Introduction
Gastric cancer is the second most common cause of
cancer-related death in the world (1). Gastric cancer is difficult to cure,
mainly because most patients present with advanced disease at
diagnosis. Patients with gastric cancer conventionally exhibit a
poorly functioning immune system, such as decreased T-cell
proliferation, a low CD4+/CD8+ ratio and a
deficient production of T-helper cytokines (2–4).
This condition of poor response does not improve significantly
after surgery (5).
Gastric adenocarcinoma often begins at a site where
the stomach lining is inflamed. Numerous experts believe that an
infection with the bacterium Helicobacter pylori is the
cause of most stomach cancers (6–8). It
has been reported that H. pylori-infected individuals have
increased levels of regulatory T cells (Tregs) in the gastric and
duodenal mucosa that express factor forkhead box protein 3 (foxp3)
mRNA. The induction of the Treg response contributes to an
equilibrium between the host and the bacterium, allowing H.
pylori to survive, while also preventing the risk of
destructive inflammation (9).
These reports suggest that FOXP3 expression may play an important
role in gastric cancer development.
FOXP3 is a member of the forkhead/winged-helix
family of transcriptional regulators and is considered to be an
important gene which functions as a regulator of thymically derived
naturally occurring regulatory T cells (nTregs) (10). Furthermore, transfection of Foxp3
allows CD4+ FOXP3− human T cells to acquire
many characteristics of Tregs (11,12).
Various studies have found the expression of FOXP3 in pancreatic
carcinoma cells, melanoma cells, hepatocellular carcinoma and other
types of tumor cells (13–15). They demonstrated not only the
existing function, but various immune functions of FOXP3 in a few
types of cancers. They described that the expression of FOXP3 has
some influence on the production of IL-10 and TGF-β2. These
cytokines are well known for playing immune inhibition roles in
tumor development and escape. Although there are contradictions
concerning the functions of FOXP3 in different types of tumors,
these results suggest another important biological characteristic
of FOXP3 in cancer genesis and development. According to these
findings, we hypothesized that FOXP3 expression is related to
gastric cancer.
In the present study, the expression and
localization of FOXP3 were identified in gastric cancer cell lines
and tissues. The distribution of FOXP3 in human gastric cancer
patients was evaluated and the relationship was analyzed between
these findings and grades of tumor differentiation.
Materials and methods
Source of normal and cancerous gastric
tissue sections and tissue array
Normal and cancerous gastric tissues were obtained
from patients (normal, 16; gastric cancer, 39 including 31 males
and 8 females, 31–78 years of age) who underwent partial
gastrectomy or gastric biopsy. Normal controls were histologically
normal tissues and were obtained from patients who underwent
partial gastrectomy for metastatic tumor or gastric biopsy.
Microarray tissues (normal 16; gastric cancer, 71 including 54
males and 17 females, 31–71 years of age) were obtained from
Cybrdi, USA. The study protocol conformed to the ethical guidelines
of the 1975 Declaration of Helsinki and received prior approval by
the Fourth Military Medical University, China.
Cell culture
Complete medium (RPMI-1640) contained RPMI-1640
supplemented with 2 mmol/l Glutamax, 100 U/ ml penicillin, 100
μg/ml streptomycin and 10 mmol/l HEPES (Invitrogen, USA) and 10%
FCS (Thermo Trace, Australia). The following additional cell lines
were obtained from the Biotechnology Center of the Fourth Military
Medical University: AGS, SGC-7901, MKN-28 and MKN-45. All tumor
cell lines were maintained in complete RPMI-1640 and passaged using
trypsin/EDTA (Invitrogen). Melanocytes were freshly prepared when
used (derived from normal human skins, from the Department of
Dermatology, Xijing Hospital, the Fourth Military Medical
University, China).
Semi-quantitative and
reverse-transcription PCR
Total RNA was isolated from gastric cancer cells and
melanocytes (as control) using TRIzol reagent (Invitrogen). A total
of 500 ng of total RNA was reverse transcribed using the kit from
Takara, Japan. PCR was performed for the Foxp3 fragment
amplification. The following primers were used (5′-3′): Foxp3
sense: CACAACATGCGACCCCCTTTCACC; Foxp3 antisense:
AGGTTGTGGCGGATGGCGTTCTTC. β-actin was used as an internal control
for normalization (primer sequences available on request).
