Introduction
Breast cancer is the most common type of cancer
among women, and a number of treatment modalities have been
aggressively investigated (1). As
with cancers of other organs, the treatment of breast cancer
patients has recently become more personalized through the detailed
analyses of various biomarkers, such as hormone receptors (estrogen
and progesterone receptor), HER-2 and other risk factors (2,3).
However, triple-negative type breast cancer (accounting for 13% of
all breast cancers) is insensitive to both endocrine and anti-HER-2
therapy and, as its prognosis is worse than those of other types of
breast cancers (3,4), suitable chemotherapy regimens are
required. Lung metastasis often causes the death of patients
suffering from breast cancer (1),
but a lung metastasis model in mice has not been widely
established.
We previously established a hormone-insensitive
breast cancer cell line, MDA-MB-435SHM, that spontaneously
metastasized to the lung after surgical extraction (5). However, its parent cell line,
MDA-MB-435, was a melanoma, not a breast cancer cell line (6). Therefore, we newly established a
human breast cancer cell line, MDA-MB-231LLM, from MDA-MB-231,
which originates from a triple-negative type human breast cancer
cell line, and performed a preliminary evaluation of
anti-metastatic activity based on the survival period after
treatment with S-1, which is commonly used against breast cancers
(8,9).
Materials and methods
Cell lines and cultures
A triple-negative type human breast cancer cell
line, MDA-MB-231 (7), was
purchased from the American Type Culture Collection (Manassas, VA,
USA). The parent MDA-MB-231 cells were routinely cultured in a
monolayer or on a microcarrier (Cytodex™ 3) in RPMI-1640 medium
supplemented with 10% fetal calf serum (FCS) at 37°C under 5%
CO2 and 100% humidity in vitro.
Reagents
FCS, BD Matrigel™ matrix (11) and Cytodex 3 were purchased from JRH
Bioscience (Lenexa, KS, USA), BD Biosciences (Bedford, MA, USA) and
GE Healthcare UK Ltd. (Amersham Place, UK), respectively.
Somunopentyl injection (sodium pentobarbital) was purchased from
Kyoritsu Seiyaku Corp. (Tokyo, Japan). Tegafur, gimeracil and
oteracil were synthesized in our laboratory. S-1 was prepared by
combining tegafur, gimeracil and oteracil at a molar ratio of
1:0.4:1 in 0.5% hydroxypropyl methylcellulose (HPMC).
The other reagents were commercially available
products of the highest grade.
Animals and establishment of the
spontaneous lung metastasis subline, MDA-MB-231LLM
The animal studies were performed according to the
guidelines and with the approval of the Institutional Animal Care
and Use Committee of Taiho Pharmaceutical Co., Ltd. Female
C.B.17/Icr SCID mice (SCID mice) (5 weeks old) and BALB/c nu/nu
mice (nude mice) were purchased from CLEA Japan Inc. (Tokyo,
Japan). The mice were housed under specific pathogen-free
conditions, and food and water were provided ad libitum.
MDA-MB-231 cultured cells were collected and
suspended in saline or 50% BD-Matrigel. MDA-MB-231 cells
(2×106 cells/body) suspended in saline were injected
intravenously, and cells suspended in 50% Matrigel or co-cultured
using Cytodex 3 were implanted subcutaneously into the mammary fat
pads (mfp) of 5-week-old female SCID mice (n=15). Metastasis nodes
in the lung were collected 42 days after the intravenous
implantation of MDA-MB-231, and 8-mm3 cubic fragments
were implanted subcutaneously into the mfp of 5-week-old female
SCID mice. The implanted tumors were extracted surgically under
anesthesia with Somunopentyl at 6 weeks after implantation, and the
metastasis nodes in the lung were collected 6–7 weeks after tumor
resection. This procedure was repeated once again, and the
spontaneous lung metastasis subline, MDA-MB-231LLM, was
established.
