Introduction
Antimicrobial peptides (AMPs) are amphiphilic,
positively charged molecules (1–4) that
have emerged as novel antimicrobial agents for use in therapeutics,
animal drugs and food preservatives. In recent years, many
different types of AMPs have been identified in various organisms,
including amphibians, mammals, plants, invertebrates and
prokaryotes (1,5–7).
Currently, the database of the National Center for Biotechnology
Information records more than 800 sequences of antibacterial
peptides and proteins from both animals and plants.
The cecropin family of antibacterial peptides is
found in the immune hemolymph of silkworm pupae (8), insects and mammals (9,10)
and plays an important role in the innate immune systems of these
animals. The cecropin family is comprised of cecropins A, B, C, D,
E and F, all of which are 35 to 39 amino acids in length and share
highly homologous sequences (11).
These peptides were originally described as having a broad spectrum
of antibacterial activity against both Gram-positive and
Gram-negative bacteria, but lacking the ability to lyse eukaryotic
cells (12). Therefore, cecropins
are considered to be valuable peptide antibiotics, particularly
against bacteria that have developed resistance to chemical
antibiotics. Moreover, previous studies (5) have demonstrated that cecropins lyse
cancer cells, opening the possibility for their use in medical
applications (13).
Over the past decade, bacterial resistance to
antibiotics has risen dramatically. Thus, there is an increasing
need to discover and introduce novel effective antibiotic drugs.
Cationic antimicrobial peptides are one potential source of novel
antibiotics. Antimicrobial peptides are naturally occurring
antibiotics (14). As part of the
innate immune system of vertebrates, these peptides have direct
antimicrobial function (15).
However, few peptide antibiotics have been tested in clinical
trials, and these few have exhibited mixed results (16–19).
ABP-CB is a promising novel antibiotic candidate. However, the
yield of ABP-CB extracted from the silkworm is fairly small. The
use of recombinant expression methods may allow for the production
of a sufficient quantity of ABP-CB in order to meet the demand. We
expressed ABP-CB in the methylotrophic yeast Pichia
pastoris, which has been widely utilized as a heterologous gene
expression system. The increasing popularity of this expression
system is attributed to three key advantages: i) it possesses a
highly regulated alcohol oxidase (AOX1) promoter gene that can be
induced by methanol and repressed by glucose and glyceroland; ii) a
minimal salt medium yields a high cell density, making this system
cost-effective; and iii) P. pastoris possesses subcellular
organelles, such as an endoplasmic reticulum and Golgi apparatus,
allowing for post-translational modifications that include protein
folding, disulfide bridge formation and glycosylation (20–24).
Here, the synthesis of the ABP-CB gene, the
extracellular expression of ABP-CB protein in P. pastoris
SMD 1168 and the characterization of the biological activity of
recombinant ABP-CB are described in detail.
Materials and methods
Materials
E. coli DH5α was used for cloning the
pPICZα-A-ABP-CB plasmid. E. coli K88 and Staphylococcus
aureus (Cowan I) were used for the antibacterial assay.
The pPICZα-A vector (Invitrogen) was used for the
expression of ABP-CB. The P. pastoris strain SMD1168
(his− mut+) was used for the expression of
the ABP-CB protein.
The restriction enzymes XhoI, XbaI and
SacI and T4 DNA ligase were purchased from Takara (Nanjing,
China).
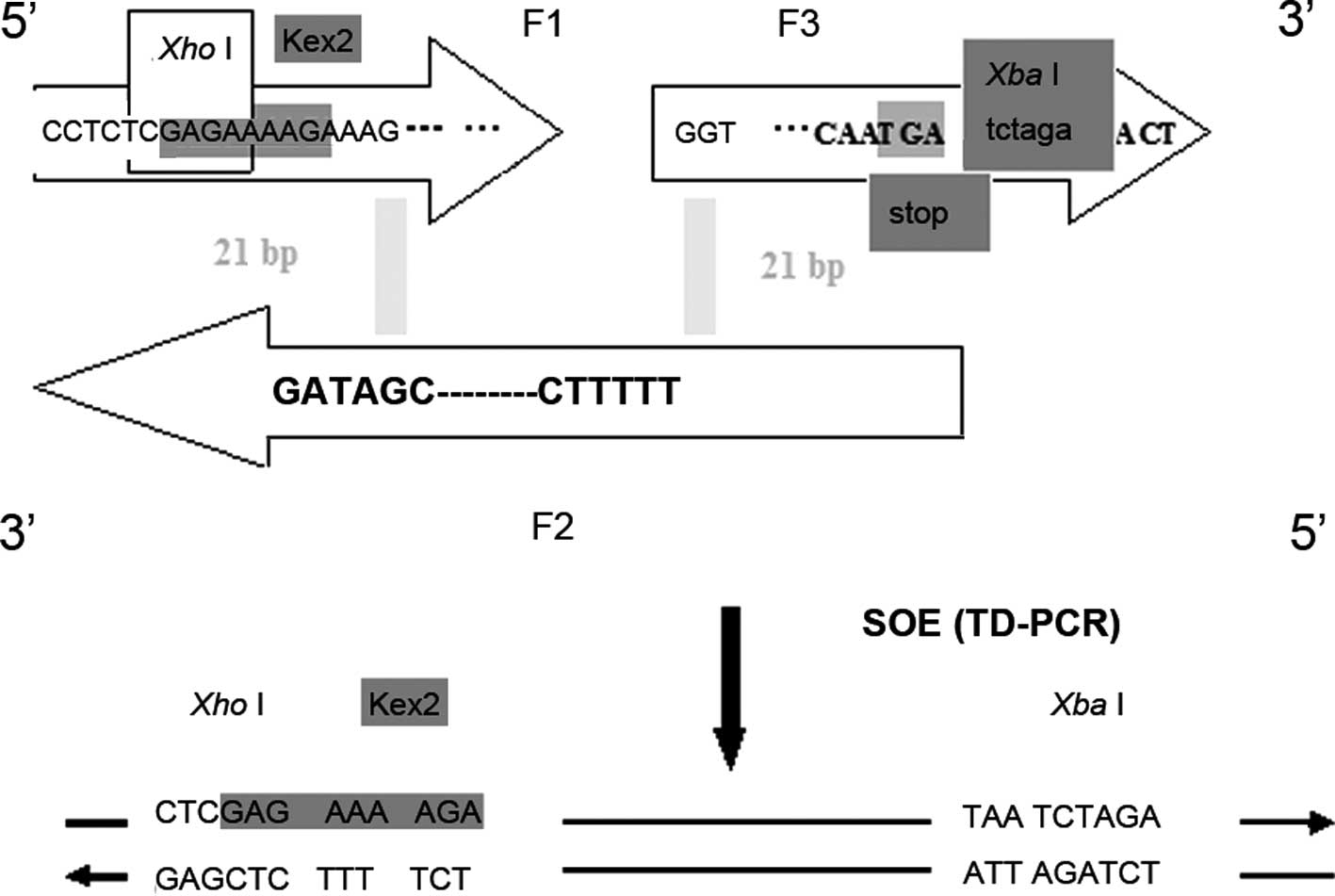
Synthesis of cecropin B
Three 60-bp oligomers with 20 bases complementary to
their 3′ ends were designed based on the CB amino acid sequence. In
order to express the native N-terminus of CB, a XhoI
restriction site was introduced into the α-factor secretion signal
of the pPICZ-A expression vector to allow in-frame cloning, and a
nucleotide sequence encoding the KEX2 cleavage site was placed
upstream of the CB gene. At the C-terminus, an Asn codon, a
XbaI restriction site and a stop codon were introduced. The
sequences of the primers were as follows: F1,
cctctcgagaaaagaaagtggaaggtcttcaagaaga tcgaaaagatgggtcgtaac; F2,
gatagctggaccagccttgacgataccgttacg gatgttacgacccatcttttc; F3,
gctggtccagctatcgctgtcctaggtgaagct aaggctctacaa tga tct aga act.
