Introduction
Glaucoma is an irreversible, chronic, blinding
disease characterized by visual field loss, optic nerve head
cupping and retinal ganglion cell loss. Glaucoma is considered the
second most prevalent cause of blindness in the world. There are
several risk factors associated with glaucoma, such as intraocular
pressure (IOP), age, race, family history and vascular disease.
Among these, increased IOP is the most common risk factor. Reducing
IOP is presently the most accepted and most practiced therapeutical
approach for glaucoma patients. However, some patients continue to
lose their sight despite apparently adequate pressure control. This
suggests that reducing IOP does not provide complete protection,
whereas other factors unrelated to IOP may be active (1–4).
Glutamate is a normal constituent of the retina, but
in high non-physiological concentrations it causes neuronal damage
and death. Normally, glutamate is rapidly removed by Müller cells
in the retina (5–8). Glutamine synthetase (GS), an
important enzyme located mainly in Müller cells, catalyzes the
amidation of glutamate to glutamine. It is one of the major
mechanisms for the clearance of extracellular glutamate and in the
past has been used as a specific label for Müller cells (8–13).
Although various studies suggest that an increase in
vitreal and retinal glutamate levels causes glaucomatous damage
(14–16), the issue remains controversial with
research data both supporting or opposing this theory. We would
expect that elevated IOP leads to an increase in glutamate, or that
Müller cells respond normally, which would lead to an increased
expression of GS (9,17–22).
This study investigated the expression of GS in Müller cells under
different pressure in vitro.
There are many animal models of glaucoma in
vivo (23). However, only one
type of method is used in vitro (24–27).
Briefly, a glass chamber or oven is re-equipped into an incubator.
This pressure system is complicated and expensive inspite of its
classical and extensive use. In the present experiment, a novel
pressure mechanism was used, which was simpler and less costly than
the previous described method.
Materials and methods
Pressure mechanism
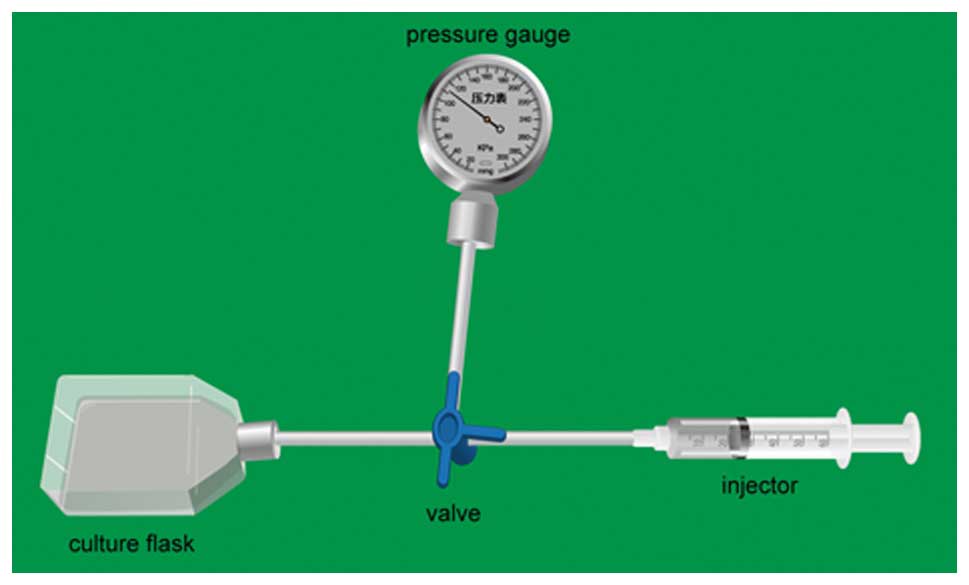
T75 culture flasks were used to construct the
pressure mechanism (Fig. 1). An
air mixture of 95% air and 5% CO2 was pumped to obtain
the relevant pressure. The pressure level of the model was 0, 20,
40, 60 and 80 mmHg, respectively, in accordance with human IOP in
glaucoma, and it was adjusted every 4 h. The time period of the
pressure was 24 h. All of the flasks were placed in an incubator at
37˚C.
