre-neoplastic lesions as
well as in human and murine medulloblastomas. This previous report
provides the earliest evidence that BTG2 acts as a tumor suppressor
in the central nervous system. Our study showed that the expression
of BTG2 mRNA in GHomas was down-regulated. We therefore
speculate that this gene plays a key role in the tumorigenesis and
progression of these tumors.
Pathway analysis
Single-gene analysis may overlook important effects
that occur on pathways, as cellular processes often affect sets of
genes acting in concert. An increased expression of 20% of all gene
members in a metabolic pathway may dramatically alter the flux
through the pathway, and may therefore be more important than a
20-fold increase in any single gene (26). Therefore, we used the KEGG pathway
database (http://www.genome.jp/kegg/pathway.html) to analyze
pathways with a p-value <0.05 (Table IV). The study suggested that the
Wnt signaling pathway and ECM-receptor interactions play key roles
in the pathogenesis of GHomas.
The Wnt signaling pathway
The signaling transduction pathway mediated by the
Wnt proteins is highly conserved between species. It influences
embryonic development, cell polarity and adhesion, apoptosis and
tumorigenesis, and it interacts with other cell signaling pathways
(27). There are at least three
different Wnt pathways: the canonical, the planar cell polarity and
the Wnt/Ca2+ pathway. In the canonical Wnt pathway, the
major effect of Wnt ligand binding to its receptor is the
stabilization of cytoplasmic β-catenin through inhibition of the
β-catenin degradation complex. Subsequently, β-catenin is then free
to enter the nucleus and activate Wnt-regulated genes through its
interaction with the T-cell factor (TCF) family of transcription
factors and concomitant recruitment of coactivators.
Receptors for the Wnt proteins are members of the
frizzled family of transmembrane proteins encoded by the frizzled
genes (Fzs). Currently, 10 or more human Fzs genes have been
identified (28). The Fzs proteins
have an N-terminal cysteine-rich extracellular domain and seven
membrane-spanning α helices linked by hydrophilic loops ending with
an intracellular C-terminus (28).
The extracellular ligand (a specific Wnt) binds to the
cysteine-rich domain (CRD) of a specific Fzs, altering the
interaction of the CRD with the transmembrane domain. These changes
result in a rearrangement of the transmembrane α helices, a
remodelling of the cytosolic face of the protein and an alteration
of downstream signalling (27). In
our study, the expression of FZD2, FZD3, FZD7
and FZD9 mRNA was down-regulated; however, the specific
mechanism by which this occurred is not clear, and requires further
study.
In the nucleus, stabilized β-catenin associates with
the TCF/lymphoid enhancer factor (LEF) family of transcription
factors to activate specific target genes. De-repression by
TCF/LEF/β-catenin complexes enables the transcription of more than
30 different genes. These include c-myc, cyclin D1,
c-jun, fra-1, matrilysin and members of the
Ap-1 family of genes, including fos, fosB and
junB (27). Our study
showed that the expression of LEF1 and CCND3 mRNA in
GHomas was up-regulated. We speculated that this plays a crucial
role in the tumorigenesis and progression of these tumors.
Secreted Wnt antagonists are involved in regulating
the Wnt pathways. These Wnt inhibitors are divided into two main
families containing either secreted frizzled-related proteins
(sFRPs) or the Dickkopf (DKK) proteins. The sFRP family is
comprised of five sFRPs and Wnt inhibitory factor 1 (WIF1). Elston
et al (29) used microarray
analysis to compare pituitary tumors to healthy pituitary glands,
demonstrating that WIF1 mRNA expression is markedly
underexpressed in all pituitary tumor subtypes. In addition, they
found significantly reduced mRNA expression of two other Wnt
pathway inhibitors, sFRP2 and sFRP4, and suggested
that the Wnt pathways are important in pituitary tumorigenesis. Our
experimental results are in agreement with their report, as we
observed that SFRP2 and WIF1 mRNA were significantly
underexpressed in GHomas. Based on the above analysis, we propose
that the Wnt pathway may play an important role in promoting the
tumorigenesis and progression of GHomas.
ECM-receptor interactions
The ECM is a three-dimensional network of proteins,
glycosaminoglycans and other macromolecules. It has a structural
support function, as well as a role in cell adhesion, migration,
proliferation and survival. ECM components, including laminins,
collagen and fibronectin, or synthetic substrates, such as
poly-D-lysine, representing a cationic polypeptide, affect the
proliferation and function of healthy and neoplastic cells
(30). Such effects have been
demonstrated in many different cultured tumor cells, including
endocrine tumor cells (31). For
example, laminin was found to regulate the development and growth
of breast, prostate, colon, thyroid and ovarian cancers (32). In our study, LAMA2,
COL11A1, COL6A1, COL6A2, COL4A6 and
COL11A2 mRNA were observed to be underexpressed in
GHomas.
