Introduction
Peroxisome proliferator-activated receptors (PPARs)
are members of the nuclear hormone receptor superfamily, first
identified by Issemann and Green in 1990 in mouse cells (1). PPARs play important roles in the
regulation of cell growth, differentiation and apoptosis. There are
three known subtypes of PPARs, namely PPARα, PPARβ and PPARγ. These
are encoded by separate genes with highly similar amino acid
sequences, particularly in the DNA- and ligand-binding domains.
Among the three subtypes, PPARγ has been the most extensively
studied (2), as it possesses
complex and diverse biological functions. In recent years, PPARγ
agonists have attracted interest due to their antitumor effect.
Mueller et al (3)
demonstrated that PPARγ ligands inhibit the growth of prostate
cancer, suggesting that the activation of PPARγ is a potential
cancer therapeutic method. Nagamine et al (4) found that rosiglitazone (RSG)
treatment increased the number of apoptotic cells in the human
gastric cancer cell lines MKN-28, −45 and −74. Thus, there is
growing evidence that PPARγ agonists exhibit significant antitumor
effects (5–7).
All-trans retinoic acid (ATRA) is one of the more
established types of retinoid drugs used in clinical chemotherapy
and cancer prevention. ATRA inhibits the growth of various types of
malignant tumor cells and is a commonly used
differentiation-inducing reagent. PPARs are known to form
heterodimers with the retinoid X receptor (RXR), bind to a specific
DNA sequence-peroxisome proliferation response element, and
regulate target gene transcription (8). Both in vitro and in
transplanted breast tumors in nude mice, Elstner et al
(9) found that ATRA assisted PPARγ
ligands in inhibiting tumor cell growth and decreased bcl-2 levels,
suggesting that the use of PPARγ agonists and retinoic acid
activates the PPARγ/RXR heterodimer and may be an effective method
for the treatment of a variety of tumors. In this study, we
investigated the effect of the combined use of highly selective
ATRA and the PPARγ agonist RSG on the proliferation and apoptosis
of the HCT-15 human colorectal cancer cell line, and further
explored the molecular mechanisms involved.
Materials and methods
Materials and reagents
The HCT-15 human colorectal cancer cell line was
purchased from the Shanghai Cell Library of the Chinese Academy of
Sciences. RSG and retinoic acid (purity >99%) were purchased
from Gaomeng Yanshan (Beijing, China), prepared as a 1 mmol/l stock
solution in DMSO and stored at −20°C. Immediately before use, the
drugs were diluted to the desired concentrations with RPMI-1640
medium containing 10% fetal bovine serum (FBS). COX-2, MMP-7 and
TIMP-1 rabbit anti-human polyclonal antibodies were purchased from
Boaosen Biotechnology (Beijing, China). The SP staining and DAB
kits were purchased from Zhongshan Golden Bridge Biotechnology
(Beijing, China).
Experimental grouping and
treatments
The MTT assay was used to examine cell
proliferation. The cells were divided into five groups: group I,
blank control group (100 μl per well of medium); group II,
vehicle control group (100 μl per well of culture medium
containing DMSO); group III, RSG only group (100 μl per well
of fresh medium with final concentrations of RSG of 6.25, 12.5, 25
or 50 μmol/l); group IV, ATRA only group, (100 μl per
well of fresh medium with a final concentration of ATRA of 2
μmol/l); group V, RSG and ATRA combined treatment group (100
μl per well of fresh medium with final concentrations of RSG
of 6.25, 12.5, 25 and 50 μmol/l, in combination with 2
μmol/l of ATRA).
Experimental methods
Cell culture
The HCT-15 human colorectal cancer cells, which are
adherent cells, were cultured in conventional RPMI-1640 medium
containing 10% FBS, 100 U/ml penicillin and 100 mg/l streptomycin
in a 5% CO2 incubator at 37°C.
MTT assay for cell proliferation
Cells in the logarithmic phase of growth were
digested with trypsin and prepared as a 5×104/ml
single-cell suspension, then were seeded into 96-well plates at a
density of 5,000 cells/well, each well containing a total volume of
100 μl. The medium was replaced on the following day, then
the cells in each group were treated as described above. At the end
of the treatment, 20 μl of MTT (5 g/l) was added to each
well, and culturing was continued in the dark for 4 h. The culture
supernatant was then removed and replaced with 150 μl of
DMSO. After shaking for 10 min until the crystals dissolved, the
absorbance (A) was measured at a wavelength of 570 nm with a
microplate reader, and the inhibition rate of the tumor cells was
calculated according to the formula: inhibition (%) = [1 - mean
A570nm of the experimental group/mean A570nm
of the control group] × 100. The IC50 was calculated as
the half inhibitory concentration. The interaction of the two drugs
was calculated as: q = E (a + b)/[Ea + (1 - Ea) × Eb], where E (a +
b) is the inhibition rate of the two drugs combined and Ea and Eb
are the inhibition rates of the drugs used alone. The effect of the
two drugs was additional at q=0.85–1.15, synergistic at q>1.15,
and antagonistic at q<0.85. The experiment was repeated three
times, and the results are represented as the mean values.
