Introduction
Cancer is a progressive disease that affects many
types of tissues, as well as organs, in the human body (1). Cancer progression and metastasis is a
complicated process. Cells move through the blood and invade
neighboring tissue by degrading the extracellular matrix (ECM)
(2). In these multiprocesses,
angiogenesis plays a pivotal role in the formation of new blood
vessels in the newly colonized areas, and thus in the formation of
additional metastatic lesions (3,4). The
ECM includes a wide variety of proteins, which can be degraded by
many matrix metalloproteinases (MMPs). MMPs are a family of highly
conserved zinc-dependent peptidases capable of degrading the ECM
(3).
Anti-tumor agents have been shown to inhibit MMP-9
activity in the ECM via the binding of the NF-κB transcription
factor with the MMP-9 promoter (5). This suggests that anti-tumor agents
play a role in anti-invasion and/or anti-angiogenesis, as well as
in caspase-3 inhibition (6). Cell
motility, and in particular cell migration, affect tumor
progression and metastasis (7).
The signaling pathways responsible for regulating cell motility
involve various nuclear transcription factors, as well as
morphological changes in the cytoskeleton that modify interactions
among membrane- and ECM-bound proteins (e.g., cdc, Rho and Rac
family proteins), which mediate cellular and membrane morphology by
controlling the polymerization-depolymerization process of the
actin cytoskeleton (8).
Cordyceps and Paecilomyces sp. are
popular entomopathogenic fungi, which have been broadly called
DongChungHaCho (winter worm summer grass) in Korea (8–11).
Paecilomyces sp. has been investigated for industrial
purposes in order to be cultivated on a large scale. However, the
precise biological mechanisms of functionality and bioavailability
require investigation.
In this study, we show that Paecilomyces
farinosus J3, which was collected and isolated from Mansusan,
Korea, decreases cell migration, invasion and tube formation,
suggesting that this activity may have originated from the pathway
of MMP-9 inhibition. Our findings, in combination with previous
studies, strongly suggest that Paecilomyces farinosus J3
should be considered a good candidate for development into an
anti-tumor therapeutic agent, as well as in anti-tumor preventive
medicine, provided that MMP-9 is the major molecular target
molecule for angiogenesis and tumor progression.
Materials and methods
Culture and separation of Paecilomyces
sp
The strain Paecilomyces farinosus J3 was
collected from Mansusan and deposited in the Collection Center of
the Department of Agricultural Biology, National Advanced Institute
of Science and Technology, RDA, Suwon, Korea. The strain was
cultured and re-isolated in PDA medium (24 g potato dextrose, 15 g
agar and 1 l distilled water) at 25°C for 2 weeks. After sufficient
maturation of the mycelial growth, the culture supernatants were
centrifuged and divided into mycelial extract and culture filtrate.
The culture filtrate was then freeze-dried under a vacuum
evaporator. The freeze-dried powder of culture filtrate was
dissolved in distilled water prior to use (12).
Cell culture
The mouse melanoma cell line B16F10 (B16; Catalog
#CRL-6323) and human umbilical vein endothelial cells (HUVECs) were
purchased from the American Type Culture Collection (ATCC;
Manassas, VA, USA). The B16F10 cells were cultured in RPMI-1640
medium supplemented with 10% FBS, while the HUVECs were cultured in
EGM-2 medium (BD Biosciences, Lake Placid, NJ, USA). The B16F10
cells were maintained in RPMI-1640 medium and subcultured by
trypsinization at 3- to 4-day intervals. The HUVECs were
subcultured every 3–4 days and were used for experiments at
passages 3–10 (13).
Wound healing assay
The strips of sterilized thin film tape (2 mm x 2
cm; 3M, Seoul, Korea) were attached to the bottom of each well of
6-well plates (Greiner, Frickenhausen, Germany), and the B16 cells
were split into plates at a concentration of 1×107
cells/well, and allowed to grow for 6 h at 37°C in a 5%
CO2 atmosphere. The tape strips were then detached,
creating linear wounds. The plates were photographed and incubated
as described above with medium containing various concentrations of
J3 (0–100 mg/ml). The plates were photographed at 24 h and the
precise wound width was calculated by use of a microruler
(http://www.eeob.iastate.edu/faculty/DrewsC/htdocs/microruler-links.htm)
as described previously (14).