Apart from being growth factors, ECM components are
considered to be the main control elements of cellular
proliferation, acting though integrin surface receptors. Integrins
are a diverse family of glycoproteins that form heterodimeric
receptors for ECM molecules. This family of proteins forms at least
25 distinct pairings among its 18 α-subunits and 8 β-subunits, with
each pairing being specific for a unique set of ligands (33). Integrin α2β1 is formed by a
non-covalent association of the α2 subunit as a monogamous partner
to the promiscuous β1 subunit. The expression of integrin α2 has
been correlated with metastatic behavior in breast cancer,
hepatocarcinoma and rhabdomyosarcoma (34). In our study, the integrin α2
(ITGA2) mRNA was expressed at a lower level in GHomas.
ECM components and integrins constitute one of the
few clear examples of factors differentially expressed during
pituitary pathogenesis (32). The
ECM likely plays a role in the inhibition of invasion and
metastasis in pituitary tumors (32). Consistent with this previous
report, our study showed that all the differentially expressed
genes involved in this pathway were underexpressed in GHomas. The
role of ECM-receptor interactions in GHomas may be to inhibit their
invasion and malignant transformation.
In conclusion, in this study we identified a number
of genes and pathways that may play an important role in the
tumorigenesis and progression of GHomas. Expression analysis of
tumors using bead-based fiber-optic arrays appears to be a valid
method for identifying genes important in tumor pathogenesis.
Moreover, further characterization of gene expression profiles
based on pathway analysis may play a vital role in elucidating the
pathogenesis of tumors.
References
|
1.
|
Pollock BE, Jacob JT, Brown PD and
Nippoldt TB: Radiosurgery of growth hormone-producing pituitary
adenomas: factors associated with biochemical remission. J
Neurosurg. 106:833–838. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Rajasoorya C, Holdaway IM, Wrightson P and
Scott DJ: Ibbertson HK determinants of clinical outcome and
survival in acromegaly. Clin Endocrinol. 41:95–102. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Beauregard C, Truong U, Hardy J and Serri
O: Long-term outcome and mortality after transsphenoidal
adenomectomy for acromegaly. Clin Endocrinol. 58:86–91. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Herman V, Fagin J, Gonsky R, Kovacs K and
Melmed S: Clonal origin of pituitary adenomas. J Clin Endocrinol
Metab. 71:1427–1433. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Farrell WE and Clayton RN: Molecular
genetics of pituitary tumours. Trends Endocrinol Metab. 9:20–26.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Ezzat S, Kontogeorgos G, Redelmeier DA,
Horvath E, Harris AG and Kovacs K: In vivo responsiveness of
morphological variants of growth hormone-producing pituitary
adenomas to octreotide. Eur J Endocrinol. 133:686–690. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Karga HJ, Alexander JM, Hedley-Whyte ET,
Klibanski A and Jameson JL: Ras mutations in human pituitary
tumors. J Clin Endocrinol Metab. 74:914–919. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Asa SL and Ezzat S: The pathogenesis of
pituitary tumors. Annu Rev Pathol. 4:97–126. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Mao S and Dong G: Discovery of highly
differentiative gene groups from microarray gene expression data
using the gene club approach. J Bioinform Comput Biol. 3:1263–1280.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Evans CO, Young AN, Brown MR, Brat DJ,
Parks JS, Neish AS and Oyesiku NM: Novel patterns of gene
expression in pituitary adenomas identified by complementary
deoxyribonucleic acid microarrays and quantitative reverse
transcription-polymerase chain reaction. J Clin Endocrinol Metab.
86:3097–3107. 2001.
|
|
11.
|
Morris DG, Musat M, Czirjak S, Hanzely Z,
Lillington DM, Korbonits M and Grossman AB: Differential gene
expression in pituitary adenomas by oligonucleotide array analysis.