Flow cytometry for the detection of
cell cycle progression
The cells were collected after digestion, washed
twice with PBS and centrifuged at 1,000 rpm for 5 min. The
supernatant was discarded. The cells were then resuspended, fixed
in ice-cold 75% ethanol and stored at 4°C. After two washes in PBS,
the cells were stained with propidium iodide (PI) and subjected to
flow cytometric analysis of the percentage of cells in G0/G1, S and
G2/M phases. Treatment with each drug concentration was conducted
in triplicate.
Annexin V/PI and flow cytometry to
detect apoptosis
The cells were resuspended in 100 μl of
binding buffer, followed by the addition of 5 μl Annexin
V-FITC and 10 μl PI. After mixing, the cells were incubated
in the dark at room temperature for 15 min. Binding buffer (400
μl) was added to the reaction tube, and the cells were
resuspended and analyzed using a flow cytometer. Treatment with
each drug concentration was performed in triplicate.
Immunocytochemical detection of
intracellular COX-2, MMP-7 and TIMP-1
Upon reaching confluence, the cells were fixed with
4% paraformaldehyde for 30 min, rinsed three times with PBS and
permeablized in PBS with 0.1% Triton X-100 at room temperature for
40 min. After being rinsed three more times with PBS, the cells
were incubated in 3% hydrogen peroxide at room temperature for 15
min, followed by an additional three rinses with PBS. The cells
were then blocked with inactivated normal goat serum at room
temperature for 15 min and then respectively incubated at 4°C
overnight with the following antibodies: rabbit polyclonal
anti-human COX-2 (1:100), MMP-7 (1:100) and TIMP-1 (1:100). After
being rinsed three times with PBS, biotinylated goat anti-rabbit
IgG was added and the incubation was continued at 37°C for 15 min.
After another three rinses in PBS, the signal was visualized by DAB
colorimetric reaction. The cells were counterstained with
hematoxylin for 2 min, differentiated in acidic ethanol, dehydrated
in an ascending series of ethanol, cleared in xylene and mounted
with neutral gum. PBS containing no primary antibody was used as
the negative control. Positive staining was determined by the
appearance of brown or brownish yellow granules on the cell
membrane or in the cytoplasm. At a high magnification (x200),
COX-2, MMP-7 and TIMP-1 protein expression was observed under a
microscope. The results were analyzed with the high-resolution
color pathological image analysis system (Nikon TE2000-U).
Statistical analysis
Data were expressed as the mean ± standard deviation
(SD). SPSS 13.0 software was used for statistical analysis, with
the t-test for comparison between two groups and one-way ANOVA for
comparison among multiple groups. P<0.05 was taken to indicate
statistical significance.
Results
Growth inhibition detected by MTT
assay
The cell growth inhibition rates of the single or
combined application of different concentrations of RSG and ATRA in
HCT-15 cells are shown in Table
I.
 | Table I.Effect of RSG, ATRA and their
combination on HCT-15 cell proliferation (mean ± SD). |
Table I.
Effect of RSG, ATRA and their
combination on HCT-15 cell proliferation (mean ± SD).