Gene splicing by overlap extension (SOE) was used
for gene synthesis (Fig. 1). An
additional pair of primers was designed for identification of the
recombinant vector and yeast. The upstream primer targeted the 5′
AOX gene region of P. pastoris, and the downstream primer
targeted the insertion region of CB; the amplification length was
496 bp. The sequences of the primers were as follows: P1: 5′ GTCTCC
ACATTGTATGCTTC3′ ; P2, 5′ CTGTGCAGGAACTTGAT3′ .
All primers were synthesized by the Shanghai Branch
Office of Invitrogen.
PCR amplification was performed according to the SOE
method using the F1, F2 and F3 elements as templates and primers.
In addition, Touchdown (TD) PCR technology was used to optimize the
PCR method in order to ensure the specificity of the SOE synthesis.
The TD-PCR reaction involved the following solutions: 5 μl 10X
Mg2+-free PCR buffer; 3 μl 25 mmol/l MgCl2; 1
μl 10 mmol/l dNTP; 2 μl each 40 pmol/l F1, F2, F3; 0.5 μl Takara
ExTaq™; and 38.5 μl sterile ultrapure water. All solutions were
mixed uniformly and centrifuged for use in TD-PCR. The reaction was
initialized at 94°C for 2 min and TD-PCR circulation was performed
at 94°C for 30 sec; the annealing temperature was decreased from 65
to 50°C in 0.5°C increments/min and maintained at 72°C for 1 min;
the temperature was then maintained at 50°C after 30 cycles, and
then another 15 cycles were performed at an optimization
temperature of 52°C; extension was performed at 72°C for 6 min.
Construction of recombinant expression
plasmids
PCR products and pPICZα-A vectors were digested with
XhoI and XbaI, gel-purified and ligated together.
E. coli DH5α was chemically transformed with the recombinant
vectors and then cultured at 37°C on low-salt LB supplemented with
25 μg/ml Zeocin for selection of recombinants. The nucleotide
sequence of the recombinant pPICZα-A-CB plasmid was verified by
restriction analysis and DNA sequencing in order to ensure 100%
identity with the expected sequence and in-frame orientation. The
positive clones were then grown for further DNA transformation.
Transformation and PCR analysis of P.
pastoris
Yeast cells were transformed using the Pichia
Expression kit. Briefly, 80 μl of competent P. pastoris
SMD1168 cells mixed with 5 μg SacI-linearized pPICZα-A-CB
were transferred into an ice-cold 0.2-cm electroporation cuvette
and incubated in an ice bath for 5 min. Immediately following
electroporation at 1.5 kV, 25 µF, 200 Ω, 1 ml of ice-cold 1 M
sorbitol was added to the cuvette, which was then incubated for 1 h
at 30°C without shaking. Aliquots of 200 μl were spread on YPDS
plates supplemented with 100 μg/ml Zeocin, and the plates were
incubated at 30°C until colonies appeared. As a negative control,
linearized pPICZα-A vector alone was transformed into P.
pastoris SMD1168 cells.
The transformants bearing the genomic integrants of
the pPICZα-A-CB plasmids were identified by genomic PCR using
primers P1 and P2. The method of ‘boiling-freezing-boiling’ was
used to prepare the Pichia genomic DNA template. PCR amplification
was performed with the following conditions: initial denaturation
at 94°C for 5 min, followed by 26 cycles at 94°C for 1 min, 46°C
for 1 min and 72°C for 1 min, and elongation at 72°C for 7 min.
Induction of ABP-CB expression in
recombinant P. pastoris using the shake-flask method
A single colony of transformants was grown in 5 ml
of buffered glycerol-complex medium [BMGY, 1% (w/v) yeast extract,
2% (w/v) peptone, 1.34% YNB, 1% glycerol, 0.4 μg/ml biotin,
buffered with 1/10 volume potassium phosphate buffer pH 6.0] in a
50-ml flask. The culture was grown at 28°C in a rotating shaker at
230 rpm for 24 h until the culture reached an OD600
equal to 5. The culture was harvested after centrifugation at 1,500
g for 5 min at room temperature. To induce ABP-CB expression, the
pellet was resuspended in 25 ml of buffered methanol-complex medium
[BMMY, 1% (w/v) yeast extract, 2% (w/v) peptone, 1.34% YNB, 0.5%
methanol, 0.4 μg/ml biotin, buffered with 1/10 volume potassium
phosphate buffer pH 6.0] in a 250-ml flask and grown at 28°C with
shaking. To maintain expression of ABP-CB, methanol was added to
the culture at a final concentration of 1%. In addition, aliquots
were sampled throughout the incubation time in order to perform
activity assays. Several parameters that could influence the level
of protein expression were analyzed. Antibacterial activity assays
were performed by measuring zones of growth inhibition of E.
coli K88 in thin agar plates, as described by Cipakova et
al (25) A total of 100 μl of
each condensed expression supernatant was added to the wells.
Determination of ABP-CB expression
efficiency and protein concentration
The efficiency of ABP-CB expression was assessed by
Tricine-SDS-PAGE. Protein concentration was determined using the
Bio-Rad dye agent with bovine serum albumin (BSA) as a standard
(23).