Invasion assay
Transwell plates (pore size 8 μm; Costar, NY,
USA) were loaded with 100 μl Matrigel (BD Biosciences),
allowed to solidify at 37°C for 2 h and then coated with 10
μl of fibronectin (200 μg/ml). The plates were loaded
with B16 cells suspended in 10% FBS (1x105 cells/well),
the samples were exposed to J3 (0–100 mg/ml) and the plates were
incubated at 37°C in 5% CO2 for 24 h. The migrated cells
were fixed with methanol, stained with hematoxylin and counted
under a microscope (15).
Tube formation assay
HUVECs (2x104 cells/well) were added to
the Matrigel-coated 24-well plates in 0.5 ml of EGM-2 medium with
various concentrations of J3 (0–100 mg/ml) and incubated for 24 h.
The cells were then visualized under a microscope and tube
formation was scored by counting the number of tubes formed
(14,15).
Gelatin zymography
Gelatin zymography was performed as described
previously (16). Proteins with
gelatinolytic activity were identified by electrophoresis in the
presence of SDS on 8% polyacrylamide gels containing 0.1% (w/v)
gelatin. In brief, cell culture medium was obtained from cultured
B16F10 cells in DMEM and PMA (100 ng), with or without different
concentrations of J3 for 24 h in 6-well culture dishes. The
cultured medium was mixed with an SDS-PAGE sample buffer in the
absence of β-mercaptoethanol and DTT. Five microliters of sample
were loaded onto a gel and electro-phoresed. After electrophoresis,
the gel was renatured twice for 30 min at room temperature in 2.5%
(v/v) Triton X-100, and subsequently transferred onto a
zymogram-developing buffer containing 50 mM Tris-HCl, 5 mM
CaCl2, 0.2 M NaCl and 0.02% Brij 35 at 37°C overnight.
After staining with Coomassie Blue R-250 (0.25%) for 30 min, the
gel was then distained in a distaining solution [methanol:acetic
acid:water (50:10:40)]. A clear band was detected and quantified
using the BioRad GelDoc system.
Statistical analysis
Data are expressed as the means ± standard deviation
(SD). Statistical significance was determined by the
Student-Newman-Keuls method for independent means, using the Sigma
Plot program (17). The critical
level of significance was set at P<0.05.
Results
In a previous study, we developed various
cultivation methods and cultivated various insect pathogenic fungi,
including Paecilomyces sp. (DongChungHaCho in Korean). The
morphology of the selected strain has a classical feature of
mycelial growth that is common in entomopathogenic fungi (data not
shown). In this study, we optimized the maintenance and culture
medium of Paecilomyces farinosus J3 (data not shown), and
investigated the biological activities of the culture filtrate of
that strain. Few strains have been studied for their anti-tumor
activities and their efficacy as culture filtrates.