Evaluation of anti-metastatic activity
and lifespan of mice treated with S-1
The metastasis nodes of MDA-MB-231LLM were collected
from the lungs and implanted subcutaneously into male nude mice,
and solid tumors were prepared. An 8-mm3 cubic fragment
of MDA-MB-231LLM was implanted into the mfp of a female SCID mouse
after 1 week of quarantine, and 27 days after implantation the
tumor was extracted under anesthesia with Somunopentyl and its
weight was measured. Seven days after extraction, the mice with no
obvious tumor recurrence were randomized according to the extracted
tumor weight (day 0).
Evaluation of the anti-metastatic activity.
The mean of the resected tumor weights was ∼1.9 g. S-1 at a dosage
of 10 mg/ kg, which has been reported to be an effective dose with
low toxicity (8), or the vehicle
(10 ml/kg) was orally administered for 28 consecutive days from day
1 (n=8). For reference, the vehicle was also administered in a
similar manner to non-tumor-bearing mice (n=8). On day 29, the mice
were sacrificed by bleeding under anesthesia, and the whole lungs
were collected and weighed.
Evaluation of the survival period. The mean
of the resected tumor weights was ∼1.3 g. S-1 (10 mg/kg) was orally
administered for 14 or 28 consecutive days from day 1 (n=9). In the
control group, the vehicle was administered in a similar manner.
The survival period of the mice was observed, and lung metastasis