The possibility that the elevated hydrostatic
pressure could alter the gas exchange was assessed by analyzing the
blood gas in the culture medium in the pressure (20, 40, 60 and 80
mmHg) and control (0 mmHg) culture groups before and after 12 h of
pressurization. The tightness was assessed by a pressure gauge.
Cell culture
Experiments were performed on newborn (0–3 days)
Sprague-Dawley (SD) rats (Slaccas Laboratory Animal Co., Ltd.). SD
rats were decapitated, and their retinas were quickly dissected in
cold D-Hank’s solution (Anresco). The mixture was transferred to
sterile centrifuge tubes and centrifuged at 1,000 rpm for 10 min.
The supernatant was discarded. Trypsin (0.125%) (Anresco) was added
for digestion. The mixture was incubated at 37˚C for 15 min. At the
end of the designated time, DMEM/F12 medium (Gibco) supplemented
with 2 mM glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin
and 10% fetal bovine serum (FBS; Sijiqing) was added to terminate
the digestion. The digests were filtered using a 200-mesh nylon
sieve and centrifuged at 1,000 rpm for 10 min. The supernatant was
discarded. The cell suspension was cultured in T75 culture flasks
at 37˚C in humidified air containing 5% CO2. When
confluent, the cells were washed once with D-Hank’s solution,
detached from the flask by treatment with 0.125% trypsin, washed
with complete cell culture medium and split 1:2 into fresh
flasks.
Immunofluorescence
The cells on the coverslips were incubated at 37˚C
for 1 day and washed three times (10 min/wash) with PBS. The cells
were fixed with 4% paraformaldehyde at room temperature for 10 min
and incubated with 0.3% Triton X-100 at 37˚C for 10 min. The cells
were washed three times (10 min/wash) with PBS, blocked with 10%
goat serum in PBS and subsequently incubated with a rabbit anti-rat
polyclonal antibody against GS (1:5,000; Abcam) as an identity
marker for Müller cells. The cells were then incubated overnight at
4˚C. The following day, the cells were incubated with the secondary
anti-rabbit IgG-Cy3 antibodies (1:200; BioLegend) at 37˚C in
darkness for 1 h. After three washes with PBS, the cells on the
coverslips were mounted on glass slides with Histomount. The cells
were viewed under an Axio microscope, and images were acquired with
a digital camera.
Western blot analysis
Fifteen samples, three at each pressure (0, 20, 40,
60 and 80 mmHg/24 h), were used for Western blotting. Cells were
washed twice in PBS. The protein concentration was determined by a
radioimmunoprecipitation assay and lysed in 2X Laemmli buffer.
Protein extracts were boiled for 10 min and centrifuged at 16,000 x
g. Proteins were separated on 12% SDS-PAGE and transferred to PVDF
membranes (Millipore). The membranes were soaked in Tris-buffered
saline (20 mmol/l Tris-Cl, 140 mmol/l NaCl, pH 7.5) containing 5%
skimmed milk and 0.1% Tween-20 for 1 h at room temperature. The
primary antibody used was GS (1:2,500; Abcam). Blots were incubated
with the primary antibody overnight at 4˚C. Anti-β-actin antibody
(1:3,000; Abcam) was used as a reference to normalize the
intensities of the immunoreactions with the different antibodies.
Then, after several washes, the membranes were incubated with a
secondary antibody against rabbit-IgG (Invitrogen) for 1 h at room
temperature in darkness. The protein bands were scanned, and images
were quantified with Odyssey (Li-CDR).
Real-time PCR (RT-PCR)
Total RNA was isolated from the individual samples
using the TRIzol® reagent (Invitrogen). The
concentration and purity of the RNA preparations were determined by
measuring the absorbance at 260/280 nm in a spectrophotometer
(Beckman). Total RNA was reversetranscribed into cDNA in a 20-μl
reaction containing 2 μg RNA, 4 μl 5X M-MLV buffer, 2 μl of dNTP, 1
μl random hexamer primer, 0.5 μl of RNase inhibitor and 1 μl of
M-MLV RTase. Reactions were performed at 25˚C for 10 min, at 42˚C
for 60 min and at 70˚C for 10 min.