| Group | Concentration
(μmol/l) |
A570nm | Inhibition
rate(%) |
|---|
| |
|
|
|---|
| | 24 h | 48 h | 24 h | 48 h |
|---|
| Control group | - | 0.91±0.10 | 1.05±0.09 | - | - |
| R1 | 6.25 | 0.81±0.03a | 0.83±0.03a | 11.23 | 20.35 |
| R2 | 12.50 | 0.70±0.11a | 0.84±0.05a | 22.71 | 19.54 |
| R3 | 25.00 | 0.59±0.04a | 0.51±0.03a | 35.64 | 41.02 |
| R4 | 50.00 | 0.46±0.04b | 0.17±0.01b | 51.88 | 73.76 |
| A | 2.00 | 0.85±0.11a | 0.82±0.03a | 6.25 | 21.65 |
| R1+A | 6.25+2 | 0.86±0.02c | 0.81±0.04c | 5.11 | 22.77 |
| R2+A | 12.5+2 | 0.72±0.05c | 0.74±0.05c | 20.36 | 29.72 |
| R3+A | 25.0+2 | 0.54±0.09c | 0.36±0.03c | 40.62 | 66.08 |
| R4+A | 50.0+2 | 0.32±0.09d | 0.09±0.01d | 65.01 | 91.22 |
For RSG treatment alone in HCT-15 cells, the
IC50 values at 24 and 48 h were 48.84 and 33.33
μmol/l, respectively. When combined with ATRA, the
IC50 values were 34.89 and 19.75 μmol/l at 24 and
48 h, respectively. RSG or ATRA alone exhibited a slight inhibitory
effect on HCT-15 cell growth (P<0.05), whereas the combined
application of RSG and ATRA showed stronger cell growth inhibition
(P<0.01). With increasing drug concentrations and reaction
times, the cell growth inhibition rate increased and the difference
was statistically significant (P<0.05). RSG and ATRA showed
significant synergy in combination (q>1.15 when 25 and 50
μmol/l of RSG were combined with ATRA).
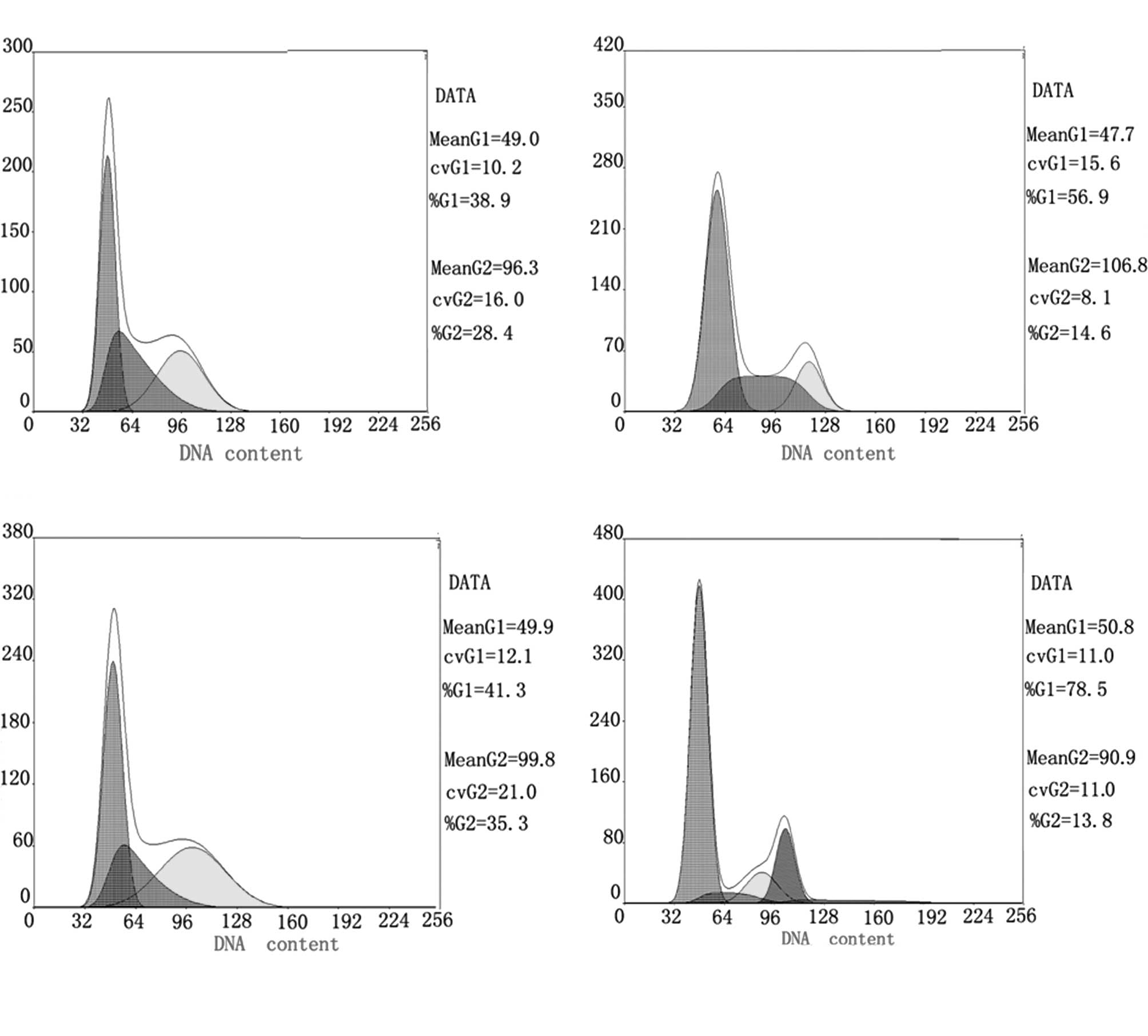
Detection of cell cycle progression by
flow cytometry
For RSG, ATRA, their combination and the control
groups, the percentages of cells in the G0/G1 phase were 56.9,
41.3, 78.5 and 38.9%, respectively; S phase cell percentages were
28.5, 27.4, 7.7 and 32.8%, respectively; G2 phase cell percentages
were 14.6, 31.3, 13.8 and 28.4%, respectively. The results showed
that, compared to the control group, the single-drug treatment
groups had an increased proportion of G0/G1 phase cells and a
decreased proportion of S phase cells (P<0.05). The combination
treatment group had an increased proportion of G1 phase cells and
decreased S phase cells (P<0.01). The results suggest that RSG
and ATRA alone or in combination cause G1 cell cycle arrest, and
that the combination enhances this G1 phase arrest (Fig. 1).
