Introduction
Human T-cell leukemia virus type 1 (HTLV-1) causes
various diseases such as adult T-cell leukemia/lymphoma (ATL),
HTLV-1-associated myelopathy/tropical spastic paraparesis
(HAM/TSP), uveitis and arthropathy (1). HTLV-1 transmission mainly occurs
through blood transfusion, sexual contact, breastfeeding or
intrauterine transfer (2).
However, not all individuals exposed to HTLV-1 develop persistent
infection. Previous reports have indicated that approximately 60%
of individuals who received infected blood or blood components and
15–30% of breast-fed children born to infected mothers developed
HTLV-1 infection (3). In the early
phase of infection, patients hold only a small percentage of HTLV-1
provirus loads in peripheral blood. In asymptomatic carriers,
proviral load is maintained for a long period, and it is currently
difficult to eradicate the infected cells. Importantly, high
proviral loads in asymptomatic carriers are associated with the
onset of ATL (4) and the
progression of motor disability associated with HAM/TSP (5). In addition, a high level of HTLV-1
provirus load in circulating lymphocytes of HTLV-1 carriers is a
risk factor for HTLV-1-related diseases (6–8).
However, the lack of knowledge of early events of HTLV-1 spread
in vivo has hindered an understanding of viral
infections.
We previously inoculated mice intraperitoneally with
MT-2 cells and reported that HTLV-1 provirus was integrated into
the mouse genome, and that HTLV-1-infected mouse cells were
distributed in various organs in the mice (9–12).
The aim of the present study was to examine whether the route of
viral infection affects HTLV-1 proliferation and host immune
response after primary HTLV-1 infection.
Materials and methods
Cells and animals
MT-2 cells, an HTLV-1-infected human T-cell line
(13), were cultured in RPMI-1640
medium supplemented with 10% fetal calf serum. Balb/c mice were
purchased from Clea, Inc., Tokyo, Japan. At 4 weeks of age, the
mice were inoculated intraperitoneally, intravenously or perorally
with 106 or 107 MT-2 cells. The experiments
were conducted in accordance with the Regulations on Animal
Experiments of the University of Tsukuba, and were approved by the
University's Animal Experiment Committee.
Quantification of HTLV-1 proviral
load
qPCR conditions were as described previously
(9). Briefly, the number of
tax (viral gene) and mouse c-myc molecules (cellular
gene control) were quantified using real-time PCR, and the HTLV-1
proviral load per 105 mouse cells was calculated as
follows: (number of tax molecules/number of mouse
c-myc molecules/2) × 105. Proviral load was
defined as zero when there was no tax signal after 50 cycles
of PCR amplification under conditions in which mouse c-myc
was amplified.
Detection of HTLV-1 antibody in
serum
The antibodies against HTLV-1 proteins in the plasma
were assayed with a particle agglutination kit (Serodia HTLV-1;
Fujirebio, Japan).
Interleukin-2 (IL-2) production
assay
Splenic T cells from native and HTLV-1-infected mice
were cultured with anti-CD3 for 2 days. The concentrations of IL-2
in the supernatants were measured by the enzyme-linked
immunosorbent assay (ELISA; Ready-SET-Go! kits, eBioscience, San
Diego, CA) according to the manufacturer's instructions. The ELISA
assay was specific for mouse-derived IL-2, since no
cross-reactivity with human IL-2 from MT-2 cells was observed.
Statistical analysis
Welch's t-test was used for statistical
analysis.
Results
Tissue distribution of the HTLV-1
provirus by various routes
As shown in Table
I, in mice infected intraperitoneally with 106 MT-2
cells, provirus was detected in most organs, and high proviral
loads were detected in peripheral blood mono-nuclear cells, spleen
and gut-associated lymphoid tissues, consistent with our previous
results (12). Among mice
inoculated intraperitoneally with different amounts of MT-2 cells,
no significant differences in organ distribution were observed,
while the mice inoculated with 107 MT-2 cells had higher
proviral loads than those inoculated with 106 MT-2
cells. In contrast to intraperitoneal infection, intravenous or
oral inoculation resulted in low proviral loads and restricted
organ distribution of infected cells. Among mice infected
intravenously, provirus was detected only in the spinal cord, while
among mice inoculated orally with 107 MT-2 cells,
provirus was detected in the kidney and liver of 2 of the 5 mice
examined. These results demonstrate that the route of viral
exposure likely affects proviral load and viral organ distribution
in mice.
 | Table I.Proviral load in organs from BALB/c
mice infected with HTLV-1 intraperitoneally, intravenously or
perorally. |
Table I.