The following targets were analyzed by RT-PCR. The
nucleotide sequences of the primers were based on published cDNA
sequences (Gene Bank). The primer sequences used for RT-PCR were as
follows: GS, 5′-ccgctcttcgtctcgttc-3′ and 5′-ctg
cttgatgcctttgtt-3′; β-actin, 5′-cccatctatgagggttacgc-3′ and 5′-ttta
atgtcacgcacgatttc-3′. RT-PCRs were performed on a LightCycler
instrument (Rotor Gene) with SYBR Green I, according to the
manufacturer’s recommendations. Cycling conditions were as follows:
initial denaturation at 94˚C for 5 min, followed by 40 cycles at
94˚C for 30 sec, 55˚C for 30 sec and 72˚C for 30 sec. The melting
curve analysis of the final PCR products was carried out from 50 to
99˚C at 1˚C intervals.
Statistical analysis
Data are presented as the mean ± SD (n=3–6 in each
group). Data were analyzed using one-way ANOVA followed by the
Turkey’s test. A p-value of <0.05 was accepted as indicative of
statistical significance.
Results
Pressure mechanism
We analyzed pH, PCO2 or PO2
from samples of the culture medium 12 h after exposure to the
different pressure levels (0, 20, 40, 60 and 80 mmHg). There was no
significant difference in these values between the control (0 mmHg)
and each pressure (20, 40, 60 and 80 mmHg) group (Table I).
 | Table I.Measurement of pressure, pH,