Purification of secreted cecropin B
After 60 h of culture, the culture medium (100 ml)
was collected by centrifugation at 12,000 g for 30 min. The
supernatant was filtrated using the Amicon ultrafiltration device.
The filtrate, which contained proteins that ranged from 3 to 10 kDa
in size, was dialyzed overnight in 0.1 M sodium acetate and then
applied to a CM-Sepharose CL-6B column (Pharmacia Biosciences, USA)
pre-equilibrated with 0.1 M sodium acetate (pH 4.5). The column was
washed with 0.1 M acetate buffer, and the proteins were eluted
using a linear gradient of 0.1–1.0 M sodium acetate (pH 4.5). The
purified proteins were analyzed by Tricine-SDS-PAGE.
Antimicrobial assays
The antimicrobial activity of CB was tested against
several Gram-positive and Gram-negative bacteria. The minimal
growth inhibition concentration (MIC), which is defined as the
lowest concentration of a peptide that can inhibit the growth of a
microorganism (26), was
determined by performing a liquid growth inhibition assay (27). A stock solution of CB was serially
diluted 10-fold in 0.01% acetic acid and 0.2% BSA. Aliquots of each
dilution (10 μl) were distributed into the wells of a 96-well
polypropylene microtiter plate, and each well was inoculated with
100 μl of a suspension of mid-log bacteria (2–7×105
CFU/ml) in LB broth. Cultures were grown for 24 h at 30°C with
vigorous shaking, and the bacterial concentration was evaluated by
measuring the absorbance of the cultures at 600 nm using a
microplate reader (Bio-Rad, USA).
Acid stability analysis of recombinant
CB
Buffers of pH ranging from 2.5 to 8.5 were prepared.
Antibacterial CB peptide (20 μl) and of the different buffers were
mixed in order to check the acid stability of CB. Buffer alone was
used as a negative control.
Heat stability of recombinant CB
Recombinant CB was heated at 60, 80 and 100°C for 5
min, and then centrifuged at 12,000 rpm for 10 min in order to
separate the precipitate. The supernatant was then used in the
antibacterial assays.
Results
Construction and transformation of
pPICZα-A-CB
Fig. 1 shows a
schematic representation of the sequence of the synthetic CB
peptide. The amplification of the CB gene was achieved through
TD-PCR, resulting in a product of 129 bp in length. After
transformation, transformants were screened by restriction enzyme
analysis using XhoI and XbaI and sequencing. The
results of restriction enzyme analysis and DNA sequencing revealed
that the CB gene was inserted correctly into the expression vector.
Transformation with the SacI-linearized version of
pPICZα-A-CB favored its insertion into the yeast genome by
homologous recombination.
Determination of the optimal conditions
for secretion of recombinant CB
The activity of the recombinant CB secreted by P.
pastoris was analyzed under several different conditions. The
supernatant was collected and its antimicrobial activity was
monitored by performing bacterial inhibition assays. In addition,
several parameters that affect the level of protein expression were
assayed. The secretion of proteases into the medium, and possibly
their release due to cell lysis, is a significant problem in many
high-cell-density cultures because it can lead to proteolytic
degradation. The induction temperature is also an important
parameter. In P. pastoris, incubation at 30, 25, 20 and 15°C
was performed in attempts to minimize extracellular proteolysis.
Lower temperatures have been shown to reduce protease levels and to
greatly enhance the yield of biologically active proteins in P.
pastoris (8,28–31).
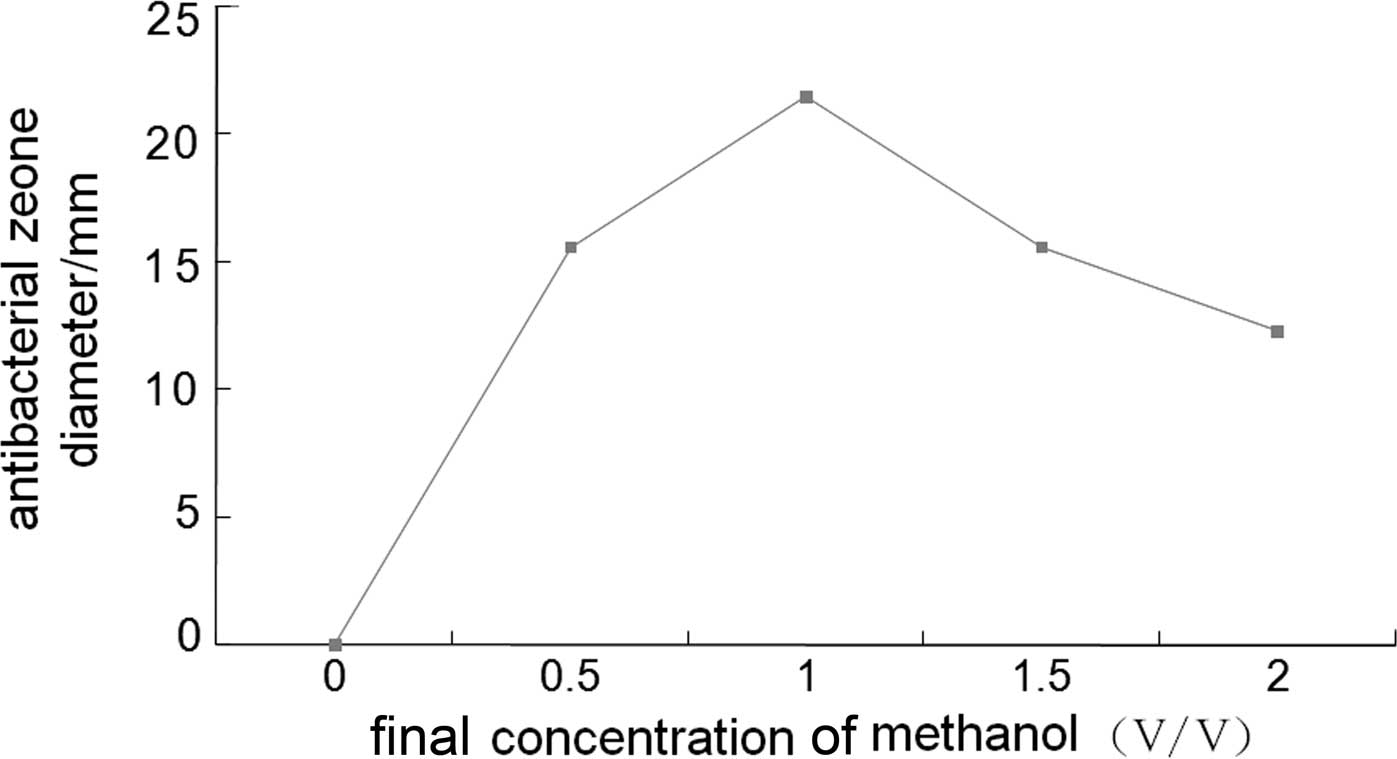
We found that reducing the incubation temperature from 30 to 20°C
during the methanol-feed phase dramatically increased the yield of
recombinant protein (data not shown). Culture of P. pastoris
on BMMY medium at pH 6.0 provided the most optimal conditions for
the expression of CB, and a methanol induction time of 60 h was the
most suitable (Figs. 2 and
3).