Proviral load in organs from BALB/c
mice infected with HTLV-1 intraperitoneally, intravenously or
perorally.
| POa
| IVa
| IPa
|
|---|
| Organs | 106
(n=6)b | 107
(n=6)b | 106
(n=5)b | 106
(n=4)b | 107
(n=5)b |
|---|
| PBMC | 0 | 0 | 0 | 25.6±12.1 | 20.4±8.9 |
| Brain | 0 | 0 | 0 | 1.3±3.1 | 0.3±0.4 |
| Salivary lymph
nodes | 0 | 0 | 0 | 7.4±3.5 | 14.4±8.4 |
| Lung | 0 | 0 | 0 | 9.0±3.3 | 13.7±9.2 |
| Thymus | 0 | 0 | 0 | 2.1±2.4 | 2.7±2.9 |
| Spleen | 0 | 0 | 0 | 21.9±8.1 | 56.1±29.0 |
| Mesenteric lymph
nodes | 0 | 0 | 0 | 38.9±9.3 | 38.0±16.1 |
| Peyer's patches | 0 | 0 | 0 | 41.7±10.2 | 78.8±25.8 |
| Liver | 0 | 1.0±1.3 | 0 | 0 | 7.0±6.7 |
| Kidney | 0 | 0.1±0.1 | 0 | 4.8±2.0 | 7.8±4.9 |
| Ovary | 0 | 0 | 0 | 5.1±2.1 | 9.1±7.8 |
| Spinal cord | 0 | 0 | 2.5±2.6 | 0.9±2.3 | 0.1±0.1 |
| Submandibular
glands | 0 | 0 | 0 | 0.9±1.4 | 0.8±1.3 |
Comparision of immune responses by
various routes against HTLV-1
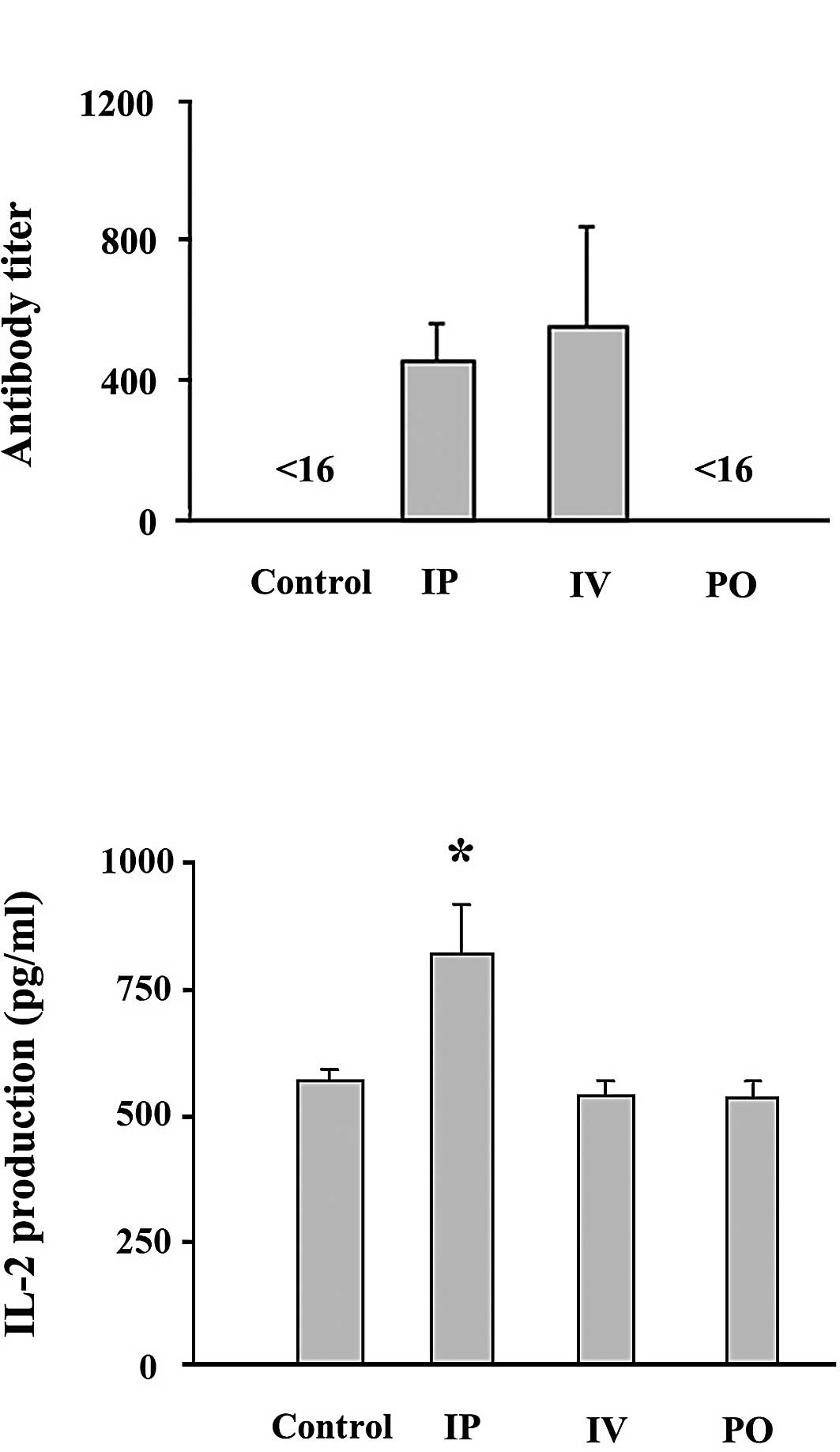
Antibody titers were determined in the sera of mice
1 month after infection using a particle agglutination kit.
Antibody titers against HTLV-1 in the mice infected orally were
below the detectable level (Fig.
1A), consistent with a previous report in which F344/N
Jcl-rnu/+ rats were inoculated orally with MT-2 cells
(14). The mice infected
intraperitoneally and intravenously exhibited similar anti-HTLV-1
titers. These results suggest that antibody titers do not correlate
with proviral load in mice 1 month after infection, and corroborate