PCO2 and PO2 after 12 h. |
Table I.
Measurement of pressure, pH,
PCO2 and PO2 after 12 h.
| | Treatment (mmHg)
|
|---|
| Control | 20 | 40 | 60 | 80 |
|---|
| Time (h) | 12 | 12 | 12 | 12 | 12 |
| Total pressure
(mmHg) | 0 | 19±3 | 41±2 | 60±2 | 78±2 |
| pH | 7.69±0.03 | 7.64±0.02 | 7.67±0.04 | 7.65±0.02 | 7.61±0.03 |
| PO2
(mmHg) | 168.0±1.9 | 166.4±1.6 | 168.0±1.6 | 164.5±1.5 | 169.1±2.0 |
| PCO2
(mmHg) | 27.5±0.4 | 27.1±0.1 | 26.5±0.4 | 25.9±0.4 | 5.6±0.2 |
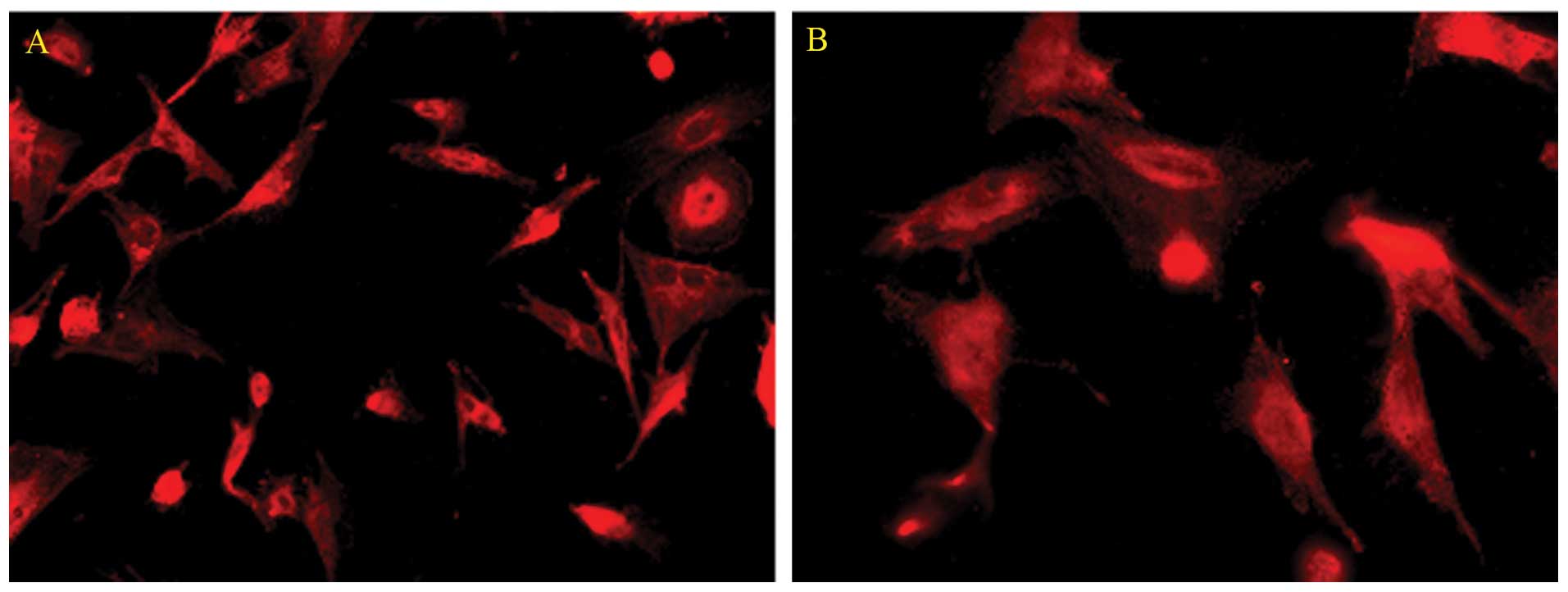
Immunofluorescence
We characterized the cultured rat Müller cells by
their expression of GS as assessed by immunocytochemical staining.
Cells in the culture system showed positive labeling for GS
(Fig. 2). Using this
immunocytochemical labeling, the cultured cells were determined to
be Müller cells.
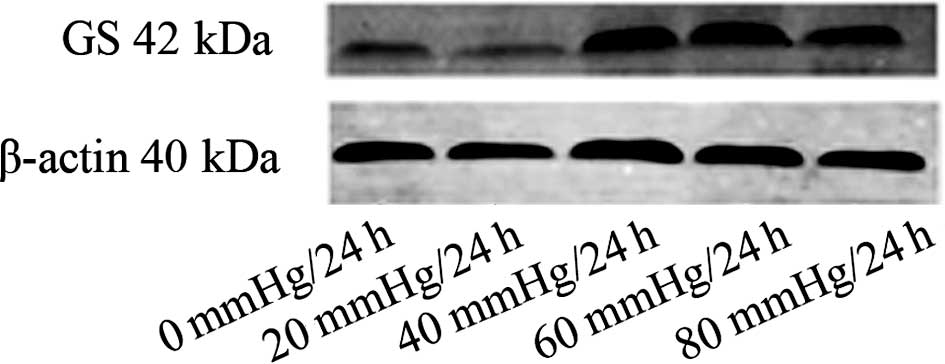
Western blot analysis
Western blot analyses of the samples at different
pressure showed that the expression of GS protein was variable. The
expression of the GS protein in the 20 mmHg/24 h group was
decreased compared to the control (0 mmHg/24 h) group. The
expression of GS protein was increased in the 40 mmHg/24 h and the
60 mmHg/24 h groups. The expression of GS protein was slightly
decreased in the 80 mmHg/24 h group (Fig. 3).