We first examined the tumor cell motility of
J3-treated B16 cells. We used a wound healing assay to assess the
in vitro effect of J3 on tumor cell migration, which is an
important phenomenon of cancer cell motility, particularly in
relation to metastatic cancer. The wound healing assay showed that
the monolayers treated with 10 mg/ml of J3 displayed a clear wound
width, while the untreated control monolayers exhibited complete
wound healing within 24 h, indicating that at J3 partially inhibits
cell migration in vitro in a dose-dependent manner (data not
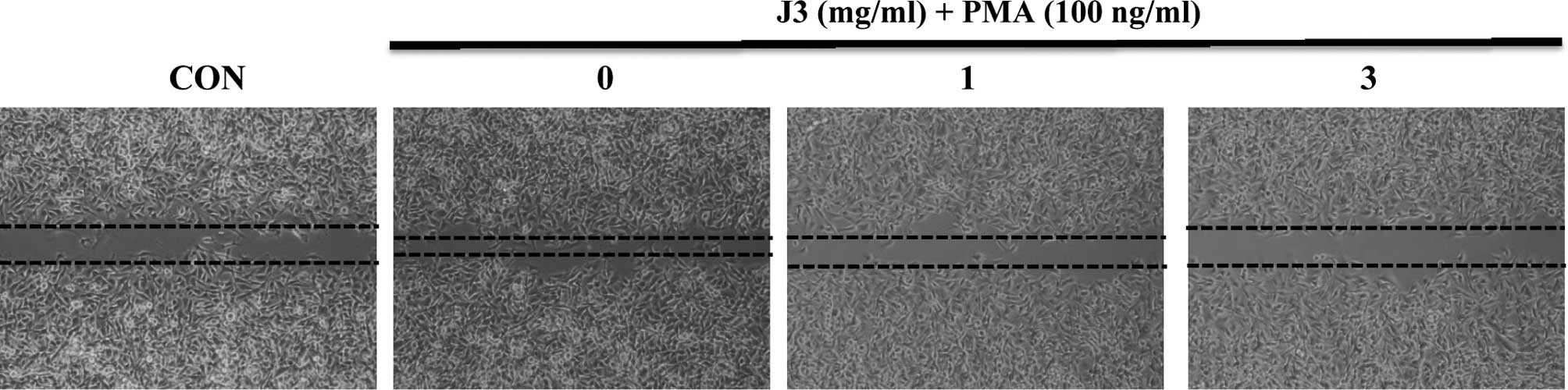
shown). Notably, the PMA-treated mono-layers also showed a cell
growth inhibitory pattern (Fig. 1,
2nd to 4th lower images) as did the control, suggesting that J3
inhibited cell migration activity with or without a cell
proliferation inducer. Similarly, the invasion assay revealed that
J3 treatment (10 mg/ml) inhibited invasive activity by >40% vs.
the control group (Fig. 2A). These
results indicate that J3 dose-dependently alleviates wound healing
and inhibits invasion in vitro in a concentration-dependent
manner (Fig. 2B).
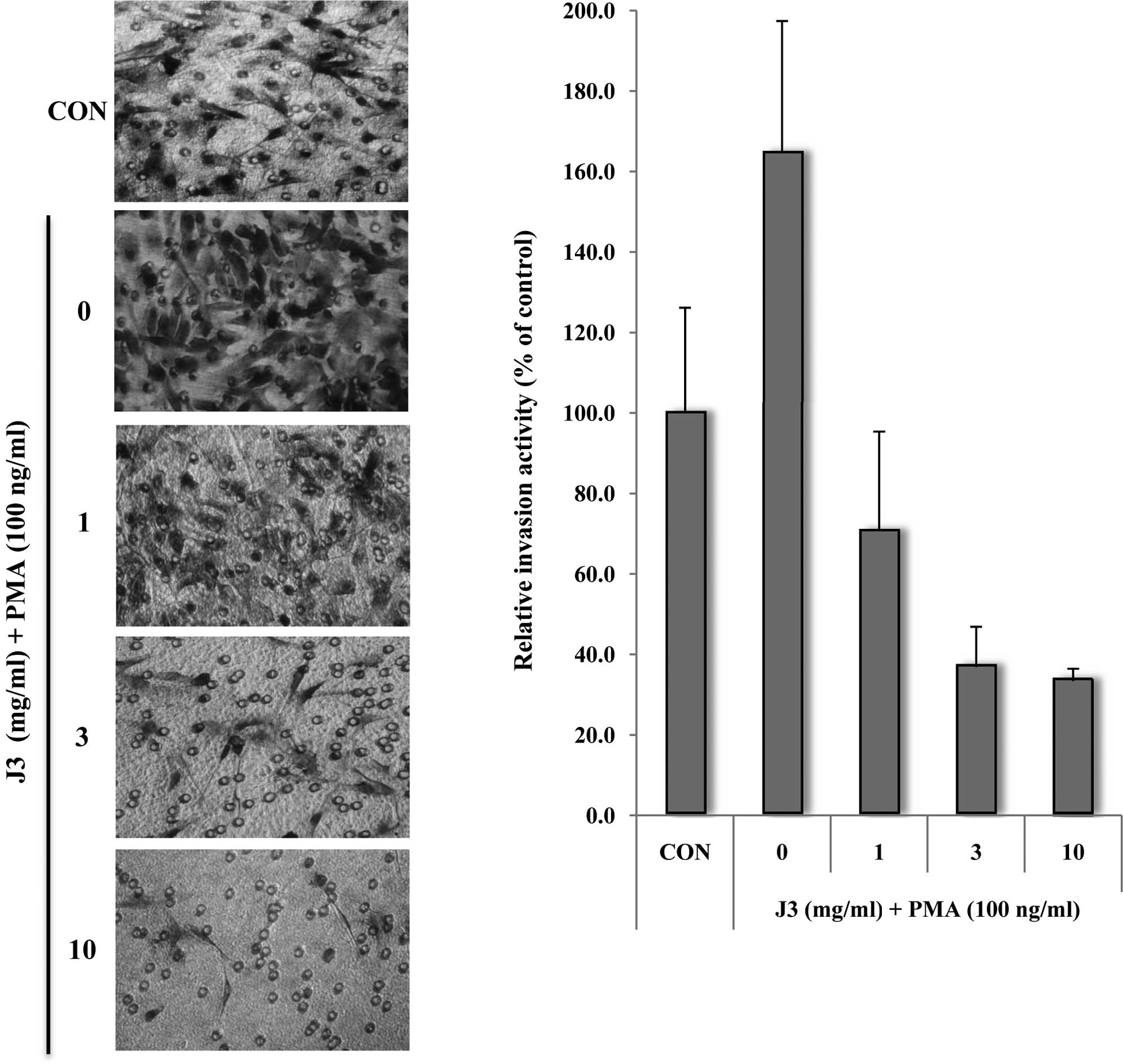
We then investigated the effect of J3 on