similar findings in our previous report, in which various syngeneic
mouse strains were used (9).
IL-2 production and proliferation of
HTLV-1-infected cells
Continuous growth of HTLV-1-infected cells in vitro
requires IL-2-mediated signaling (15), and IL-2 also likely drives the
proliferation of HTLV-1-infected cells in vivo (16). We investigated whether IL-2
production is involved in the proliferation of HTLV-1-infected
cells in BALB/c mice inoculated with MT-2 cells. The mice infected
intraperitoneally showed significantly higher IL-2 production than
the mice infected intravenously or perorally, or than the
uninfected mice (Fig. 1B).
Exclusion of MT-2 cells in inoculated
mice
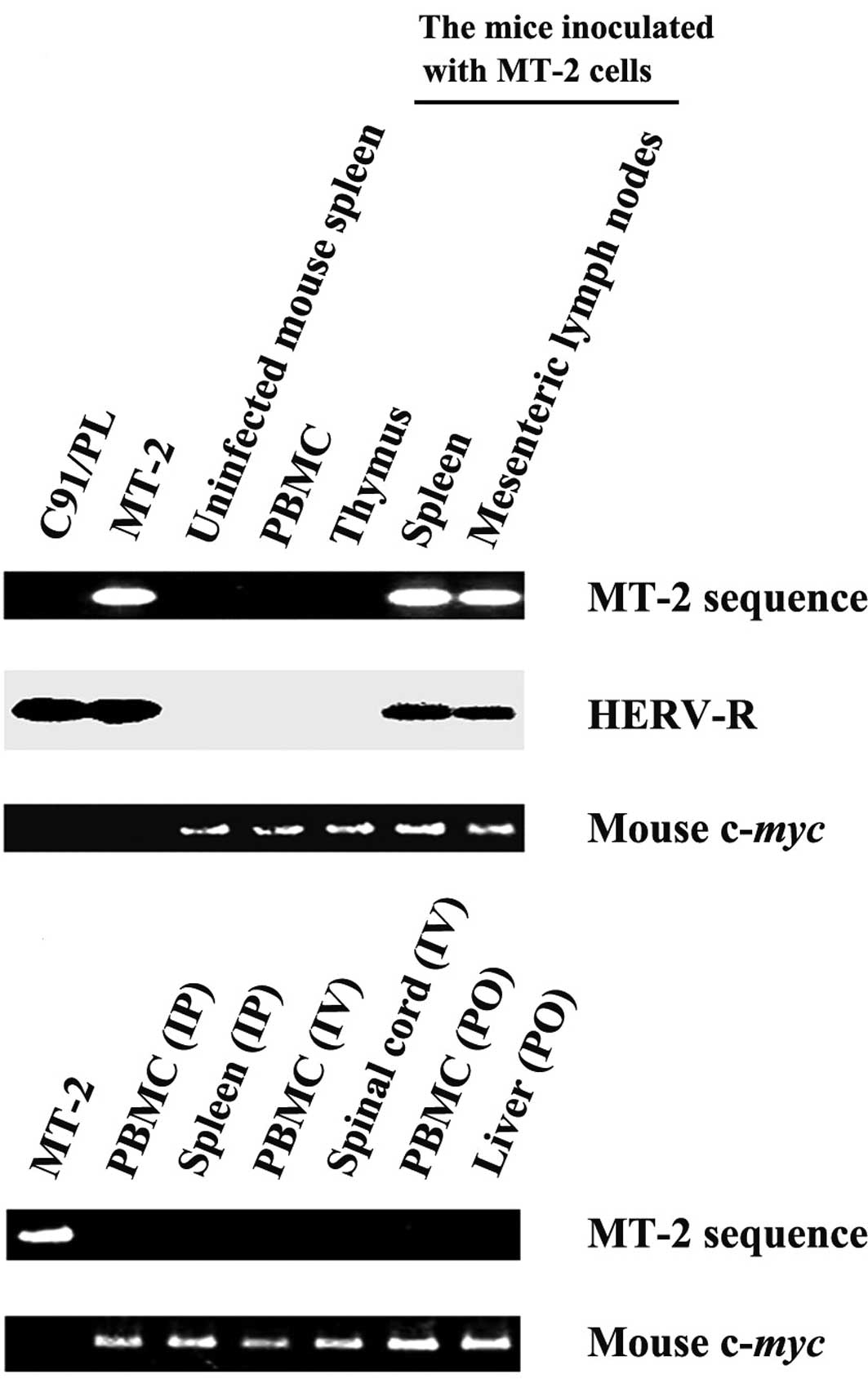
To determine HTLV-1 proliferation in animals
inoculated with HTLV-1-infected cells, the inoculated cells must be
distinguished from newly infected host cells. Therefore, we
previously cloned and sequenced the cellular DNA sequence flanking
the 3′ LTR of the HTLV-1 provirus in the MT-2 cells and established
a PCR method to specifically amplify the human sequence flanking
the 3′ LTR of HTLV-1 provirus in the MT-2 cells (9). As shown in Fig. 2A, the MT-2-specific sequence was
detected in a mouse inoculated with MT-2 cells intraperitoneally
and sacrificed 12 h after infection, while this sequence was not
detected in C91/PL cells (17),
another HTLV-1-infected human cell line having different proviral
integration sites, nor in the spleen of an uninfected control