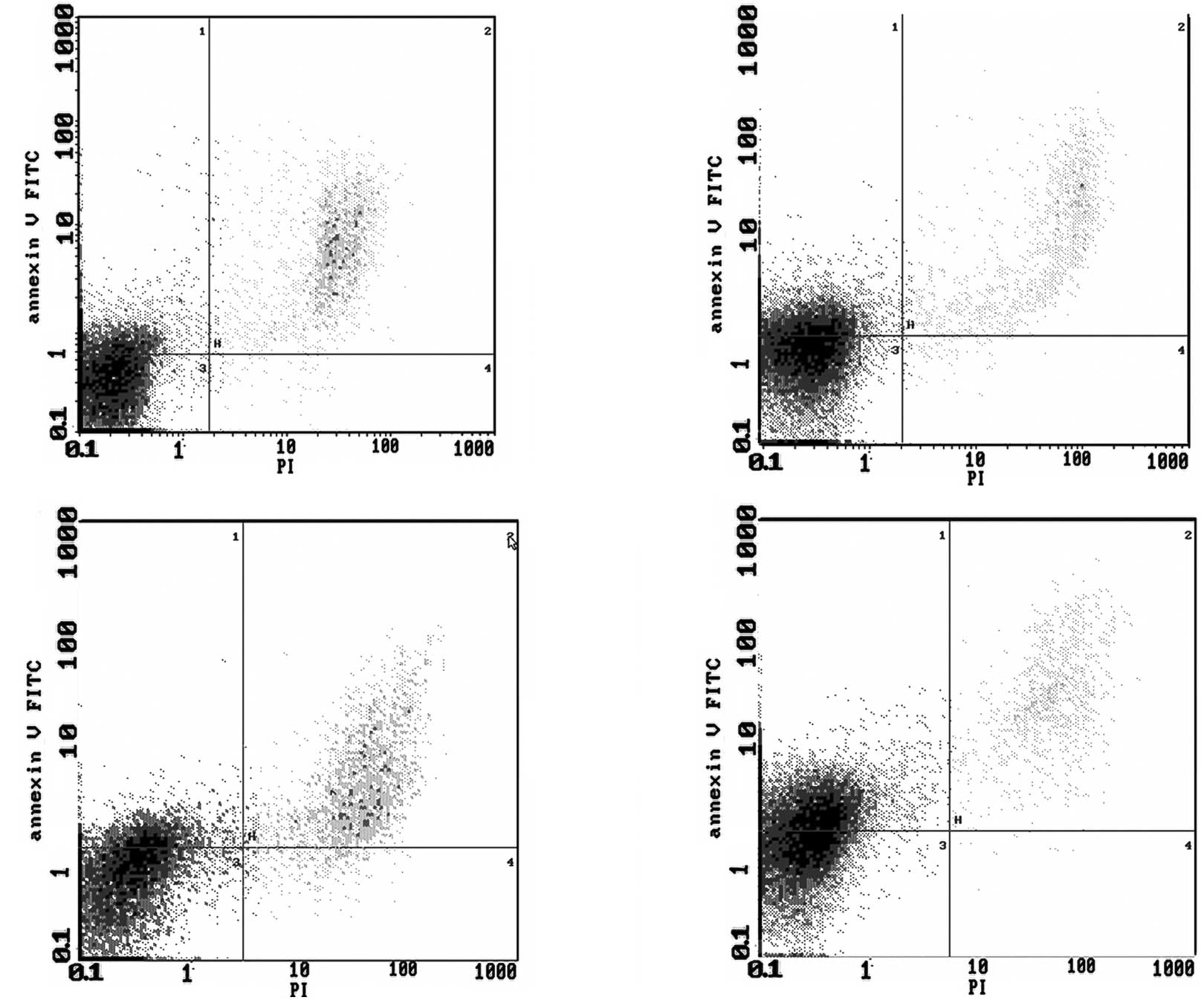
Detection of apoptosis by flow
cytometry
For RSG, ATRA, their combination and the control
groups, the apoptosis rates were 27.4, 14.5, 41.5 and 12.7%,
respectively. The results showed that, compared to the control
group, RSG and ATRA alone or in combination induced the apoptosis
of HCT-15 cells, and the difference was statistically significant
(P<0.05). The combination treatment was also statistically
significant compared to the single-drug treatments (P<0.05)
(Fig. 2).