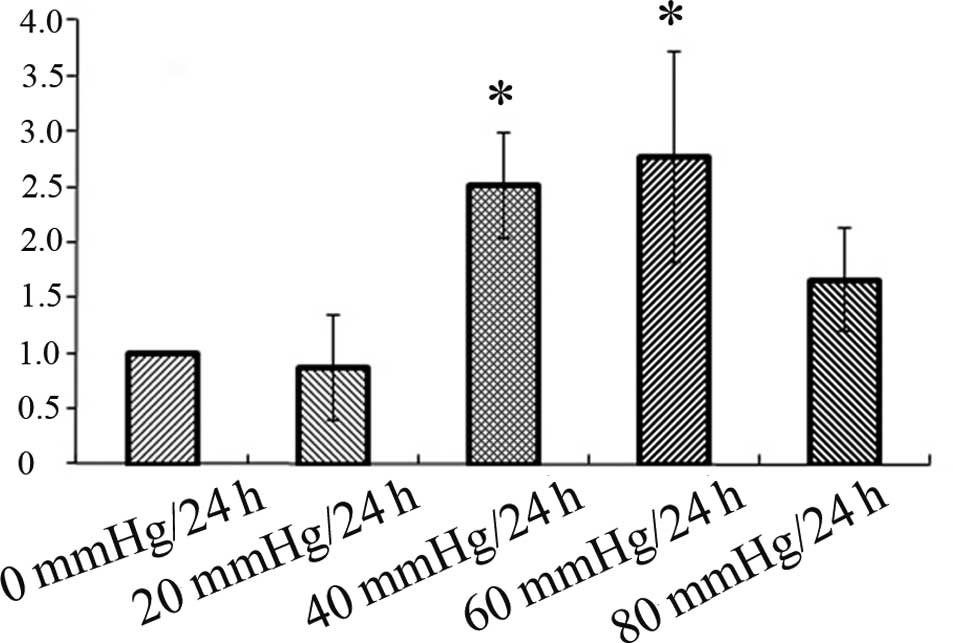
RT-PCR analysis
RT-PCR was performed to examine whether the mRNA
expression levels of GS were different among the different pressure
groups. Data showed that the expression of GS mRNA was decreased in
the 20 mmHg/24 h group compared to the control (0 mmHg/24 h) group.
Hereafter, the GS mRNA expression was increased in the 40 mmHg/24 h
group which further increased in the 60 mmHg/24 h group, and then
decreased in the 80 mmHg/24 h group. However, the difference was
only statistically significant between the control (0 mmHg/24 h)
and the 40 mmHg/24 h and 60 mmHg/24 h groups, and there was no
statistical significance between the control (0 mmHg/24 h) group
and the 20 mmHg/24 h and 80 mmHg/24 h groups (Fig. 4). The results of the expression of
GS mRNA were in accord with the results of the expression of the GS
protein.
Discussion
The present results demonstrated that i) the
pressure mechanism that we constructed was effective and that ii)
moderate pressure promotes the up-regulation of GS in active Müller
cells.
In recent years, a number of experimental techniques
have been used to chronically raise the IOP in a short period of
time. These techniques include laser photocoagulation of the
trabecular meshwork, episcleral vein cauterization, episcleral vein
injection of hypertonic saline and injection of substances into the
anterior chamber to obstruct aqueous outflow (28). Compared to glaucomatous models
in vivo, in vitro models are fewer. In this study,
many precautions were taken to limit artifacts from the
experimental method. Laboratory film (Pechiney) was used to seal up
the interfaces. To avoid artifacts due to ‘on-off’ changes in
pressure, all of the operations involving refreshment of the medium
or adjustment of pressure were carried out within 5 min. The
results (Table I) showed that the
pressure mechanism was effective.
Müller cells are the major glial cells of the retina
and constitute a functional link between neurons and vessels. They
maintain the integrity of the blood-retinal barrier, remove
metabolic waste and maintain the balance of the retinal
extracellular environment (ions, water, pH, neurotransmitters). In
the normal retina, Müller cells play a crucial role in degrading
the level of glutamate. Glutamate taken up by Müller cells is
converted, in large part, intracellularly, to glutamine through GS
(29–32).
In the present study, the change in GS expression in
the Müller cells was variable depending on the change of pressure.
At 20 mmHg of pressure, the expression of GS was lower than that in
the control. When the pressure was increased to 40 mmHg, the
expression of GS was markedly higher (Figs. 3 and 4). The expression level of GS was the
highest at a pressure of 60 mmHg and from then the level declined.
The present results showed that at an increased hydrostatic
pressure, Müller cells may malfunction. However, by further
elevating the hydrostatic pressure, Müller cells reacted rapidly,
with a return of functionality. Yet, at an excessively high
pressure, Müller cells may be damaged. Since Müller cells are the
main storage of glycogen (33) and
obtain ATP from glycolysis rapidly, longterm exposure to noxious
stress (extensively high hydrostatic pressure) which induces ATP
depletion, inhibits GS activity.