mouse, confirming the specificity of the MT-2-based primers. As
expected, the MT-2 sequence was not detected in any of the mice 1
month after infection (Fig. 2B),
consistent with previous studies showing that MT-2 cells cannot
proliferate in various mouse strains containing natural killer
activity, including SCID mice (9,10,18,19).
Together, these results indicated that the MT-2 cells were rejected
in BALB/c mice 1 month after infection, and that the proviral load
in these mice represented HTLV-1-infected mouse cells.
Discussion
Difficulty in obtaining human specimens has hindered
the understanding of the mechanisms involved in HTLV-1
proliferation in asymptomatic carriers. Therefore, to understand
viral transmission, host immune responses and the relationship
between genetic background and initial viral proliferation, it is
essential to establish animal models for HTLV-1 infection. A
variety of animal models of HTLV-1 infection have been developed
such as monkey, rabbit, rat and hamster (20). However, since mice are better
characterized, particularly with respect to genetic information,
and are more easily maintained than the aforementioned animal
models, they are most commonly used. Previous studies examining the
inoculation of immunodeficient mice, such as SCID or
NOD/SCID/gammacnull mice, with ATL cells or
HTLV-1-infected cell lines, have demonstrated that these mouse
models are useful for characterizing HTLV-1-transformed cells and
for screening drugs for ATL patients in vivo (21,22).