angiogenesis, which is a key feature of metastasis. HUVECs were
treated with or without various concentrations of J3, and tube
formation was measured in terms of tube size and number. The data
demonstrated that J3 inhibits HUVEC tube formation in vitro
in a dose-dependent manner (Fig.
3; 0.1–3 mg/ml). Notably, the cell tubular numbers were
dramatically decreased by various doses of J3 (Fig. 3B, compare columns 2 and 5).
Collectively, these findings indicate that J3 inhibits cell
migration, cell invasiveness and tube formation in vitro,
suggesting that this extract can function as an anti-tumor
component in vitro by decreasing metastatic and/or
angiogenic potential.
 | Figure 3.Paecilomyces farinosus J3
alleviates HUVEC tube formation on Matrigel. (A) HUVECs were plated
at 2x104 cells/well in a Matrigel-coated 24-well plate,
and then exposed to 0, 0.1, 0.3, 1 or 3 mg/ml of Paecilomyces
farinosus J3 culture filtrate. After 24 h, the culture medium
was removed and the cells were fixed with 10% formalin. The cell
morphology infiltrated into the Matrigel (A), and the relative
tubular numbers (B) of the formed tubes were calculated. Bar, 10
μm. The results are shown as the average per field ± SD of
three independent experiments. CON, control.