Although the relationship between the elevated
hydrostatic pressure and the increase in glutamate was not
determined in the present study, GS up-regulation in Müller cells
at a moderate hydrostatic pressure suggests that conversion ability
of glutamate in the Müller cells was reinforced. Therefore, we
propose that Müller cells adaptively react to GS up-regulation to
degrade the level of glutamate at an elevated but moderate
hydrostatic pressure.
Acknowledgements
This study was supported by the
National Natural Science Foundation of China (no. 81070728), the
Shanghai Leading Academic Discipline Project (no. S30205), the
Shanghai Municipal Education Committee Project (no. 10YZ38) and the
Shanghai Natural Science Foundation (no. 08RZ1413900).
References
|
1.
|
John C and Morrison MD: Integrins in the
optic nerve head: potential roles in glaucomatous optic neuropathy.
Trans Am Ophthalmol Soc. 104:453–477. 2006.PubMed/NCBI
|
|
2.
|
Bull ND, Limb GA and Martin KR: Human
Müller stem cell (MIO-M1) transplantation in a rat model of
glaucoma: survival, differentiation and integration. Invest
Ophthalmol Vis Sci. 49:3449–3456. 2008.
|
|
3.
|
Quigley HA: New paradigms in the
mechanisms and management of glaucoma. Eye. 19:1241–1248. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Sommer A: Intraocular pressure and
glaucoma. Am J Ophthalmol. 107:186–188. 1989. View Article : Google Scholar
|
|
5.
|
Carter-Dawson L, Shen F, Harwerth RS,
Smith EL, Crawford ML and Chang A: Glutamine immunoreactivity in
Müller cells of monkey eyes with experimental glaucoma. Exp Eye
Res. 66:537–545. 1998.
|
|
6.
|
Massey SC: Cell types using glutamate as a
neurotransmitter in the vertebrate retina. Progress in Retinal
Research. Osborne NN and Chader CJ: Pergamon; Oxford: pp. 399–425.
1990, View Article : Google Scholar
|
|
7.
|
Pow DV and Robinson SR: Glutamate in some
retinal neurons is derived solely from glia. Neuroscience.
60:355–366. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Ambati J, Chalam KV and Chawla DK:
Elevated gammaaminobutyric acid, glutamate, and vascular
endothelial growth factor levels in the vitreous of patients with
proliferative diabetic retinopathy. Arch Ophthalmol. 115:1161–1166.
1997. View Article : Google Scholar
|
|
9.
|
Shen F, Chen B, Danias J, Lee KC, Lee H
and Su Y: Glutamate-induced glutamine synthetase expression in
retinal Müller cells after short-term ocular hypertension in the
rat. Invest Ophthalmol Vis Sci. 45:3107–3112. 2004.
|
|
10.
|
Riepe RE and Norenberg MD: Müller cell
localization of glutamine synthetase in the rat retina. Nature.
268:654–655. 1977.
|
|
11.
|
Rauen T and Wiebner M: Fine tuning of
glutamate uptake and degradation in glial cells: common
transcriptional regulation of GLAST1 and GS. Neurochem Int.
37:179–189. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Linser PJ, Sorrentino M and Moscona AA:
Cellular compartmentalization of carbonic anhydrase-C and glutamine
synthethase in developing and mature mouse neural retina. Brain
Res. 315:65–71. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Newman E and Reichenbach A: The Müller
cells: a functional element of the retina. Trends Neurosci.
19:307–312. 1996.
|
|
14.
|
Dreyer EB, Zurakowski D, Schumer RA, Podos
SM and Lipton SA: Elevated glutamate in vitreous body of humans and
monkeys with glaucoma. Arch Opthalmol. 114:299–305. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Brooks DE, Garcia GA, Dreyer EB,
Zurakowski D and Franco-Bourland RE: Vitreous body glutamate
concentration in dogs with glaucoma. Am J Vet Res. 58:864–867.