was confirmed macroscopically. The increase in the life span (ILS)
was calculated using the following formula: ILS = [(median survival
period of the treated group)/ (median survival period of the
control group) - 1] × 100%.
Statistical analysis
The anti-metastatic activity was analyzed based on
the mean of the whole lung weight between the control, treated and
normal groups on day 29 using a generalized two-tailed Wilcoxon
test. Significant differences in the survival periods between the
treated and control groups were analyzed using a log-rank test with
EXSAS Ver. 7.11 (Arm Systex Co., Ltd., Osaka, Japan).
Results
Suitable implantation route for
establishing a selective lung metastasis subline
Lung metastasis was observed in 11/15 female SCID
mice after intravenous implantation, but no metastasis was observed
after implantation using Matrigel, and only 1/15 mice exhibited
metastasis after implantation using Cytodex 3. Therefore, lung
metastasis nodes developing after intravenous implantation were
collected and implanted into the mfp of female SCID mice. Next, the
incidence of lung metastasis was compared between a primary
tumor-resected group and a not-resected group. The incidences of
lung metastasis were 90 and 40% in the resected and notresected
groups, respectively. As in the following experiment, lung
metastasis was observed in the SCID mice in which a lung metastasis
node had been implanted into the mfp after surgical resection, and
the resulting lung metastasis nodes were defined as the metastatic
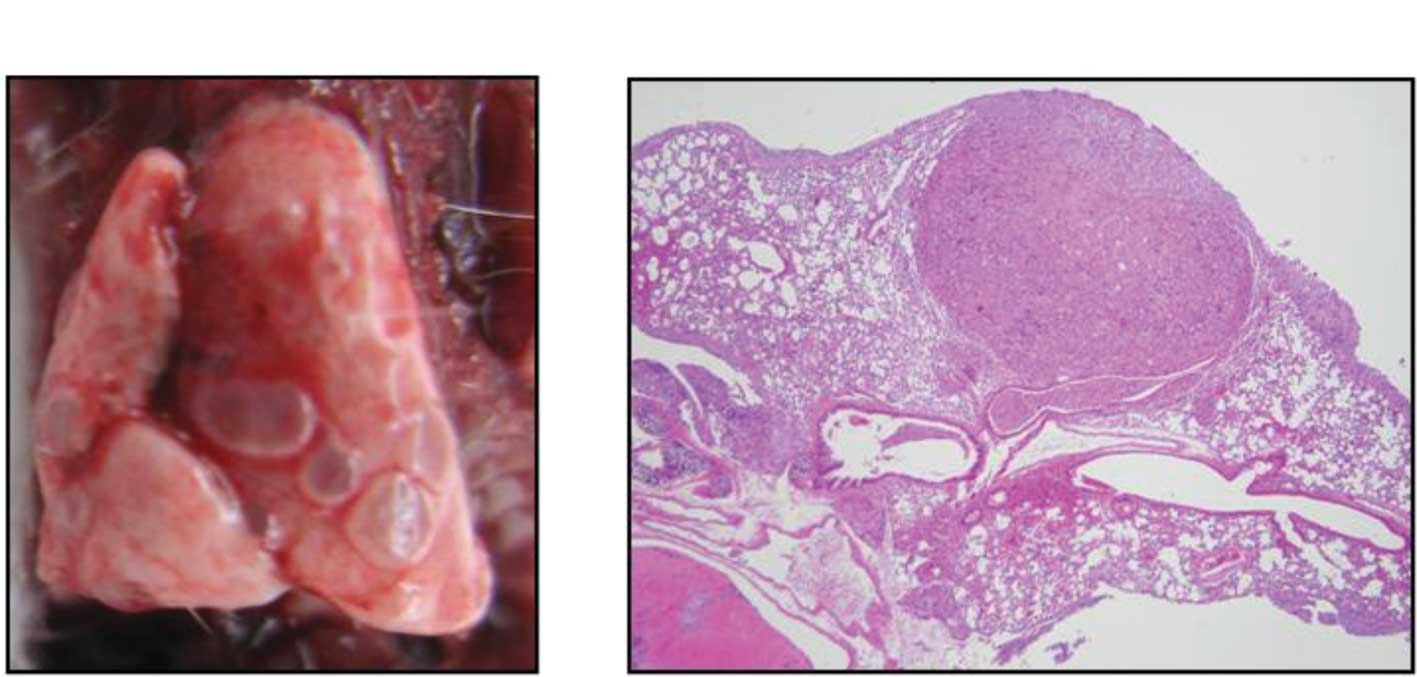
subline, MDA-MB-231LLM. Fig. 1
shows a typical pulmonary metastasis of a MDA-MB-231LLM tumor both
macroscopically and after H&E staining. Metastatic nodes were
observed around the vessels in the pulmonary alveoli.
When nude mice were used, no lung metastasis in the
whole lung was observed.
Evaluation of anti-metastatic activity of
S-1
For the mice in the control group metastases were
observed in the whole lung and, prior to the final evaluation, 3/8
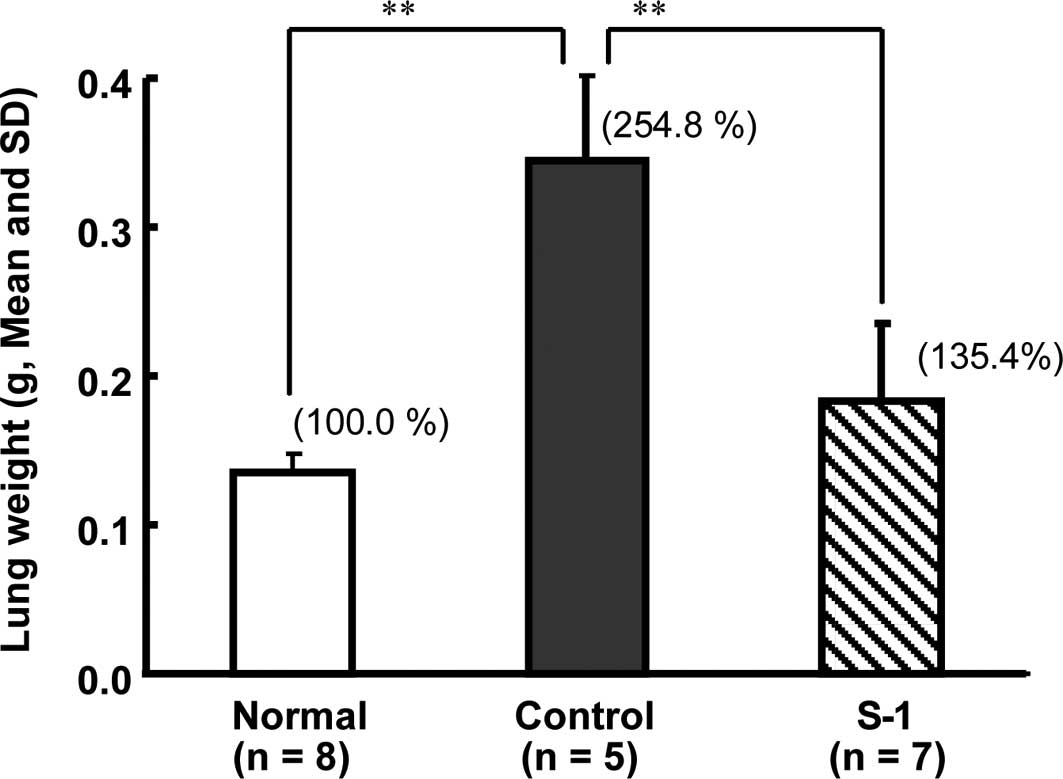
mice had died as a result of lung dysfunction. On day 29, the lung
weight of the control group (0.344±0.057 g, n=5) was significantly
(P<0.01) higher than that of the normal group (0.135±0.013 g,
n=8; Fig. 2A). Among the mice
treated with S-1, the number of metastasis nodes was smaller, and
the lung weight (0.183±0.052 g, n=7) was significantly (P<0.01)
lower than that of the control group (Figs. 2B and 3).
Evaluation of the survival period after
treatment with S-1
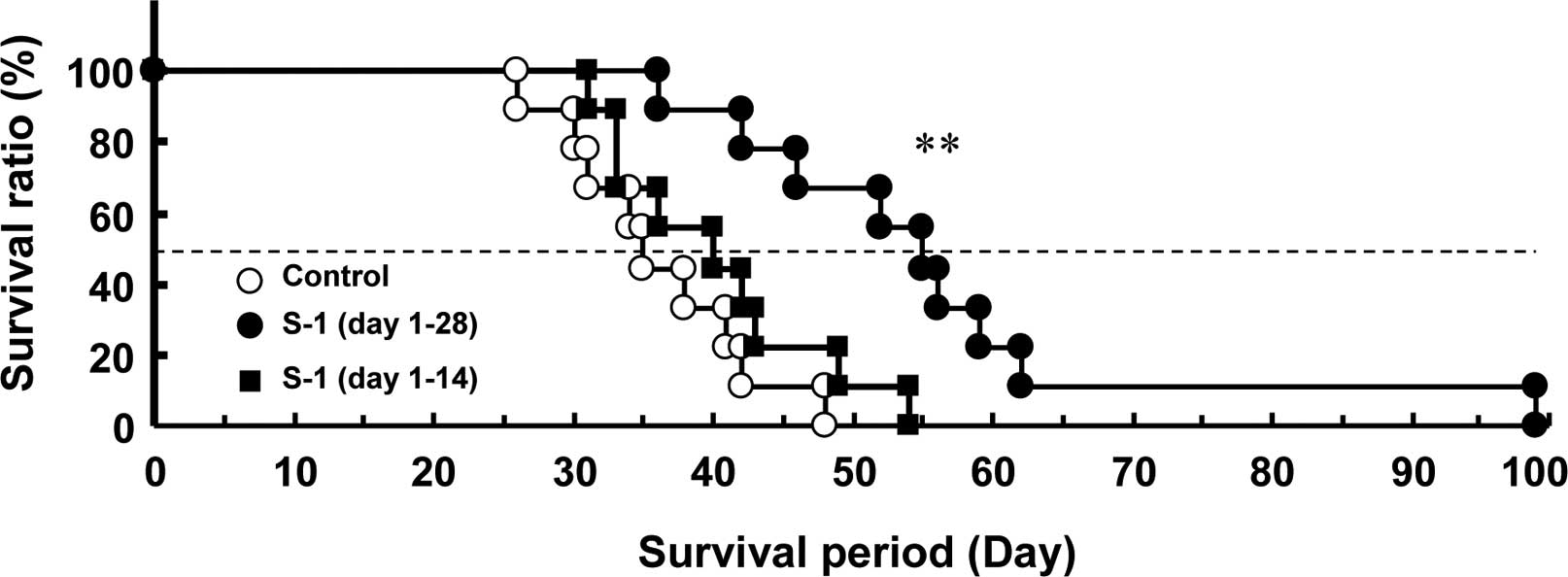
A Kaplan-Meier survival curve was plotted for the
mice. The median survival period of the control mice was 35 days,
and all of the mice died as a result of lung dysfunction after 48
days. The survival period of the 28-day and 14-day consecutive S-1
administration groups was 55 days (P<0.01) and 40 days,
respectively (Fig. 4 and Table I).