However these models might not be suitable for studying host-virus
interactions.
A few studies have compared initial routes of
infection with the extent of HTLV-1 proliferation, but the effects
of the routes of viral exposure differ depending on the animal
model. In this report, we introduced MT-2 cells into BALB/c mice
and demonstrated that intraperitoneal infection resulted in higher
proviral loads compared with intravenous or peroral viral
infection. In rabbits, injection of the HTLV-1 molecular clone K30p
into muscle resulted in systemic HTLV-1 infection, but no provirus
was detected in nervous tissue (23). It was found that the frequencies of
provirus detection in peripheral blood were comparable in rats
inoculated with MT-2 cells orally, intravenously or
intraperitoneally (14). Another
report using rats demonstrated that oral inoculation of rats with
5×106 MT-2 cells resulted in higher proviral loads
compared with rats infected intraperitoneally, although proviral
loads were similar among rats inoculated orally or
intraperitoneally with 5×101 MT-2 cells (24). The factors controlling HTLV-1
proliferation in response to different routes of viral exposure
remain unknown; however, viral or proviral loads differ greatly
among the mouse strains used in retroviral infection studies
(9), and endogenous retroviruses
appear to influence retroviral proliferation (25,26).
We assessed the effects of the initial viral amount and the routes
of HTLV-1 infection in mice, and HTLV-1 provirus was detected only
in the spinal cord via intravenous infection (Table I). Osame et al reported that
blood transfusion was associated with the onset of neurological
symptoms of HAM/TSP (27). These
results suggest that host-virus interactions, host immune
responses, and interference from endogenous host retroviruses
likely influence HTLV-1 proliferation after viral exposure through
various routes of inoculation, consistent with the different levels
of cytokine production observed among the infected mice in the
present study (Fig. 1).
To better understand HTLV-1 proliferation shortly
after viral infection via the primary routes of transmission, we
inoculated mice with MT-2 cells intravenously and orally. Although
we found that HTLV-1 was transmitted to the mice through both
intravenous and oral routes, low proviral loads were observed 1
month after infection. In humans, transmission of HTLV-1-infected
cells occurs via various cellular components, such as
prostaglandins, lactoferrin, and transforming growth factor-ß,
found in breast milk and serum. These compounds enhance expression
of glucose transporter-1 and the HTLV-1 receptor, and activate the
HTLV-1 long-terminal repeat promoter (28–30).
Since we inoculated BALB/c mice with MT-2 cells suspended in
phosphate-buffered saline, it is possible that efficient HTLV-1
proliferation following intravenous or oral inoculation failed
owing to the absence of factors known to enhance transmission and
replication of HTLV-1.
In conclusion, HTLV-1 carrier mouse models are
useful for testing various anti-HTLV-1 therapeutic approaches.
Furthermore, these findings have expanded the understanding of the
role of viral gene products and host factors that determine
HTLV-1-related pathogenesis.
Acknowledgements
This work was supported in part by a
Grant-in-Aid for Cancer Research from the Ministry of Health,
Labour and Welfare, Japan. T.N. was supported by individual awards
from the Yasuda Medical Research Foundation and the Ishidu Shun
Memorial Scholarship. M.T. and B.S. were supported in part by the
Japan Society for the Promotion of Science fellowship program.
References
|
1.
|
Uchiyama T: Human T cell leukemia virus
type I (HTLV-I) and human diseases. Annu Rev Immunol. 15:15–37.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Grant C, Barmak K, Alefantis T, Yao J,
Jacobson S and Wigdahl B: Human T cell leukemia virus type I and
neurologic disease: events in bone marrow, peripheral blood, and
central nervous system during normal immune surveillance and
neuroinflammation. J Cell Physiol. 190:133–159. 2002. View Article : Google Scholar
|
|
3.
|
Okochi K, Sato H and Hinuma Y: A
retrospective study on transmission of adult T cell leukemia virus
by blood transfusion: seroconversion in recipients. Vox Sang.