Semi-quantitative RT-PCR of FOXP3 transcripts was carried out by
comparing the signal intensities of PCR products of Foxp3 gene to
that of the β-actin gene from the same RNA sample using agarose gel
electrophoresis. The intensities of the product bands were
quantified by densitometric scanning of the gels (Pharmacia
Biotech) using ‘Total Image’ 1D GEL Analysis software. DNA marker
(Takara) was run in each gel to confirm the size of the PCR
product.
Western blot analysis
To examine the protein expression level of FOXP3 in
gastric cancer cells, whole cell lysates were subjected to SDS-PAGE
electrophoresis, followed by blotting on a nitrocellulose membrane.
For FOXP3 detection, membranes were probed with goat anti-human
FOXP3 polyclonal antibody overnight at 4°C followed by incubation
with a secondary horseradish peroxidase-conjugated antibody. The
mouse anti-human β-actin monoclonal antibody was used as an
internal control (R&D, USA). Chemiluminescent detection was
performed with the enhanced chemiluminescence detection kit (Anmei,
China).
Flow cytometry
To determine the expression of FOXP3, gastric cancer
cells were stained for FOXP3 and analyzed by flow cytometry. Cells
were washed in PBS containing 1% bovine serum albumin (BSA) and
0.1% NaN3 before antibody staining, followed by fixation
with 1% paraformaldehyde. Fluorescein isothiocyanate
(FITC)-conjugated rat anti-human FOXP3 monoclonal antibody was
purchased from eBiosciences, USA. A total of 105 events
were collected using Becton Dickinson FACScaliber (Becton
Dickinson, USA). Analysis was performed using the WinMDI 2.8
program (Purdue University Cytometry Laboratories, USA).
Confocal microscopic analysis
For double-labeled immunofluorescence,
formalin-fixed slides of the cell lines were treated in 3% hydrogen
peroxide in methanol for 10 min. Following three rinses in PBS, the
slides were treated for 1 h in blocking solution (Zhongshan,
China). Slides were incubated for 1 h at room temperature with the
FITC-conjugated rat anti-human FOXP3 monoclonal antibody
(eBioscience, USA) diluted in 1% bovine serum albumin in TBS with
0.1% Tween-20. Following PBS rinsing, the slides were treated with
a 1:1,000 dilution of diamidino-phenylindole (DAPI) (stock solution
1 mg/ml) (Sigma-Aldrich, USA) for 30 min which was used to
visualize nuclei. The slides were mounted with the Prolong
Anti-Fade mounting medium. Slides were examined with a Leica TCS-SP
laser scanning confocal microscope (Leica, German). All images were
collected using a pinhole of 1 Airy unit.
Immunohistochemistry
This study was approved by the Department of
Pathology, Xijing Hospital, the Fourth Military Medical University,
China. Paraffin-embedded resected gastric cancer specimens (n=55)
from 2006 through 2008 were retrieved from the Xijing Hospital
tissue bank. Rat anti-human FOXP3 monoclonal antibody was applied
to paraffin-embedded sections after microwave antigen retrieval for
10 min in 0.01 mol/l citrate buffer (pH 6.0). Specimens were
treated with 0.3% hydrogen peroxide in methanol for 15 min after
incubation with the primary antibody to block endogenous peroxidase
activity and blocked with human serum to minimize background
reactivity. The secondary antibody of horseradish
peroxidase-labeled goat anti-rat antibody (Zhongshan, China) was
diluted with 10% human serum and incubated for 1 h. These slides
were examined systematically using an image analyzer system
(Olympus BH-2 microscope; Japan).