Expression of CB in P. pastoris
The highest CB-producing P. pastoris clone
was selected for subsequent large-scale protein expression under
the most optimal conditions of 28°C with 1.0% methanol for 60 h.
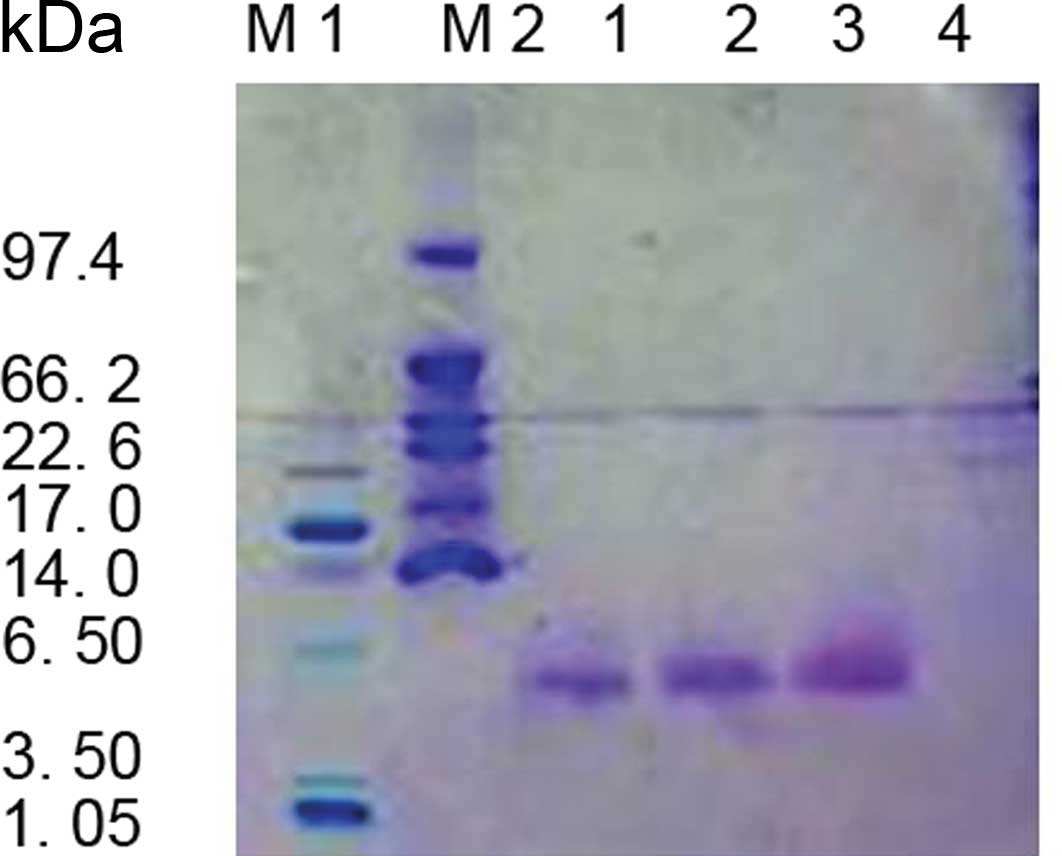
Approximately 50 mg of ABP-CB was secreted into 1 l of medium. The
resultant protein appeared as a single homogeneous band on a
Coomassie blue-stained Tricine-SDS-PAGE gel (Fig. 4). The size of the recombinant CB
was consistent with the expected molecular weight of 4.7 kDa
calculated from the amino acid sequence.
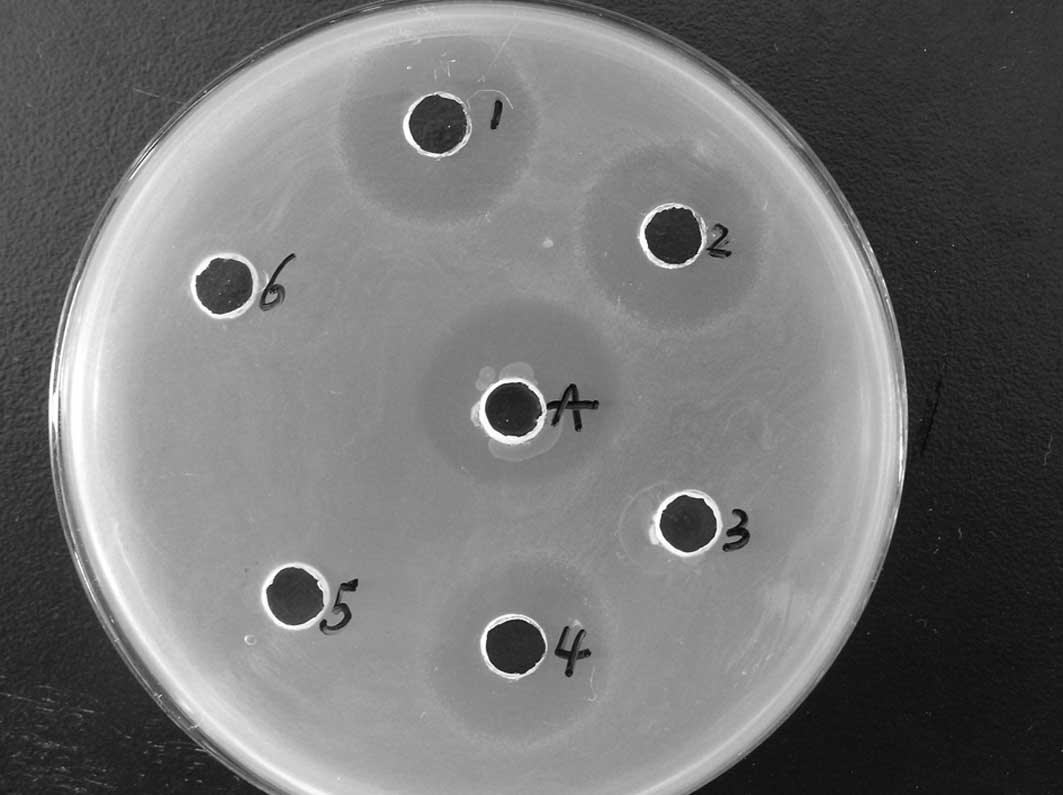
Antimicrobial assay
The antibacterial activity of recombinant CB was
qualitatively determined with a radial diffusion assay using E.