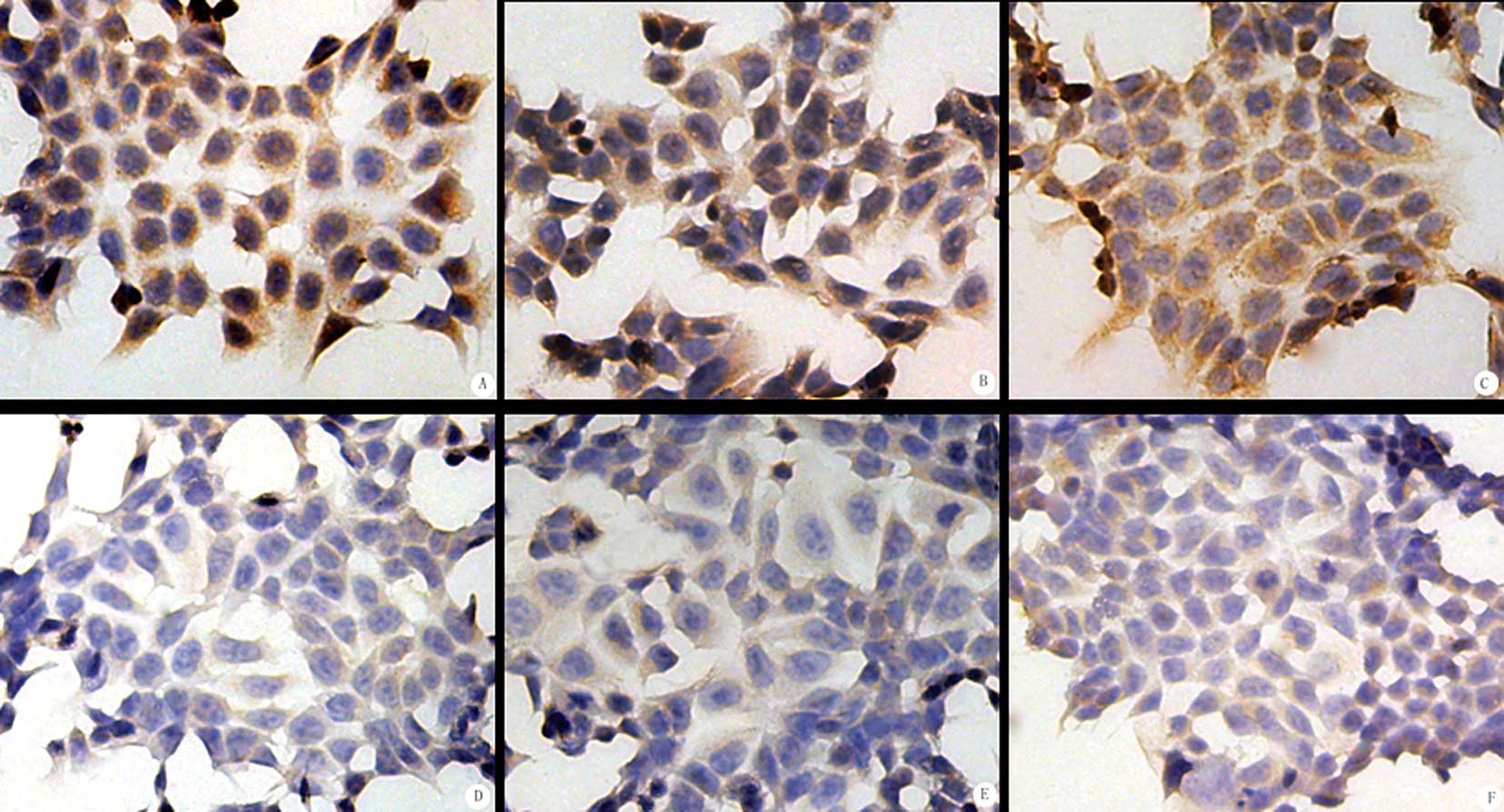
SP immunocytochemistry
COX-2, MMP-7 and TIMP-1 were all expressed in the
HCT-15 cells (Table II). Under a
light microscope, brown particles were located in the cytoplasm of
the tumor cells. In the control group, the expression was strong. A
higher number of positive cells was noted and more brown granules
in the cytoplasm and darker staining were observed. The positive
rates of COX-2, MMP-7 and TIMP-1 protein expression in the control
cells were 66.79, 73.21 and 64.08%, respectively (Fig. 3). In the experimental groups, the
expression was rather weak, characterized by fewer positive cells
and lighter staining. In the RSG group, the positive rates of
COX-2, MMP-7 and TIMP-1 expression were 50.02, 49.78 and 43.37%,
respectively. In the ATRA group, the positive rates were 46.67,
48.58 and 48.89%, respectively; significantly lower than in the
control group (P<0.05). In the combination group, the positive
rates of COX-2, MMP-7 and TIMP-1 expression were 19.33, 20.58 and
13.13%, respectively; significantly lower than in the control group
(P<0.01) (Fig. 3).
 | Table II.COX-2, MMP-7 and TIMP-1 protein
expression in each group (mean ± SD). |
Table II.
COX-2, MMP-7 and TIMP-1 protein
expression in each group (mean ± SD).
| Positive rate (%)
in each group
|
|---|
| RSG (25
μmol/l) | ATRA (2
μmol/l) | RSG + ATRA (25 + 2
μmol/l) | Control group |
|---|
| COX-2 | 50.02±3.10a | 46.67±2.58a | 19.33±1.21b | 66.79±6.10 |
| MMP-7 | 49.78±2.09a | 48.58±2.78a | 20.58±1.04b | 73.21±6.62 |
| TIMP-1 | 43.37±2.01a | 48.89±3.19a | 13.13±1.09b | 64.08±4.28 |
Discussion
With the advancement of research on the genetic and
molecular mechanisms of malignant tumors, drugs that target
specific structures, functional areas, molecular groups, enzymes
and signal transduction pathways in tumor cells have been
increasingly used in the clinical setting. These targeted therapies
have become important treatment modalities in addition to
conventional surgery, chemotherapy and radiotherapy, and have
achieved encouraging results (10).
PPARs have diverse biological functions, including
the regulation of lipid and glucose metabolism. PPARs have been
found to induce tumor cell differentiation and apoptosis and to