46:245–253. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Okayama A, Stuver S, Matsuoka M, Ishizaki
J, Tanaka G, Kubuki Y, Mueller N, Hsieh CC, Tachibana N and
Tsubouchi H: Role of HTLV-1 proviral DNA load and clonality in the
development of adult T-cell leukemia/lymphoma in asymptomatic
carriers. Int J Cancer. 110:621–625. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Matsuzaki T, Nakagawa M, Nagai M, Usuku K,
Higuchi I, Arimura K, Kubota H, Izumo S, Akiba S and Osame M:
HTLV-I proviral load correlates with progression of motor
disability in HAM/TSP: analysis of 239 HAM/TSP patients including
64 patients followed up for 10 years. J Neurovirol. 7:228–234.
2001.PubMed/NCBI
|
|
6.
|
Taylor GP, Tosswill JH, Matutes E, Daenke
S, Hall S, Bain BJ, Davis R, Thomas D, Rossor M, Bangham CR and
Weber JN: Prospective study of HTLVI infection in an initially
asymptomatic cohort. J Acquir Immune Defic Syndr. 22:92–100. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Hisada M, Okayama A, Shioiri S, Spiegelman
DL, Stuver SO and Mueller NE: Risk factors for adult T-cell
leukemia among carriers of human T-lymphotropic virus type I.
Blood. 92:3557–3561. 1998.PubMed/NCBI
|
|
8.
|
Okayama A and Stuver SO: Long-term
follow-up of HTLV-1 carriers. Gann Monogr Cancer Res. 50:127–139.
2003.
|
|
9.
|
Nitta T, Tanaka M, Sun B, Hanai S and Miwa
M: The genetic background as a determinant of human T-cell leukemia
virus type 1 proviral load. Biochem Biophys Res Commun.
309:161–165. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Nitta T, Tanaka M, Sun B, Sugihara E,
Kimura M, Kamada Y, Takahashi H, Hanai S, Jiang SW, Fujisawa J and
Miwa M: Reduction of human T-cell leukemia virus type-1 infection
in mice lacking nuclear factor-kappaB-inducing kinase. Cancer Sci.
99:872–878. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Tanaka M, Sun B, Fang J, Nitta T, Yoshida
T, Kohtoh S, Kikukawa H, Hanai S, Uchida K and Miwa M: Human T-cell
leukemia virus type 1 (HTLV-1) infection of mice: proliferation of
cell clones with integrated HTLV-1 provirus in lymphoid organs. J
Virol. 75:4420–4423. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Fang J, Kushida S, Feng R, Tanaka M,
Kawamura T, Abe H, Maeda N, Onobori M, Hori M, Uchida K and Miwa M:
Transmission of human T-cell leukemia virus type 1 to mice. J
Virol. 72:3952–3957. 1998.PubMed/NCBI
|
|
13.
|
Miyoshi I, Kubonishi I, Yoshimoto S, Akagi
T, Ohtsuki Y, Shiraishi Y, Nagata K and Hinuma Y: Type C virus
particles in a cord T-cell line derived by co-cultivating normal
human cord leukocytes and human leukaemic T cells. Nature.
294:770–771. 1981. View
Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Kato H, Koya Y, Ohashi T, Hanabuchi S,
Takemura F, Fujii M, Tsujimoto H, Hasegawa A and Kannagi M: Oral
administration of human T-cell leukemia virus type 1 induces immune
unresponsiveness with persistent infection in adult rats. J Virol.
72:7289–7293. 1998.PubMed/NCBI
|
|
15.
|
Persaud D, Munoz JL, Tarsis SL, Parks ES
and Parks WP: Time course and cytokine dependence of human T-cell
lymphotropic virus type 1 T-lymphocyte transformation as revealed
by a microtiter infectivity assay. J Virol. 69:6297–6303. 1995.
|
|
16.
|
Satoh M, Toma H, Sugahara K, Etoh K,
Shiroma Y, Kiyuna S, Takara M, Matsuoka M, Yamaguchi K, Nakada K,
Fujita K, Kojima S, Hori E, Tanaka Y, Kamihira S, Sato Y and
Watanabe T: Involvement of IL-2/IL-2R system activation by parasite
antigen in polyclonal expansion of CD4(+)25(+) HTLV-1-infected
T-cells in human carriers of both HTLV-1 and S. stercoralis.