*Significant difference from the control
(Paecilomyces farinosus J3, 0 mg/ml), P<0.05. |
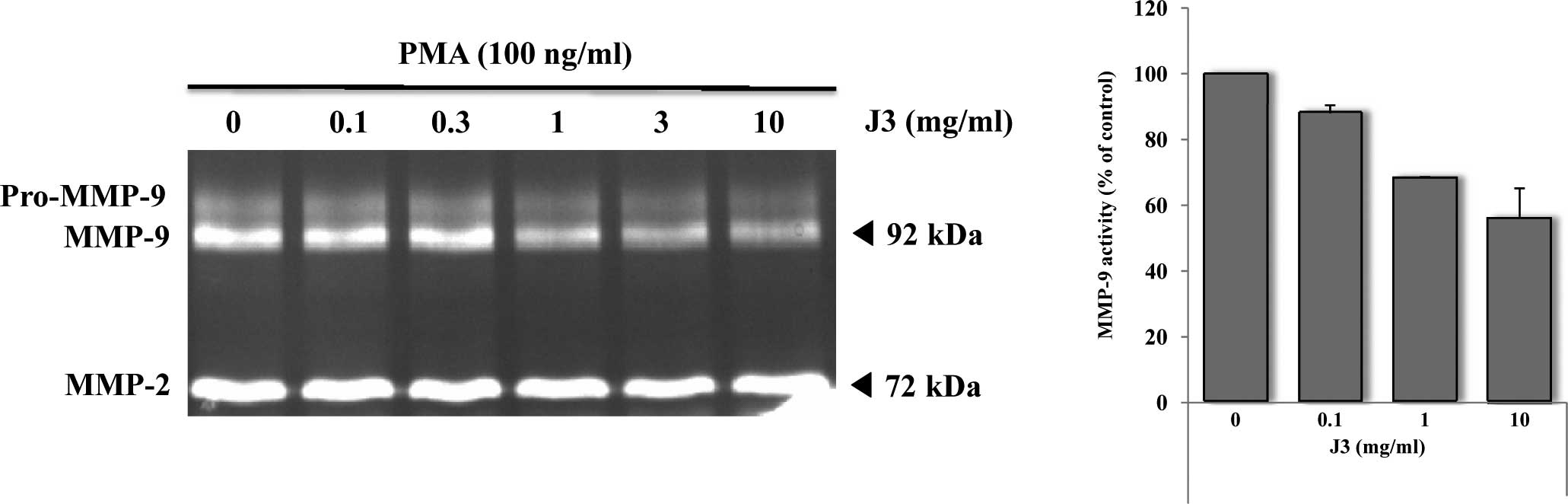
Zymographic analysis revealed that the treatment of
cells with 0.1–10 mg/ml of J3 inhibited gelatinase (MMP-9; 92 kDa)
by ∼45% (Fig. 4A), suggesting that
J3 has a strong potential to hamper the secretion of the active
form of MMP-9 rather than the activity level per se. The
relative MMP-9 activity reached a level of 55% of the control at a
concentration of 10 mg/ml (Fig.
4B). MMP-2 activity did not change by J3 treatment with or
without PMA (Fig. 4B; data not
shown). Additionally, the expression of other MMPs, as well as
other tumor-related molecules (uPAR, Timp-1, Timp-2, Paxillin, Src
and ARF-2), did not change after treatment with J3 (data not
shown). However, the precise up- and down-stream signaling pathway
involved needs to be investigated.
Discussion
Traditional medicine utilizingplant and/or herbal
extracts is believed to inhibit cancer cell growth and control the
homeostasis of malignant cells in tissues or organs (1,2). For
many years, herbal extracts have been used as functional food
materials in Oriental medicine, in order to maintain homeostasis in
the body and to mitigate the symptoms of degenerative diseases
(18,19). Moreover, the tyrosinase-inhibitory
activity of Cordyceps sp. extracts leads to the suppression
of melanin production (20); these
extracts therefore have a potential use in cosmetics. Additionally,
the anti-depressant, anti-oxidative, anti-hepatitic, anti-diabetic
and anti-aging activities of various extracts of Cordyceps
sp. lead to a wide range of bioactivities in vitro, as
well as in vivo (21–25).
Paecilomyces sp. is also a type of entomogeneous fungus. The
bioavailability and functionality of Paecilomyces sp. are
more useful and potent than those of Cordyceps sp., in that
the strain has beneficial effects on artificial cultivation and
usefulness in host infections (26–29).
This study was undertaken to investigate the anti-tumor effects of
the culture filtrate of Paecilomyces farinosus J3. The
results of a wound healing assay, invasion assay, HUVEC assay and
gelatin zymography indicated that J3 inhibited B16 melanoma cell
growth, thus demonstrating its anti-tumor potential. We therefore
sought to examine the mechanisms involved in this cell growth
inhibition by J3.