Semi-quantitative analysis of
immunohistochemistry
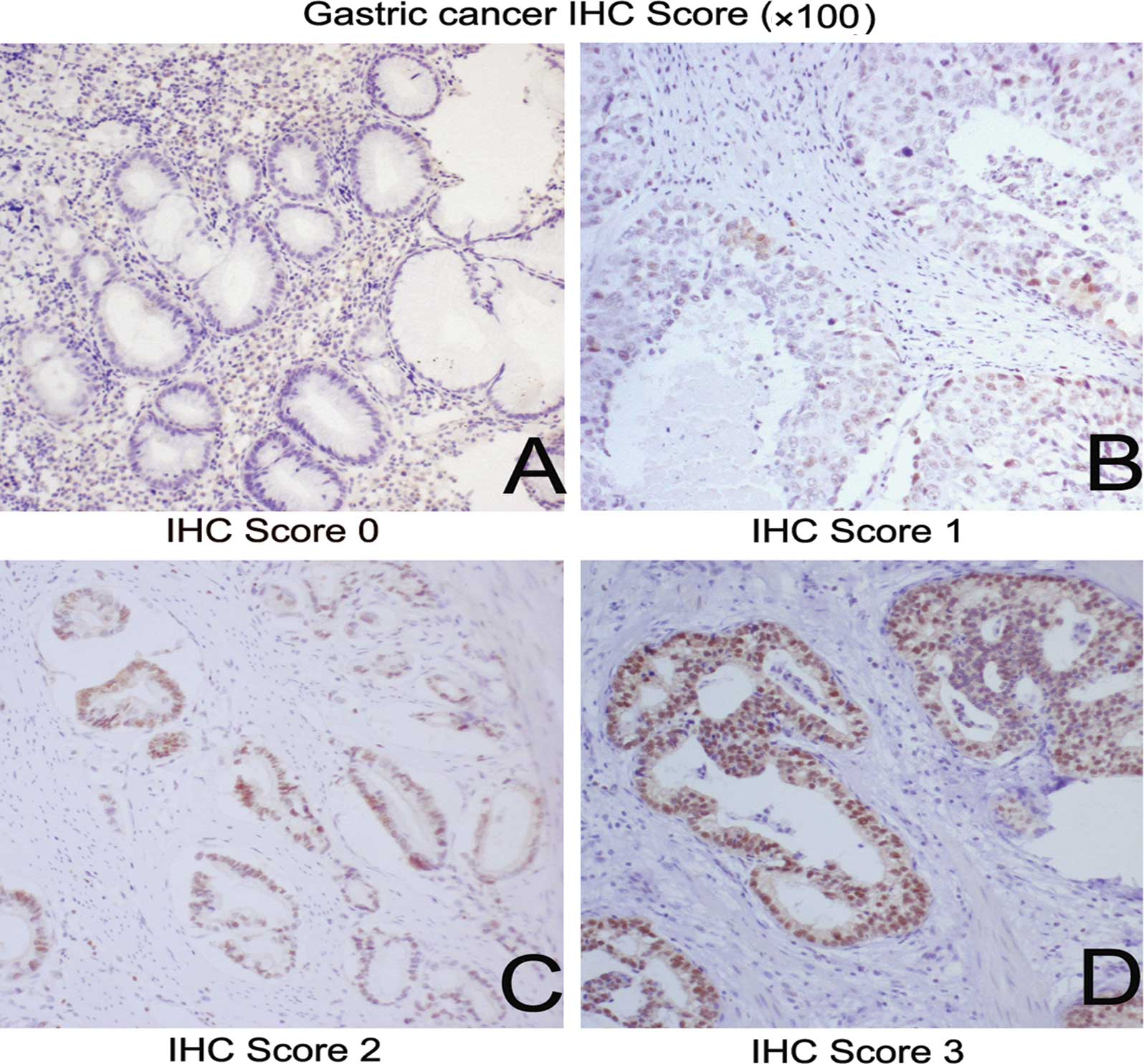
Slides were reviewed under light microscopy at x100
magnification by two pathologists separately. Semi-quantitative
analysis of FOXP3 staining was assessed as 0, 1+, 2+ and 3+ as
previously established (16,17).
Grade 0 was defined as the complete absence or weak FOXP3
immunostaining in <1% of tumor cells; grade 1+ was focal
positivity in 1–10% of tumor cells; grade 2+ was positive FOXP3
immunostaining in 11–50% of tumor cells; and grade 3+ was positive
FOXP3 immunostaining in >50% of tumor cells. A global assessment
of the entire tumor was carried out without selection for the
invasive front or areas of active tumor growth. The frequency and
semi-quantitative analysis of positive tumors for all regions were
calculated for statistical comparisons.
Statistical analysis
Differences in proportions were compared using the
Pearson Chi-square test or Fisher’s Exact test, as appropriate. The
statistic correlations between the grades of gastric cancer
differentiation and the staining level of FOXP3 were analyzed using
the Cochran-Mantel-Haenszel test. Differences with a P-value
<0.05 were considered to be statistically significant. All
analyses were carried out using SAS statistical software version
9.1 (Cary, NC, USA).