coli as a representative Gram-negative bacterium. CB was found
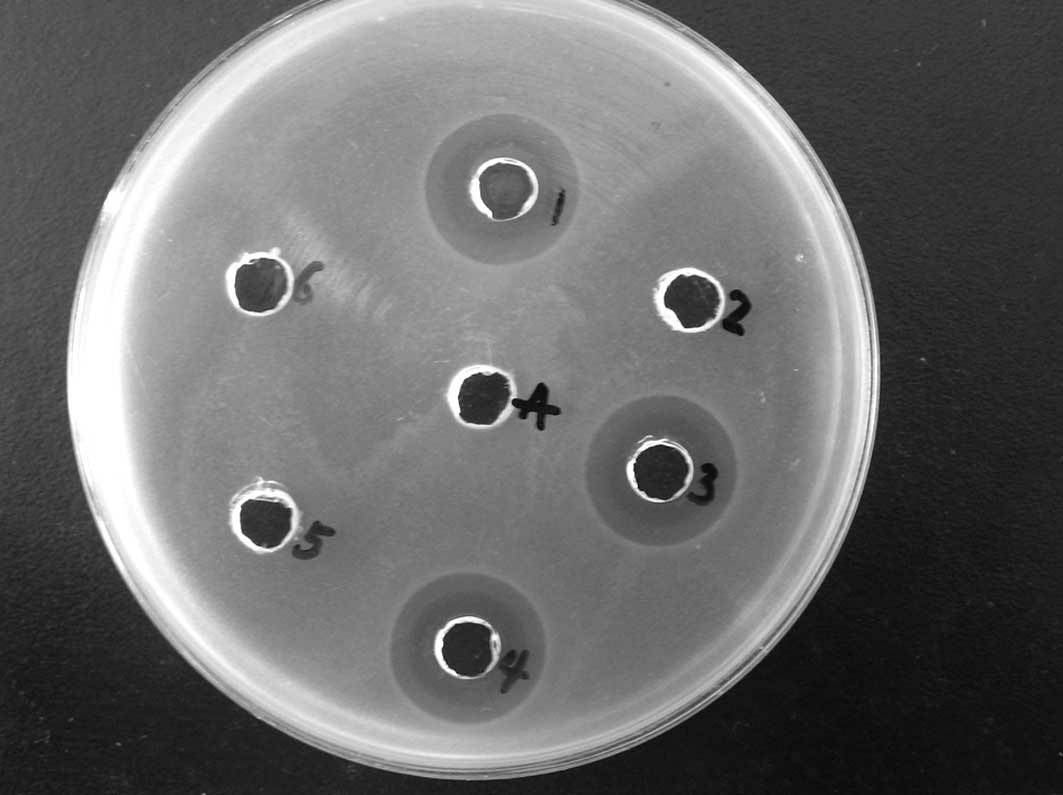
to be highly effective against E. coli (Fig. 5). Similar results were obtained
from radial diffusion assays using the Gram-positive bacterium
S. aureus (Fig. 6, Table I).
 | Table I.Antibacterial activity of cecropin B
(MIC) expressed as the final concentration in μM. |
Table I.
Antibacterial activity of cecropin B
(MIC) expressed as the final concentration in μM.
| Microorganism | MIC (μM) |
|---|
| Gram-positive
bacteria | |
| Staphylococcus
aureus | 0.89 |
| Bacillus
subtilis | 0.98 |
|
Sporosarcina | 0.88 |
|
Curtobacterium | 0.97 |
| Gram-negative
bacteria | |
| E.
coli | 0.50 |
| Salmonella
pullorum | 0.78 |
| Pseudomonas
aeruginosa | 0.98 |
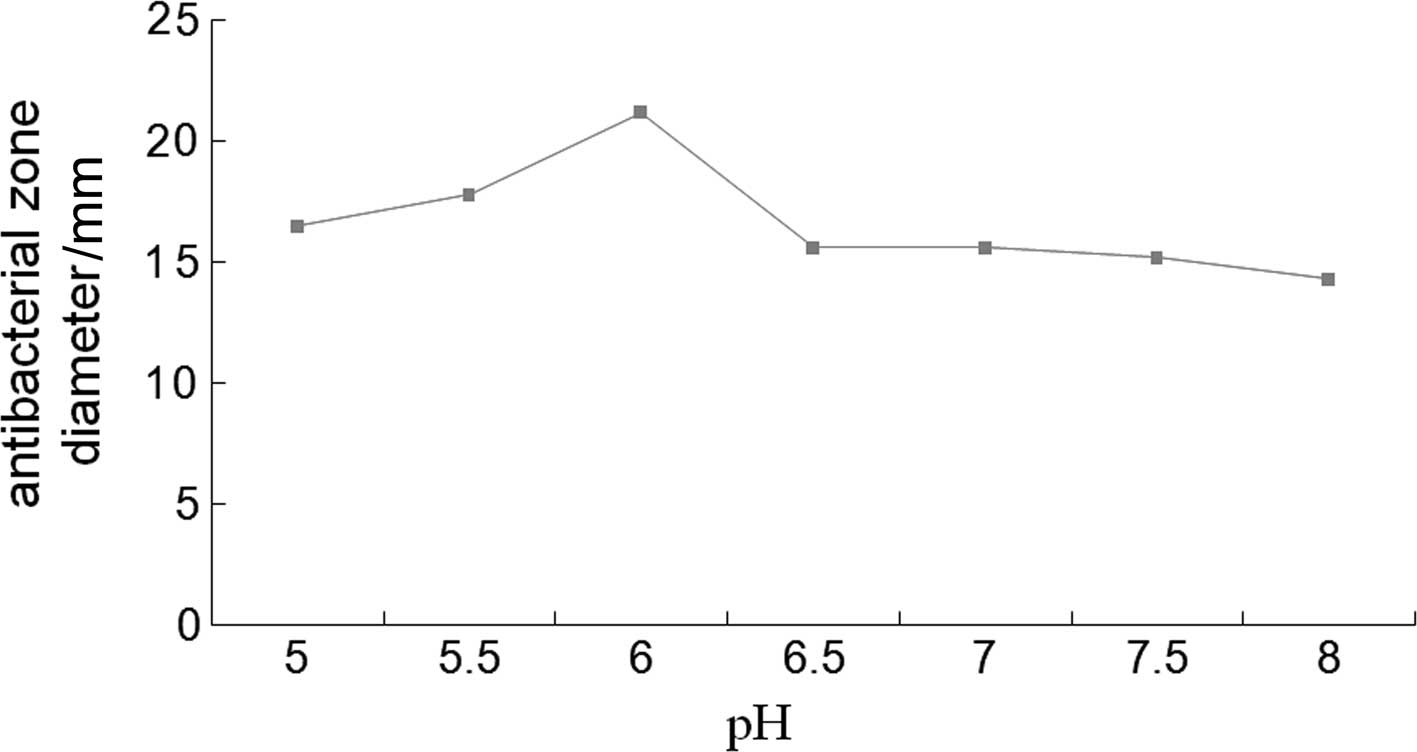
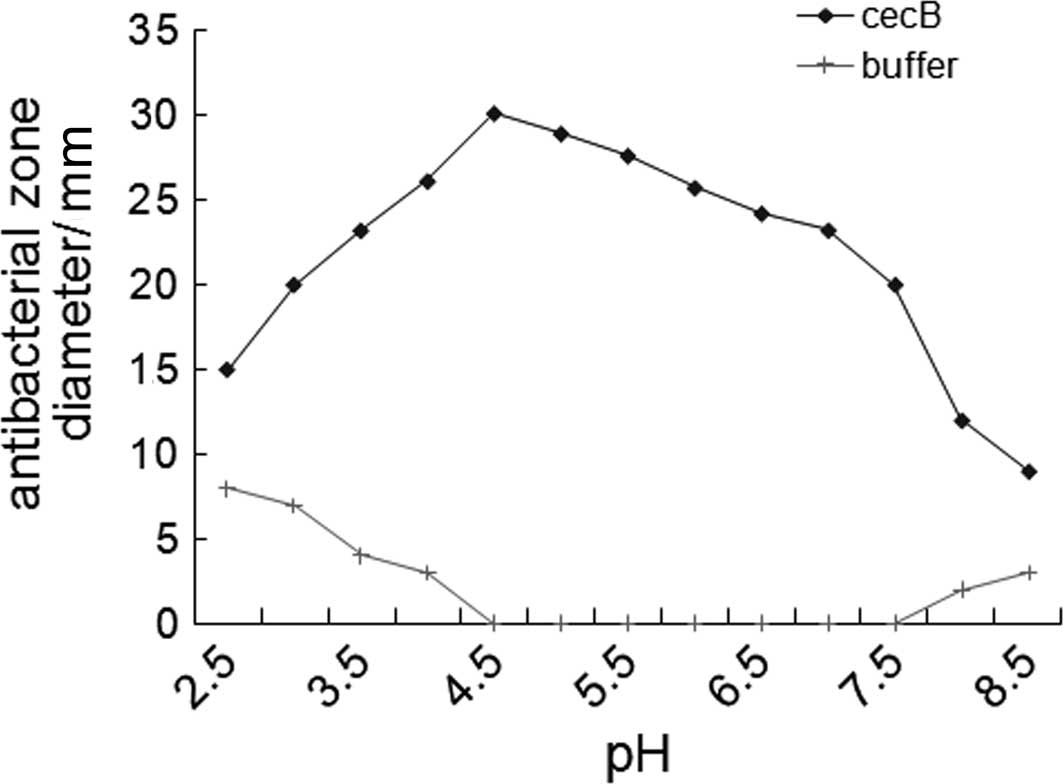