 | Table I.The median survival period of the
control and S-1-treated mice. |
Table I.
The median survival period of the
control and S-1-treated mice.
| Group | Median survival
period (days) | ILS (%) |
|---|
| Control | 35.0 | 0.0 |
| S-1 (10 mg/kg, days
1–28) | 55.0a | 57.1 |
| S-1 (10 mg/kg, days
1–14) | 40.0 | 14.3 |
Discussion
Standard chemotherapy against triple-negative type
breast cancer is warranted. Since the prognosis of breast cancer
with lung metastasis is poor (1),
effective chemotherapy against metastatic triple-negative type
breast cancer is required. Using human xenograft models, a number
of chemotherapeutic agents against human breast cancers have been
evaluated. However, the antitumor activity against human breast
cancers other than those which have been subcutaneously implanted
is not sufficient. Since the microenvironment of the organ in which
tumor cells metastasize and grow is thought to be important
(12), a suitable implantation
route was first examined. The incidence of lung metastasis after
the implantation of the parental MDA-MB-231 cell line into the mfp
using Matrigel or Cytodex 3 was lower than that after intravenous
implantation. Since a subline that grows in a distant organ is
thought to possess a different affinity from those of other
variants (13), lung metastasis
nodes following intravenous implantation were collected, and the
above-described procedure was repeated an additional two times. As
a result, the highly selective lung metastasis subline,
MDA-MB-231LLM, was established within a shorter period than that
described in previous reports (5,14).
Consequently, the selection of the route of first implantation may
be important.
The control of micro-metastasis is a critical
problem for prolonging the progression-free survival and overall
survival periods of patients. Therefore, we attempted to examine
the possibility of evaluating chemotherapeutic agents using this
model based on the survival period. As the parent MDA-MB-231 cell
line is reported to possess a high level of dihydropyrimidine
dehydrogenase (DPD) activity (16), we measured the DPD activity in
MDA-MB-231LLM and confirmed a high enzyme activity (data not
shown). The anti-metastatic activity of S-1, which inhibits DPD,
has been reported to be effective in an adjuvant setting (17–19);
consequently, the efficacy of S-1 was evaluated using this model.
Long-term S-1 treatment from the early stage, when no macroscopic
metastasis was observed, contributed to a prolonged survival
period. These data suggest that this MDA-MB-231LLM orthotropic
implantation model may be useful for evaluating the anti-metastatic
activity of drugs in mice based on the survival period in addition
to the lung weight.
Acknowledgements
We thank Fumio Nakagawa, Katsuhiko
Izumi, Yukari Yamada and Minoru Sakata for the technical assistance
in this experiment.
References
|
1.
|
Chaudary MA: Patterns and recurrence in
western and Japanese women with breast cancer. Breast Cancer Res
Treat. 18:S115–S118. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Early Breast Cancer Trialists’
Collaborative Group: Tamoxifen for early breast cancer: an overview
of the randomised trials. Lancet. 351:1451–1467. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Spitale A, Mazzola P, Soldini D, et al: A
breast cancer classification according to immunohistochemical
markers: clinicopathologic features and short-term survival
analysis in a population-based study from the South of Switzerland.