First, we examined the effect of the culture
filtrate of J3 on cell migration using a wound healing assay in
B16F10 melanoma cells. This cell line is a good model for the
assessment of cell morphology, motility and proliferation (13–15).
B16 cells migrated into the damaged wound, suggesting that
migration is associated with various events in the cytoskeletal
structure, such as actin polymerization and changes in the cell
motility-related framework (30).
J3 exerted a dose- and time-dependent inhibition of wound healing
in B16 cells. This phenomenon strongly suggests that certain
components in J3 are able to restrict cell motility, by means of
cytoskeletal change and/or morphological features.
We investigated whether J3 can influence HUVEC
differentiation into capillary-like tube formation. As metastatic
potential is characterized by endothelial cell differentiation,
in vitro angiogenic assays were performed to assess this
potential with HUVECs by incubating the cells for 24 h with J3 on
Matrigel. As capillary-like structures were observed in the HUVECs
in the control, we attempted to confirm this by treatment with J3.
As shown in Fig. 3A, in cell
morphology the J3-treated HUVECs was markedly decreased in a
dose-dependent manner, suggesting that the tube formation was
affected by J3 in a concentration- and time-dependent manner
(Fig. 3B and data not shown).
Invasion assay are well-recognized in vitro
angiogenic method for assessing invasion in B16F10 melanoma cells.
Fig. 2 elucidates the J3
dose-dependent inhibition of cell invasion in B16F10 melanoma cells
on Matrigel in a transwell assay. PMA-activated B16F10 cells
underwent a 1.6-fold growth increase compared to the PMA
non-treated control cells, whereas J3-treated cells (10 mg/ml) were
inhibited via the process of the breakdown of the matrix at a level
of 35% compared to the control, and a level of 21% compared to the
PMA-treated cells, although the activity was milder than that of 50
μM dykellic acid (5,6; data not shown).
There are many types of matrix-degrading proteases
in numerous types of cells (3). Of
these, gelatinase is now well-known for its role in angiogenesis
development, as the enzyme is activated during its passage through
the blood vessels when tumor cells are under hypoxic conditions
(4). We therefore investigated the
effects of J3 on MMPs. RT-PCR analysis of MMP-1 to -28 revealed
that only the expression of MMP-9 was reduced (data not shown). In
Fig. 4A, by treatment with J3, the
MMP-9 level (92 kDa) was decreased in a dose-dependent manner,
whereas the MMP-2 level was not. B16F10 melanoma cells did not
secrete their pro-MMP-9 form into the medium, and this was not
detected in the zymogram (Fig. 4A,
above each 92 kDa band). We previously showed that Oriental herbal
medicines exhibit various anti-tumor activities. As chemicals from
herbal extracts, such as dykellic acid (5,6),
methylselenol (31), gall extract
of Wisteria floribunda (13), methylene chloride fraction of
Geum japonicum Thunberg (14) and aqueous extract of Gastrodia
elata Blume (15) are
potentially toxic to tissues or organs, we sought to identify
natural substances or food-borne biomaterial(s). Among the
candidates is J3, since this fungus has no toxicity, as shown by
animal experiments and single dose acute/chronic acute toxicity
tests (data not shown).
In conclusion, we herein demonstrate that
Paecilomyces farinosus J3 dose-dependently inhibits B16 cell
migration and motility, and inhibits HUVEC tube formation in
vitro, resulting in decreased levels of MMP-9. These findings,
in combination with those of previous studies indicating that J3
inhibits a PMA-induced increase in MMP-9 expression, suggest that
J3 may be a promising candidate for future development as an
anti-tumor agent.
Acknowledgements
This study was supported by the
BioGreen 21 (Agenda) Projects, RDA, Korea
(2009101-060-005-001-02-00), and also in part by the Technology
Development Program for Agriculture and Forestry, The Ministry of
Food, Agriculture, Forestry, and Fisheries, Korea (on
anti-asthmatic assay).