Acid stability of recombinant CB
Recombinant CB had the strongest antibacterial
activity at pH 4.0. There were no obvious changes observed in the
diameters of the antibacterial zone at pH values between 3.5 and
7.5. The diameter of the antibacterial zone decreased gradually at
pH values >7.5. Recombinant CB was stable at low (3.5) and high
pH (8.5), resulting in suppressed antibacterial activity (Fig. 7).
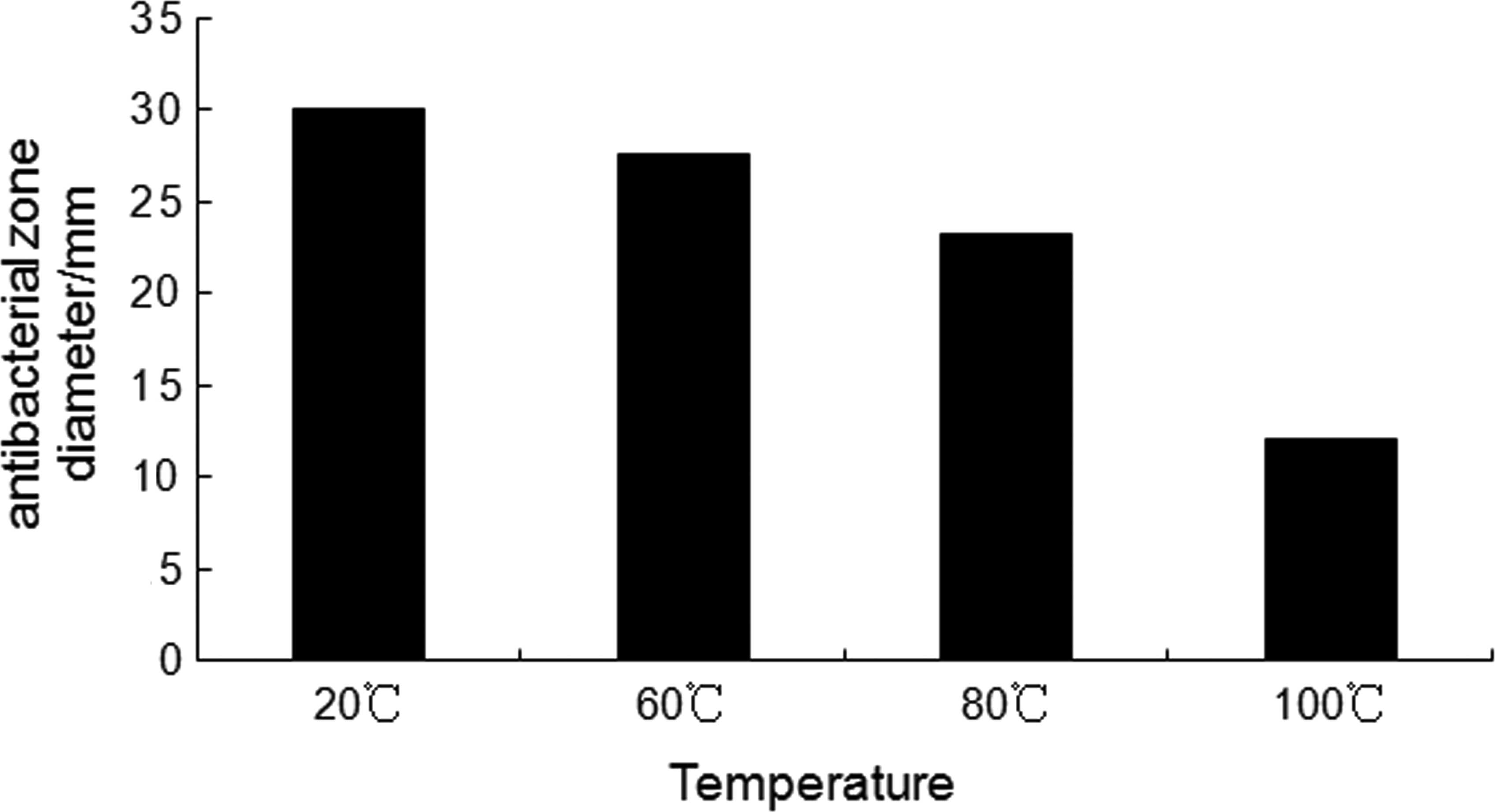
Heat stability of recombinant CB
Incubation of the CB supernatant at 60°C for 5 min
had no effect on its antibacterial activity. We found that there
were many ingredients in the supernatant of the CB peptide, which
were removed by treatment at high temperature. Boiling the
supernatant for 30 min removed approximately 60% of the mixed
proteins found in the supernatant without changing the heat
resistance of CB (Fig. 8).