inhibit tumor angiogenesis. In particular, PPARs are closely
related to gastrointestinal tumors. Recent studies have found that
PPARγ is expressed not only in adipose tissue, where it is involved
in lipid metabolism (11), but
also in a variety of tumors (12).
Upon activation by its specific ligands, PPARγ inhibits the growth
of tumor cells (13–16). Although the exact mechanism has not
been fully elucidated, it is thought to be related to the induction
of apoptosis and cell cycle arrest (17).
Our results showed that the application of the PPARγ
agonist RSG inhibited the proliferation of HCT-15 human colon
cancer cells. At a low dose of 6.25 μmol/l, the inhibition
was not significant; however, when the dose was increased from 25
to 50 μmol/l, the inhibition was gradually increased,
achieving a significant difference compared to the vehicle control
group. This inhibition of proliferation showed a marked dose-effect
relationship, and when RSG was combined with ATRA the inhibition
was markedly enhanced. The IC50 values for the HCT-15
cells at 24 and 48 h were 48.84 and 33.33 μmol/l,
respectively, when RSG was applied alone, and 34.89 and 19.75
μmol/l, respectively, when RSG was combined with ATRA. These
results revealed that the combined drug treatment exerted a
stronger effect than the single drug treatment in inhibiting HCT-15
cell proliferation. Moreover, within a certain concentration range,
this effect was dose- and time-dependent. In addition, after
intervention with RSG, ATRA or their combination, the percentage of
cells in the G1 phase increased and the percentage of cells in the
S phase decreased; cells were prevented from entering the division
cycle and instead entered a resting state. The apoptosis assay also
showed that, compared to the control group, RSG, ATRA or their
combination induced the apoptosis of HCT-15 cells, and their
combined use had a stronger effect than the single treatments. The
results revealed that RSG in combination with ATRA arrested HCT-15
cells in the G1 phase, halted mitosis and increased the percentage
of tumor cell apoptosis. Therefore, the combination of RSG and ATRA
inhibits proliferation and induces the apoptosis of HCT-15 human
colon cancer cells, consistent with a previous report (18).