1997.PubMed/NCBI
|
|
16.
|
Dkhissi O, Chanut E and Wasowicz M:
Retinal TUNEL-positive cells and high glutamate levels in vitreous
humor of mutant quail with a glaucoma-like disorder. Invest
Ophthalmol Vis Sci. 40:990–995. 1999.PubMed/NCBI
|
|
17.
|
Carter-Dawson L, Shen F, Harwerth RS,
Smith EL, Crawford ML and Chuang A: Glutamine immunoreactivity in
Müller cells of monkey eyes with experimental glaucoma. Exp Eye
Res. 66:537–545. 1998.
|
|
18.
|
Li S, Wang J, Wang D and Bai H: The
experimental study of effect of pressure on rat Müller cells in
vitro. Chin J Ophthamol. 41:325–329. 2005.
|
|
19.
|
Woldemussie E, Wijono M and Ruiz G: Müller
cell response to laser-induced increase in intraocular pressure in
rats. Glia. 47:109–119. 2004.
|
|
20.
|
Dreyer EB, Zurakowski D, Schumer RA, Podos
SM and Lipton SA: Elevated glutamate in vitreous body of humans and
monkeys with glaucoma. Arch Opthalmol. 114:299–305. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Carter-Dawson L, Crauford ML and Harwerth
RS: Vitreal glutamate concentration in monkeys with experimental
glaucoma. Invest Opthalmol Vis Sci. 43:2633–2637. 2002.PubMed/NCBI
|
|
22.
|
Honkanen RA, Baruah S and Zimmerman MB:
Vitreous amino acid levels in patients with glaucoma undergoing
vitrectomy. Arch Ophthalmol. 121:183–188. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Johnson TV and Tomarev SI: Rodent models
of glaucoma. Brain Res Bull. 81:349–358. 2010. View Article : Google Scholar
|
|
24.
|
Agar A, Yip SS, Hill MA and Coroneo MT:
Pressure-related apoptosis in neuronal cell lines. J Neurosci Res.
60:495–503. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Sappington RM, Chan M and Calkins DJ:
Interleukin-6 protects retinal ganglion cells from pressure-induced
death. Invest Opthalmol Vis Sci. 47:2932–2942. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Salvador-Silva M, Ricard CS, Agapova OA,
Yang P and Hernandez MR: Expression of small heat shock proteins
and intermediate filaments in the human optic nerve head astrocytes
exposed to elevated hydrostatic pressure in vitro. J Neurosci Res.
66:59–73. 2001. View
Article : Google Scholar
|
|
27.
|
Tezel G and Wax MB: Hypoxia-inducible
factor 1α in the glaucomatous retina and optic nerve head. Arch
Ophthalmol. 122:1348–1356. 2004.
|
|
28.
|
Reichenbach A, Wurm A, Pannicke T, Landiev
I, Wiedemann P and Bringmann A: Müller cells as players in retinal
degeneration and edema. Graefe’s Arch Clin Exp Ophthalmol.
245:627–636. 2007.
|
|
29.
|
Walsh N, Valter K and Stone J: Cellular
and subcellular patterns of expression of bFGF and CNTF in the
normal and light stressed adult rat retina. Curr Eye Res.
72:495–501. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Harada T, Harada C and Kohsaka S:
Microglia-Müller glia cell interactions control neurotrophic factor
production during lightinduced retinal degeneration. Neurosei.
22:9228–9236. 2002.
|
|
31.
|
Pow DV and Crook DK: Direct
immunocytochemical evidence for the transfer of glutamine from
glial cells to neurons: use of specific antibodies directed against
the D-steroisomers of glutamate and glutamine. Neuroscience.
70:295–302. 1996. View Article : Google Scholar
|
|
32.
|
Johnson TV and Tomarev SI: Rodent models
of glaucoma. Brain Research Bulletin. 81:349–358. 2010. View Article : Google Scholar
|
|
33.
|
Tsacopoulos M: Metabolic signaling between
neurons and glial cells: a short review. J Physiol Paris.
96:283–288. 2002. View Article : Google Scholar : PubMed/NCBI
|