Oncogene. 21:2466–2475. 2002.PubMed/NCBI
|
|
17.
|
Popovic M, Lange-Wantzin G, Sarin PS, Mann
D and Gallo RC: Transformation of human umbilical cord blood T
cells by human T-cell leukemia/lymphoma virus. Proc Natl Acad Sci
USA. 80:5402–5406. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Imada K, Takaori-Kondo A, Akagi T,
Shimotohno K, Sugamura K, Hattori T, Yamabe H, Okuma M and Uchiyama
T: Tumorigenicity of human T-cell leukemia virus type I-infected
cell lines in severe combined immunodeficient mice and
characterization of the cells proliferating in vivo. Blood.
86:2350–2357. 1995.PubMed/NCBI
|
|
19.
|
Ishihara S, Tachibana N, Okayama A, Murai
K, Tsuda K and Mueller N: Successful graft of HTLV-I-transformed
human T-cells (MT-2) in severe combined immunodeficiency mice
treated with anti-asialo GM-1 antibody. Jpn J Cancer Res.
83:320–323. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Lairmore MD, Silverman L and Ratner L:
Animal models for human T-lymphotropic virus type 1 (HTLV-1)
infection and transformation. Oncogene. 24:6005–6015. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Dewan MZ, Terashima K, Taruishi M,
Hasegawa H, Ito M, Tanaka Y, Mori N, Sata T, Koyanagi Y, Maeda M,
Kubuki Y, Okayama A, Fujii M and Yamamoto N: Rapid tumor formation
of human T-cell leukemia virus type 1-infected cell lines in novel
NOD-SCID/gammac(null) mice: suppression by an inhibitor against
NF-kappaB. J Virol. 77:5286–5294. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Ohsugi T, Horie R, Kumasaka T, Ishida A,
Ishida T, Yamaguchi K, Watanabe T, Umezawa K and Urano T: In vivo
antitumor activity of the NF-kappaB inhibitor
dehydroxymethyl-epoxyquinomicin in a mouse model of adult T-cell
leukemia. Carcinogenesis. 26:1382–1388. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Simpson RM, Zhao TM, Schmidt HB, Said W
and Kindt TJ: Source and route of exposure influence infectivity of
a molecular clone of human T cell leukemia virus type I. AIDS Res
Hum Retroviruses. 14:711–715. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Hasegawa A, Ohashi T, Hanabuchi S, Kato H,
Takemura F, Masuda T and Kannagi M: Expansion of human T-cell
leukemia virus type 1 (HTLV-1) reservoir in orally infected rats:
inverse correlation with HTLV-1-specific cellular immune response.
J Virol. 77:2956–2963. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Jude BA, Pobezinskaya Y, Bishop J, Parke
S, Medzhitov RM, Chervonsky AV and Golovkina TV: Subversion of the
innate immune system by a retrovirus. Nat Immunol. 4:573–578. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Panoutsakopoulou V, Little CS, Sieck TG,
Blankenhorn EP and Blank KJ: Differences in the immune response
during the acute phase of E-55+ murine leukemia virus
infection in progressor BALB and long term nonprogressor C57BL
mice. J Immunol. 161:17–26. 1998.PubMed/NCBI
|
|
27.
|
Osame M, Janssen R, Kubota H, Nishitani H,
Igata A, Nagataki S, Mori M, Goto I, Shimabukuro H and Khabbaz R:
Nationwide survey of HTLV-1-associated myelopathy in Japan:
association with blood transfusion. Ann Neurol. 28:50–56. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Jones KS, Akel S, Petrow-Sadowski C, Huang
Y, Bertolette DC and Ruscetti FW: Induction of human T cell
leukemia virus type I receptors on quiescent naive T lymphocytes by
TGF-beta. J Immunol. 174:4262–4270. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Moriuchi M and Moriuchi H: A milk protein
lactoferrin enhances human T cell leukemia virus type I and
suppresses HIV-1 infection. J Immunol. 166:4231–4236. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Moriuchi M and Moriuchi H: Transforming
growth factor-beta enhances human T-cell leukemia virus type I
infection. J Med Virol. 67:427–430. 2002. View Article : Google Scholar : PubMed/NCBI
|