Many studies have shown that COX-2 overexpression is
closely related to cancer occurrence and development in the
digestive system. COX-2 promotes cell adhesion, inhibits cell
apoptosis, induces tumor angiogenesis and promotes tumor invasion
and metastasis (19). MMP-7 is the
smallest of the MMP family members and its expression is related to
the occurrence and development of various malignant human tumors,
such as the invasion and metastasis of colorectal cancer (20,21).
As MMP inhibitors, the primary function of TIMPs is to counter the
activity of MMPs, by which they limit the degradation of the
matrix. Tumor invasion and metastasis are probably due to the
inhibition of TIMP secretion in tumor cells (22). Our results showed that COX-2, MMP-7
and TIMP-1 were expressed at a high level in the control group,
with the positive rates of 66.79, 73.21 and 64.08%, respectively.
RSG or ATRA treatment alone decreased the expression levels of
COX-2, MMP-7 and TIMP-1; RSG and ATRA in combination further
significantly decreased the expression of COX-2, MMP-7 and TIMP-1.
This result revealed that the combined use of RSG and ATRA
significantly inhibited the expression of COX-2, MMP-7 and TIMP-1,
genes related to tumor cell growth, invasion and metastasis,
therefore the potential for tumor cell growth, development,
invasion and metastasis was greatly reduced. The mechanisms behind
the anti-proliferative and growth inhibitory effect of the
combination of RGS and ATRA leading to apoptosis in the HCT-15
human colorectal cell line may involve the down-regulation of
COX-2, MMP-7 and TIMP-1 expression.
In the present study, through a series of in
vitro experiments, we investigated whether the PPARγ agonist
RSG and ATRA inhibited the proliferation and growth of HCT-15 human
colorectal cancer cells in vitro, and determined that the
combination of the two targeted drugs was more effective. Through
the detection of genes related to tumor growth and metastasis, the
possible molecular mechanism behind the inhibition of HCT-15 human
colorectal cancer cell growth by the two drugs was investigated,
and was found to potentially involve the down-regulation of the
expression of COX-2, MMP-7 and TIMP-1. However, the correlation
between TIMP-1 and MMP-7 in HCT-15 cells and their related
signaling pathways requires further investigation in future
studies.
Acknowledgements
The authors are grateful to their
colleagues and students. Through their assistance this research
study was successfully completed.
Abbreviations:
|
ATRA,
|
all-trans retinoic acid;
|
|
PPARs,
|
peroxisome proliferator-activated
receptors;
|
|
RSG,
|
rosiglitazone
|
References
|
1.
|
Issemann I and Green S: Activation of a
member of the steroid hormone receptor superfamily by peroxisome
proliferators. Nature. 347:645–650. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Kersten S, Desvergne B and Wahli W: Roles
of PPARs in health and disease. Nature. 40:421–424. 2000.
|
|
3.
|
Mueller E, Smith M, Sarraf P, Kroll T,
Aiyer A, Kaufman DS, Oh W, Demetri G, Figg WD, Zhou XP, Eng C,
Spiegelman BM and Kantoff PW: Effects of ligand activation of
peroxisome proliferator-activated receptor gamma in human prostate
cancer. Proc Natl Acad Sci USA. 97:10990–10995. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Nagamine M, Okumura T, Tanno S, Sawamukai
M, Motomura W, Takahashi N and Kohgo Y: PPARgamma ligand-induced
apoptosis through a p53-dependent mechanism in human gastric cancer
cells. Cancer Sci. 94:338–343. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Heaney AP, Fernando M and Melmed S:
PPAR-gamma receptor ligands: novel therapy for pituitary adenomas.
J Clin Invest. 111:1381–1388. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Yoshimura R, Matsuymma M, Segawa Y, Hase
T, Mitsuhashi M, Tsuchida K, Wada S, Kawahito Y, Sano H and
Nakatani T: Expression of peroxisome proliferator-activated
receptors (PPARs) in human urinary bladder carcinoma and growth
inhibition by its agonists. Int J Cancer. 104:597–602. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Guan YF, Zhang YH, Breyer RM, Davis L and
Breyer MD: Expression of peroxisome proliferator-activated receptor
gamma (PPARgamma) in human transitional bladder cancer and its role
in inducing cell death. Neoplasia. 1:330–339. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Faueonnet S, Lascombe I, Chabannes E,
Adessi GL, Desvergne B, Wahli W and Bittard H: Differential
regulation of vascular endothelial growth factor expression by
peroxisome proliferator-activated receptors in bladder cancer
cells. J Biol Chem. 277:23534–23543. 2002. View Article : Google Scholar
|
|
9.