References
|
1.
|
Khatami M: Yin and Yang in inflammation:
duality in innate immune cell function and tumorigenesis. Expert
Opin Biol Ther. 8:1461–1472. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Mahabeleshwar GH and Byzova TV:
Angiogenesis in melanoma. Semin Oncol. 34:555–565. 2007. View Article : Google Scholar
|
|
3.
|
Stamenkovic I: Extracellular matrix
remodelling: the role of matrix metalloproteinases. J Pathol.
200:448–464. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Jakob C, Sterz J, Zavrski I, Heider U,
Kleeberg L, Fleissner C, Kaiser M and Sezer O: Angiogenesis in
multiple myeloma. Eur J Cancer. 42:1581–1590. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Woo JH, Park JW, Lee SH, Kim YH, Lee IK,
Gabrielson E, Lee SH, Lee HJ, Kho YH and Kwon TK: Dykellic acid
inhibits phorbol myristate acetate-induced matrix
metalloproteinase-9 expression by inhibiting nuclear factor kappa B
transcriptional activity. Cancer Res. 63:3430–3434. 2003.
|
|
6.
|
Lee SH, Youk ES, Lee HJ, Kho YH, Kim HM
and Kim SU: Dykellic acid inhibits drug-induced caspase-3-like
protease activation. Biochem Biophys Res Commun. 302:539–544. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Coghlin C and Murray GI: Current and
emerging concepts in tumour metastasis. J Pathol. 222:1–15. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Bourguignon LYW: Hyaluronan-mediated CD44
activation of RhoGTPase signaling and cytoskeleton function
promotes tumor progression. Semin Cancer Biol. 18:251–259. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Rao YK, Fang SH and Tzeng YM: Evaluation
of the anti-inflammatory and anti-proliferation tumoral cells
activities of Antrodia camphorate, Cordyceps
sinensis, and Cinnamomum osmophloeum bark extracts. J
Ethnopharmacol. 114:78–85. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Lee HM, Kim YJ, Kim HW, Lee DH, Sung MK
and Park TS: Induction of apoptosis by Cordyceps militaris
through activation of caspase-3 in leukemia HL-60 cells. Biol Pharm
Bull. 29:670–674. 2006.
|
|
11.
|
Alves SB, Rossi SR, Lopes RB, Tamai MA and
Pereira RM: Beauveria bassiana yeast phase on agar medium
and its pathogenicity against Diatraea saccharalis
(Lepidoptera: Crambidae) and Tetranychus urticae
(Acari: Tetranychidae). J Invertebr Pathol. 81:70–77. 2002.
View Article : Google Scholar
|
|
12.
|
Russell R and Paterson M: Cordyceps
– a traditional Chinese medicine and another fungal therapeutic
biofactory? Phytochemistry. 69:1469–1495. 2008. View Article : Google Scholar
|
|
13.
|
Heo JC, Park JY, Lee JM, Kwon TK, Kim SU,
Chung SK and Lee SH: Wisteria floribunda gall extract
inhibits cell migration in mouse B16F1 melanoma cells by regulating
CD44 expression and GTP-RhoA activity. J Ethnopharmacol. 102:10–14.
2005. View Article : Google Scholar
|
|
14.
|
Heo JC, Woo SW, Kweon MA, Son M, Yoon EK,
Lee HK, Choi WS, Cho KJ and Lee SH: A methylene chrolide fraction
of Geum japonicum Thurnberg inhibits metastatic and
angiogenic potential. Oncol Rep. 19:1399–1404. 2008.
|
|
15.
|
Heo JC, Woo SU, Son M, Park JY, Choi WS,
Chang KT, Kim SU, Yoon EK, Kim YH, Shin HM and Lee SH: Anti-tumor
activity of Gastrodia elata Blume is closely associated with
a GTP-Ras-dependent pathway. Oncol Rep. 18:849–853. 2007.
|
|
16.