Eur J Endocrinol. 153:143–151. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Ellestad LE, Carre W, Muchow M, Jenkins
SA, Wang X, Cogburn LA and Porter TE: Gene expression profiling
during cellular differentiation in the embryonic pituitary gland
using cDNA microarrays. Physiol Genomics. 25:414–425. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Denko N, Schindler C, Koong A, Laderoute
K, Green C and Giaccia A: Epigenetic regulation of gene expression
in cervical cancer cells by the tumor microenvironment. Clin Cancer
Res. 6:480–487. 2000.PubMed/NCBI
|
|
15.
|
Lewis MT: Homeobox genes in mammary gland
development and neoplasia. Breast Cancer Res. 2:158–169. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Lappin TR, Grier DG, Thompson A and
Halliday HL: HOX genes: seductive science, mysterious mechanisms.
Ulster Med J. 75:23–31. 2006.PubMed/NCBI
|
|
17.
|
Wang HL, Deng CS, Lin J, Pan DY, Zou ZY
and Zhou XY: Expression of angiopoietin-2 is correlated with
vascularization and tumor size in human colorectal adenocarcinoma.
Tohoku J Exp Med. 213:33–40. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Nag S, Nourhaghighi N, Venugopalan R, Asa
SL and Stewart DJ: Angiopoietins are expressed in the normal rat
pituitary gland. Endocr Pathol. 16:67–73. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Hu B, Guo P, Fang Q, et al: Angiopoietin-2
induces human glioma invasion through the activation of matrix
metalloprotease-2. Proc Natl Acad Sci USA. 100:8904–8909. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Jang TJ, Ji YS and Jung KH: Decreased
expression of 15-hydroxyprostaglandin dehydrogenase in gastric
carcinomas. Yonsei Med J. 49:917–922. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Coggins KG, Latour A, Nguyen MS, Audoly L,
Coffman TM and Koller BH: Metabolism of PGE2 by prostaglandin
dehydrogenase is essential for remodeling the ductus arteriosus.
Nat Med. 8:91–92. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Wolf I, O’Kelly J, Rubinek T, et al:
15-hydroxyprostaglandin dehydrogenase is a tumor suppressor of
human breast cancer. Cancer Res. 66:7818–7823. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Rouault JP, Falette N, Guehenneux F, et
al: Identification of BTG2, an antiproliferative p53-dependent
component of the DNA damage cellular response pathway. Nat Genet.
14:482–486. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Cortes U, Moyret-Lalle C, Falette N, et
al: BTG gene expression in the p53-dependent and -independent
cellular response to DNA damage. Mol Carcinog. 27:57–64. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Farioli-Vecchioli S, Tanori M, Micheli L,
et al: Inhibition of medulloblastoma tumorigenesis by the
antiproliferative and prodifferentiative gene PC3. FASEB J.
21:2215–2225. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Subramanian A, Tamayo P, Mootha VK, et al:
Gene set enrichment analysis: a knowledge-based approach for
interpreting genome-wide expression profiles. Proc Natl Acad Sci
USA. 102:15545–15550. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Karim R, Tse G, Putti T, Scolyer R and Lee
S: The significance of the Wnt pathway in the pathology of human
cancers. Pathology. 36:120–128. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Jones SE and Jomary C: Secreted
Frizzled-related proteins: searching for relationships and
patterns. Bioessays. 24:811–820. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Elston MS, Gill AJ, Conaglen JV, et al:
Wnt pathway inhibitors are strongly down-regulated in pituitary
tumors. Endocrinology. 149:1235–1242. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Danen EH and Yamada KM: Fibronectin,
integrins, and growth control. J Cell Physiol. 189:1–13. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Huet C, Pisselet C, Mandon-Pepin B, Monget
P and Monniaux D: Extracellular matrix regulates ovine granulosa
cell survival, proliferation and steroidogenesis: relationships
between cell shape and function. J Endocrinol. 169:347–360. 2001.
View Article : Google Scholar
|
|
32.
|
Paez-Pereda M, Kuchenbauer F, Arzt E and
Stalla GK: Regulation of pituitary hormones and cell proliferation
by components of the extracellular matrix. Braz J Med Biol Res.
38:1487–1494. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Gerger A, Hofmann G, Langsenlehner U, et
al: Integrin alpha-2 and beta-3 gene polymorphisms and colorectal
cancer risk. Int J Colorectal Dis. 24:159–163. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Sawhney RS, Cookson MM, Omar Y, Hauser J
and Brattain MG: Integrin alpha2-mediated ERK and calpain
activation play a critical role in cell adhesion and motility via
focal adhesion kinase signaling: identification of a novel
signaling pathway. J Biol Chem. 281:8497–8510. 2006. View Article : Google Scholar
|