Discussion
Cecropins are a family of small basic polypeptides
that are mainly present in the hemolymph of insects (30). CB exhibits significant
antibacterial activity against Gram-positive and Gram-negative
bacteria, and also has potent antitumor activity against certain
transformed tumor cells, but rather than the hemolytic effect on
human erythrocytes. To date, potent CB analogues have been
synthesized and have been proven to exhibit effective antibacterial
activity; however, heterologous expression of recombinant cecropin
has seldom been reported (32,33).
Heterologous expression of recombinant proteins results in toxicity
for the host cells (27). For a
peptide to be used as an antibiotic, the fusion protein partner
must be removed from the bacterial expression system; this process
is expensive and laborious. Therefore, in the present study, the
methylotrophic yeast P. pastoris was used as a host for high
level expression and secretion of recombinant CB. Recombinant CB
was successfully secreted by P. pastoris into the culture
supernatant, and 5.0 mg of recombinant CB was obtained from 100 ml
of crude extract. The large-scale production of antibiotic
peptides, such as cecropin, will require further exploration
regarding the manufacturing methods.
Previously, it was reported that the recombinant
protein Psd1, which originates from the C-terminus of the α-factor
secretion signal of S. cerevisiae and contains four
additional amino acids (EAEA) at the N-terminal region, was
produced by P. pastoris. In addition, recombinant Psd1 was
shown to decrease antifungal activity against A. niger by at
least 10-fold (22,34). In the present study, we cloned the
gene encoding CB, with only the KEX2 cleavage site, by recursive
PCR in order to obtain CB protein with its native N-terminus.
Tricine-SDS-PAGE revealed that CB was successfully secreted by the
P. pastoris α-mating factor signal sequence into the culture
supernatant. Activity assays demonstrated that CB had low MIC
against all Gram-negative and Gram-positive bacteria tested.
Additionally, our results showed that the signal peptide was
efficiently processed by the KEX2 protease through the P.
pastoris secretory pathway.
Expression of a foreign gene, in this case CB, in
P. pastoris and secretion of the active antibacterial
peptide suggest that the CB gene could be introduced into animal
cells in order to enhance their resistance to bacterial pathogens.
Previously, we showed that both CB and cecropin P1 produced by
transgene constructs under the control of the cytomegalovirus (CMV)
promoter in Chinook salmon embryo cells (CHSE-214) exhibited
bactericidal activity against three pathogens (35). In addition, the expression of
cecropin in yeast and fish cells demonstrates that the cecropin
gene can be introduced into animal cells. Moreover, the expression
of CB by transgenic plants has also been reported (36,37).
However, further studies are required to address the feasibility of
generating cecropin-expressing transgenic animals for the purpose
of increasing their resistance to microbial diseases. In this
study, we showed that heterologous CB can be expressed in P.
pastoris under the promoter for the secretion signal of the
yeast-mating factor. Recombinant CB was expressed at a
concentration of 50 mg/l in culture medium, and it exhibited strong
antimicrobial activity. Our results show that P. pastoris is
a robust system that can be used for the expression of secreted CB,
which exhibits antibacterial activity against both Gram-positive
and Gram-negative bacteria (7–11).
References
|
1.
|
Devine DA and Hancock RE: Cationic
peptides: distribution and mechanisms of resistance. Curr Pharm
Des. 8:703–714. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Ganz T: Defensins: antimicrobial peptides
of innate immunity. Nat Rev Immunol. 3:710–720. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Segrest JP, De Loof H, Dohlman JG,
Brouillette CG and Anantharamaiah GM: Amphipathic helix motif:
classes and properties. Proteins. 8:103–117. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Zasloff M: Antimicrobial peptides of
multicellular organisms. Nature. 415:389–395. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Boman HG: Peptide antibiotics and their
role in innate immunity. Annu Rev Immunol. 13:61–92. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Noga EJ and Silphaduang U: Piscidins: a
novel family of peptide antibiotics from fish. Drug News Perspect.
16:87–92. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Steiner H, Hultmark D, Engstrom A, Bennich
H and Boman HG: Sequence and specificity of two antibacterial
proteins involved in insect immunity. Nature. 292:246–248. 1981.
View Article : Google Scholar
|
|
8.
|
Boman HG and Hultmark D: Cell-free
immunity in insects. Annu Rev Microbiol. 41:103–126. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Lee JY, Boman A, Sun CX, Andersson M,
Jornvall H, Mutt V and Boman HG: Antibacterial peptides from pig
intestine: isolation of a mammalian cecropin. Proc Natl Acad Sci
USA. 86:9159–9162. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Hultmark D, Engstrom A, Bennich H, Kapur R
and Boman HG: Insect immunity: isolation and structure of cecropin
D and four minor antibacterial components from Cecropia
pupae. Eur J Biochem. 127:207–217. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Boman HG, Wade D, Boman IA, Wahlin B and
Merrifield RB: Antibacterial and antimalarial properties of
peptides that are cecropin-melittin hybrids. FEBS Lett.