|
Giannopoulou E, Dimitropoulos K, Argyriou
AA, Koutras AK, Dimitrakopoulos F and Kalofonos HP: An in vitro
study, evaluating the effect of sunitinib and/or lapatinib on two
glioma cell lines. Invest New Drugs. 28:554–560. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Falkeholm L, Grant CA, Magnusson A and
Moller E: Xylenefree method for istological preparation: a
multicentre evaluation. Lab Invest. 81:1213–1221. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Halsted CH: Dietary supplements and
functional foods: 2 sides of a coin? Am J Clin Nutr. 77(Suppl 4):
1001–1007. 2003.PubMed/NCBI
|
|
19.
|
Ferrari CK: Functional foods, herbs and
nutraceuticals: towards biochemical mechanisms of healthy aging.
Biogerontology. 5:275–289. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Ji DB, Ye J, Li CL, Wang YH, Zhao J and
Cai SQ: Anti-aging effect of Cordyceps sinensis extract.
Phytother Res. 23:116–122. 2009.
|
|
21.
|
Hsu CH, Sun HL, Sheu JN, Ku MS, Hu CM,
Chan Y and Lue KH: Effects of the immunomodulatory agent
Cordyceps militaris on airway inflammation in a mouse asthma
model. Pediatr Neonatol. 49:171–178. 2008.PubMed/NCBI
|
|
22.
|
Ohta Y, Lee JB, Hayashi K, Fujita A, Park
DK and Hayashi T: In vivo anti-influenza virus activity of an
immunomodulatory acidic polysaccharide isolated from Cordyceps
militaris grown on germinated soybeans. J Agric Food Chem.
55:10194–10199. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Nishizawa K, Torii K, Kawasaki A, Katada
M, Ito M, Terashita K, Aiso S and Matsuoka M: Antidepressant-like
effect of Cordyceps sinensis in the mouse tail suspension
test. Biol Pharm Bull. 30:1758–1762. 2007.
|
|
24.
|
Shi B, Wang Z, Jin H, Chen YW, Wang Q and
Qian Y: Immunoregulatory Cordyceps sinensis increases
regulatory T cells to Th17 cell ratio and delays diabetes in NOD
mice. Int Immunopharmacol. 9:582–586. 2009.
|
|
25.
|
Ko WS, Hsu SL, Chyau CC, Chen KC and Peng
RY: Compound Cordyceps TCM-700C exhibits potent
hepatoprotective capability in animal model. Fitoterapia. 81:1–7.
2010.
|
|
26.
|
Zhang C, Zou X, Leluo G, Xu J and Xiang M:
Prevention of type 1 diabetes by immature dendritic cells treated
with an ethanol extract of Paecilomyces hepiali Chen
mycelium. Methods Find Exp Clin Pharmacol. 30:421–429. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Lu HE, Jian CH, Chen SF, Chen TM, Lee ST,
Chang CS and Weng CF: Hypoglycaemic effects of fermented mycelium
of Paecilomyces farinosus (G30801) on high-fat fed rats with
streptozotocin-induced diabetes. Indian J Med Res. 131:696–701.
2010.PubMed/NCBI
|
|
28.
|
Lang G, Blunt JW, Cummings NJ, Cole AL and
Munro MH: Paecilosetin, a new bioactive fungal metabolite from a
New Zealand isolate of Paecilomyces farinosus. J Nat Prod.
68:810–811. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Cheng Y, Schneider B, Riese U, Schubert B,
Li Z and Hamburger M: Farinosones A-C, neurotrophic alkaloidal
metabolites from the entomogenous deuteromycete Paecilomyces
farinosus. J Nat Prod. 67:1854–1858. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Hata K, Hori K, Murata J and Takahashi S:
Remodeling of actin cytoskeleton in lupeol-induced B16 2F2 cell
differentiation. J Biochem. 138:467–472. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Kim A, Jung JY, Son M, Lee SH, Lim JS and
Chung AS: Long exposure of non-cytotoxic concentrations of
methylselenol suppresses the invasive potential of B16F10 melanoma.
Oncol Rep. 20:557–565. 2008.PubMed/NCBI
|