|
Elstner E, Williamson EA, Zang C, Fritz J,
Heber D, Fenner M, Possinger K and Koeffler HP: Novel therapeutic
approach: ligands for PPARgamma and retinoid receptors induce
apoptosis in bcl-2-positive human breast cancer cells. Breast
Cancer Res Treat. 74:155–165. 2002. View Article : Google Scholar
|
|
10.
|
Zhou X, Zheng Q, Zhao X and Ma H: The
inhibitory effect of elemene combined with tamoxifen on breast
cancer cell line MCF-7. J Xi’an Jiaotong Univ Med Sci. 28:74–77.
2007.
|
|
11.
|
Chawla A, Schwarz EJ, Dimaculangan DD and
Lazar MA: Peroxisome proliferator activated receptor (PPAR) gamma:
adipose-predominant expression and induction early in adipocyte
differentiation. Endocrinology. 135:798–800. 1994.
|
|
12.
|
Theocharis S, Giaginis C, Parasi A,
Margeli A, Kakisis J, Agapitos E and Kouraklis G: Expression of
peroxisome proliferator-activated receptor-gamma in colon cancer:
correlation with histopathological parameters, cell cycle-related
molecules, and patient survival. Dig Dis Sci. 52:2305–2311. 2007.
View Article : Google Scholar
|
|
13.
|
Elstner E, Muller C, Koshizuka K,
Williamson EA, Park D, Asou H, Shintaku P, Said JW, Heber D and
Koeffler HP: Ligands for peroxisome proliferator-activated receptor
gamma and retinoic acid receptor inhibit growth and induce
apoptosis of human breast cancer cells in vitro and in BNX mice.
Proc Natl Acad Sci USA. 95:8806–8811. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Shimada T, Kojima K, Yoshiura K, Hiraishi
H and Terano A: Characteristics of the peroxisome
proliferator-activated receptor γ (PPARγ) ligand induced apoptosis
in colon cancer cells. Gut. 50:658–664. 2002.
|
|
15.
|
Chang TH and Szabo E: Induction of
differentiation and apoptosis by ligands of peroxisome
proliferator-activated receptor γ in non-small cell lung cancer.
Cancer Res. 60:1129–1138. 2000.
|
|
16.
|
Motomura W, Okumura T, Takahashi N, Obara
T and Kohgo Y: Activation of peroxisome proliferator-activated
receptorγ by troglitazone inhibits cell growth through the
increases of P27kipl in human pancreatic carcinoma cells. Cancer
Res. 60:5558–5564. 2000.
|
|
17.
|
Liu JJ, Liu PQ, Lin DJ, Xiao RZ, Huang M,
Li XD, He Y and Huang RW: Downregulation of cyclooxygenase-2
expression and activation of caspase-3 are involved in peroxisome
proliferator-activated receptor-gamma agonist-induced apoptosis in
human monocyte leukemia cells in vitro. Ann Hematol. 86:173–183.
2007. View Article : Google Scholar
|
|
18.
|
Yoshida K, Tanabe K, Fujii D, Oue N, Yasui
W and Toge T: Induction mechanism of apoptosis by troglitazone
through peroxisome proliferator-activated receptor-gamma in gastric
carcinoma cells. Anticancer Res. 23:267–273. 2003.
|
|
19.
|
Murata H, Kawano S, Tsuji S, Tsuji M,
Sawaoka H, Kimura Y, Shiozaki H and Hori M: Cyclooxygenase-2
overexpression enhances lymphatic invasion and metastasis in human
gastric carcinoma. Am J Gastroenterol. 94:451–460. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Yoshimoto M, Itoh F, Yamamoto H, Hinoda Y,
Imai K and Yachi A: Expression of MMP-7 mRNA in human colorectal
cancers. Int J Cancer. 54:614–619. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
McDonnell S, Naver M, Coffey RJ Jr and
Matrisian LM: Expression and localization of the matrix
metalloproteinase pump21 (MMP-7) in human gastric and colon
carcinomas. Mol Carcinog. 4:527–536. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Baker AH, George SJ, Zaltsman AB, Murphy G
and Newby AC: Inhibition of invasion and induction of apoptotic
cell death of cancer cell lines by over-expression of TIMP-3. Br J
Cancer. 79:1347–1355. 1999. View Article : Google Scholar : PubMed/NCBI
|