259:103–106. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Moore AJ, Devine DA and Bibby MC:
Preliminary experimental anticancer activity of cecropins. Pept
Res. 7:265–269. 1994.PubMed/NCBI
|
|
13.
|
Hancock RE and Lehrer R: Cationic
peptides: a new source of antibiotics. Trends Biotechnol. 16:82–88.
1998.PubMed/NCBI
|
|
14.
|
Hancock RE and Patrzykat A: Clinical
development of cationic antimicrobial peptides: from natural to
novel antibiotics. Curr Drug Targets Infect Disord. 2:79–83. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Diamond G: Natures antibiotics: the
potential of antimicrobial peptides as new drugs. Biologist.
48:209–212. 2001.PubMed/NCBI
|
|
16.
|
Andres E and Dimarcq JL: Cationic
antimicrobial peptides: from innate immunity study to drug
development. Med Mal Infect. 37:194–199. 2007.PubMed/NCBI
|
|
17.
|
Cereghino JL and Cregg JM: Heterologous
protein expression in the methylotrophic yeast Pichia
pastoris. FEMS Microbiol Rev. 24:45–66. 2000. View Article : Google Scholar
|
|
18.
|
Sreekrishna K, Brankamp RG, Kropp KE,
Blankenship DT, Tsay JT, Smith PL, Wierschke JD, Subramaniam A and
Birkenberger LA: Strategies for optimal synthesis and secretion of
heterologous proteins in the methylotrophic yeast Pichia
pastoris. Gene. 190:55–62. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Cregg JM, Cereghino JL, Shi J and Higgins
DR: Recombinant protein expression in Pichia pastoris. Mol
Biotechnol. 16:23–52. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Boze H, Celine L, Patrick C, Fabien R,
Christine V, Yves C and Guy M: High-level secretory production of
recombinant porcine follicle-stimulating hormone by Pichia
pastoris. Process Biochem. 36:907–913. 2001. View Article : Google Scholar
|
|
21.
|
Lee CY, Lee SJ, Jung KH, Katoh S and Lee
K: High dissolved oxygen tension enhances heterologous protein
expression by recombinant Pichia pastoris. Process Biochem.
38:1147–1154. 2003. View Article : Google Scholar
|
|
22.
|
Almeida MS, Cabral KS, de Medeiros LN,
Valente AP, Almeida FC and Kurtenbach E: cDNA cloning and
heterologous expression of functional cysteine-rich antifungal
protein Psd1 in the yeast Pichia pastoris. Arch Biochem
Biophys. 395:199–207. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Ausubel FM, Brent R and Kingston RE:
Protocols in molecular biology. Trends Cell Biol. 6:366–367.
1996.
|
|
24.
|
Lehrer RI, Rosenman M, Harwig SS, Jackson
R and Eisenhauer P: Ultrasensitive assays for endogenous peptides.
J Immunol Methods. 137:167–173. 1991. View Article : Google Scholar
|
|
25.
|
Cipakova I, Hostinova E, Gasperik J and
Velebny V: High-level expression and purification of a recombinant
hBD-1 fused to LMM protein in Escherichia coli. Protein Expr
Purif. 37:207–212. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Shi X, Karkut T, Chamankhah M, Alting-Mees
M, Hemmingsen SM and Hegedus D: Optimal conditions for the
expression of a single-chain antibody (scFv) gene in Pichia
pastoris. Protein Express Purif. 28:321–330. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Jahic M, Gustavsson M, Jansen AK,
Martinelle M and Enfors SO: Analysis and control of proteolysis of
a fusion protein in Pichia pastoris fed-batch processes. J
Biotechnol. 102:45–53. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Li SZ, Xiong F and Lin Q: Low-temperature
increases the yield of biologically active herring antifreeze
protein in Pichia pastoris. Protein Express Purif.
21:438–445. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Bencurova M, Rendic D, Fabini G, Kopecky
EM, Altmann F and Wilson IB: Expression of eukaryotic
glycosyltransferases in the yeast Pichia pastoris.
Biochimie. 85:413–422. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Skosyrev VS, Kulesskiy EA, Yakhnin AV,
Temirov YV and Vinokurov LM: Expression of the recombinant
antibacterial peptide sarcotoxin IA in Escherichia coli
cells. Protein Expr Purif. 28:350–356. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Aly R, Granot D, Mahler-Slasky Y, Halpern
N, Nir D and Galun E: Saccharomyces cerevisiae cells
harboring the gene encoding sarcotoxin IA secrete a peptide that is
toxic to plant pathogenic bacteria. Protein Expr Purif. 16:120–124.
1999. View Article : Google Scholar
|
|
32.
|
Yamada K, Nakajima Y and Nator S:
Production of recombinant sarcotoxin IA in Bombyx mori
cells. Biochem J. 272:633–636. 1990.PubMed/NCBI
|
|
33.
|
Rao XC, Li S, Hu JC, Jin XL, Hu XM, Huang
JJ, Chen ZJ, Zhu JM and Hu FQ: A novel carrier molecule for
high-level expression of peptide antibiotics in Escherichia
coli. Protein Expr Purif. 36:11–18. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Cabral KM, Almeida MS, Valente AP, Almeida
FC and Kurtenbach E: Production of the active antifungal Pisum
sativum defensin 1 (Psd1) in Pichia pastoris: overcoming
the inefficiency of the STE13 protease. Protein Expr Purif.
31:115–122. 2003.PubMed/NCBI
|
|
35.
|
Sarmasik A and Chen TT: Bactericidal
activity of cecropin B and cecropin P1 expressed in fish cells
(CHSE-214): application in controlling fish bacterial pathogens.
Aquaculture. 220:183–194. 2003. View Article : Google Scholar
|
|
36.
|
Sessitsch A, Kan FY and Pfeifer U:
Diversity and community structure of culturable Bacillus
spp. populations in the rhizospheres of transgenic potatoes
expressing the lytic peptide cecropin B. Appl Soil Ecol.
22:149–158. 2003.
|
|
37.
|
Sharma A, Sharma R, Imamura M, Yamakawa M
and Machii H: Transgenic expression of cecropin B, an antibacterial
peptide from Bombyx mori, confers enhanced resistance to
bacterial leaf blight in rice. FEBS Lett. 484:7–11. 2000.
View Article : Google Scholar : PubMed/NCBI
|