Ann Oncol. 20:628–635. 2009. View Article : Google Scholar
|
|
4.
|
Brandes AA, Franceschi E, Tosoni A, et al:
Trastuzumab and lapatinib beyond trastuzumab progression for
metastatic breast cancer: strategies and pitfalls. Expert Rev
Anticancer Ther. 10:179–184. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Nukatsuka M, Fujioka A, Nakagawa F, et al:
Antimetastatic and anticancer activity of S-1, a new oral
dihydropyrimidinedehydrogenase-inhibiting fluoropyrimidine, alone
and in combination with paclitaxel in an orthotopically implanted
human breast cancer model. Int J Oncol. 25:1531–1536. 2004.
|
|
6.
|
Rae JM, Creighton CJ, Meck JM, et al: MD
MDA-MB-435 cells are derived from M14 melanoma cells – a loss for
breast cancer, but a boon for melanoma research. Breast Cancer Res
Treat. 104:13–19. 2007.PubMed/NCBI
|
|
7.
|
Cailleau R, Young R, Olivé M and Reeves WJ
Jr: Breast tumor cell lines from pleural effusions. J Natl Cancer
Inst. 53:661–674. 1974.PubMed/NCBI
|
|
8.
|
Fukushima M, Satake H, Uchida J, et al:
Preclinical antitumor efficacy of S-1: a new oral formulation of
5-fluorouracil on human tumor xenografts. Int J Oncol. 13:693–698.
1998.PubMed/NCBI
|
|
9.
|
Saeki T, Takashima S, Sano M, et al: A
phase II study of S-1 in patients with metastatic breast cancer – a
Japanese trial by the S-1 Cooperative Study Group, Breast Cancer
Working Group. Breast Cancer. 11:194–202. 2002.
|
|
10.
|
Shien T, Shimizu C, Akashi-Tanaka S, et
al: Clinical efficacy of S-1 in pretreated metastatic breast cancer
patients. Jpn J Clin Oncol. 38:172–175. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Price JE, Polyzos A, Zhang RD, et al:
Tumorigenicity and metastasis of human breast carcinoma cell lines
in nude mice. Cancer Res. 50:717–721. 1990.PubMed/NCBI
|
|
12.
|
Kleinman HK, McGarvey ML, Liotta LA, et
al: Isolation and characterization of type IV procollagen, laminin,
and heparan sulfate proteoglycan from the EHS sarcoma. Biochem.
21:6188–6193. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Fidler IJ: Rationale and methods for the
use of nude mice to study the biology and therapy of human cancer
metastasis. Cancer Metastasis Rev. 5:29–49. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Paget S: The distribution of secondary
growths in cancer of the breast. Cancer Metastasis Rev. 8:98–101.
1989.PubMed/NCBI
|
|
15.
|
Munoz R, Man S, Shaked Y, et al: Highly
efficacious nontoxic preclinical treatment for advanced metastatic
breast cancer using combination oral UFT-cyclophosphamide
metronomic chemotherapy. Cancer Res. 66:3386–3391. 2006. View Article : Google Scholar
|
|
16.
|
Ishikawa T, Sekiguchi F, Fukase Y, et al:
Positive correlation between the efficacy of capecitabine and
doxifluridine and the ratio of thymidine phosphorylase to
dihydropyrimidine dehydrogenase activities in tumors in human
cancer xenografts. Cancer Res. 58:685–690. 1998.
|
|
17.
|
Ajani JA, Rodriguez W, Bodoky G, et al:
Multicenter phase III comparison of cisplatin/S-1 with
cisplatin/infusional fluorouracil in advanced gastric or
gastroesophageal adenocarcinoma study: the FLAGS trial. J Clin
Oncol. 28:1547–1553. 2010. View Article : Google Scholar
|
|
18.
|
Ito Y, Nakanishi H, Kodera Y, et al:
Characterization of a novel lymph node metastasis model from human
colonic cancer and its preclinical use for comparison of
anti-metastatic efficacy between oral S-1 and UFT/ LV. Cancer Sci.
101:1853–1860. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Van den Brande J, Schöffski P, Schellens
JH, et al: EORTC Early Clinical Studies Group early phase II trial
of S-1 in patients with advanced or metastatic colorectal cancer.
Br J Cancer. 88:648–653. 2003.
|