Results
FOXP3 is widely expressed in gastric
cancer cell lines
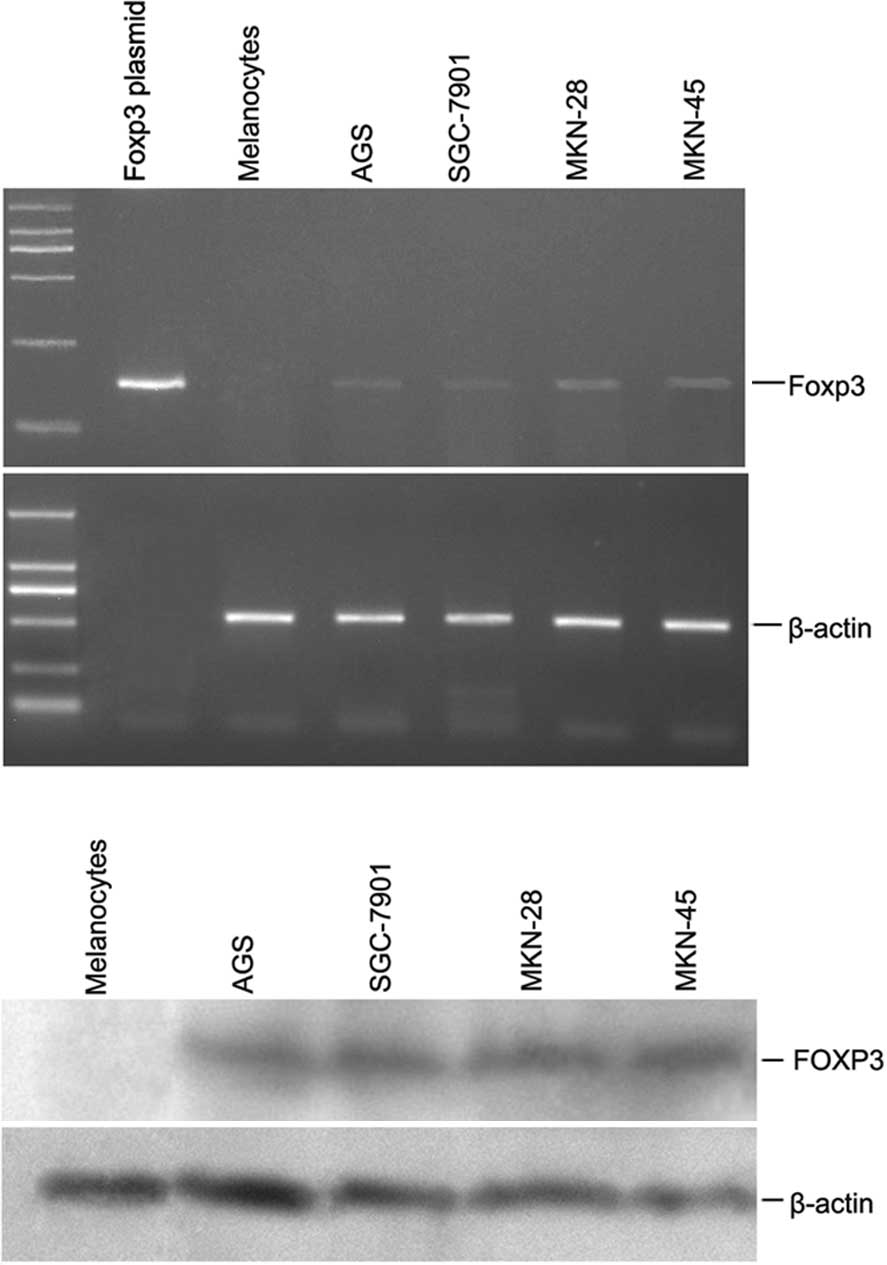
In an initial analysis of RT-PCR, Foxp3 was found in
several gastric cell lines. To further validate these results,
semi-quantitative RT-PCR analysis of the four gastric cancer cell
lines and melanocytes was conducted. The results revealed a
significant overexpression of Foxp3 in the gastric cancer specimens
compared to the non-malignant melanocytes (Fig. 1A). The cell lines shown in Fig. 1A (AGS, SGC-7901, MKN-28 and MKN-45)
were uniformly positive for Foxp3. Staining of melanocytes was
negative, as expected. Foxp3 full length plasmid was conducted and
kept by us (data not shown).
Moreover, as shown in Fig. 1B, FOXP3 protein was detected in the
gastric cancer cell lines by Western blotting. Since FOXP3
expression in the gastric cancer cells was a novel observation, we
confirmed the staining results in the Western blotting using two
different anti-FOXP3 antibodies (eBioscience and R&D; data not
shown). Thus, FOXP3 was widely expressed in the gastric cancer cell
lines (but not in the normal melanocytes); however, the intensity
of expression was variable.
FOXP3 localization in the gastric cancer
cell lines
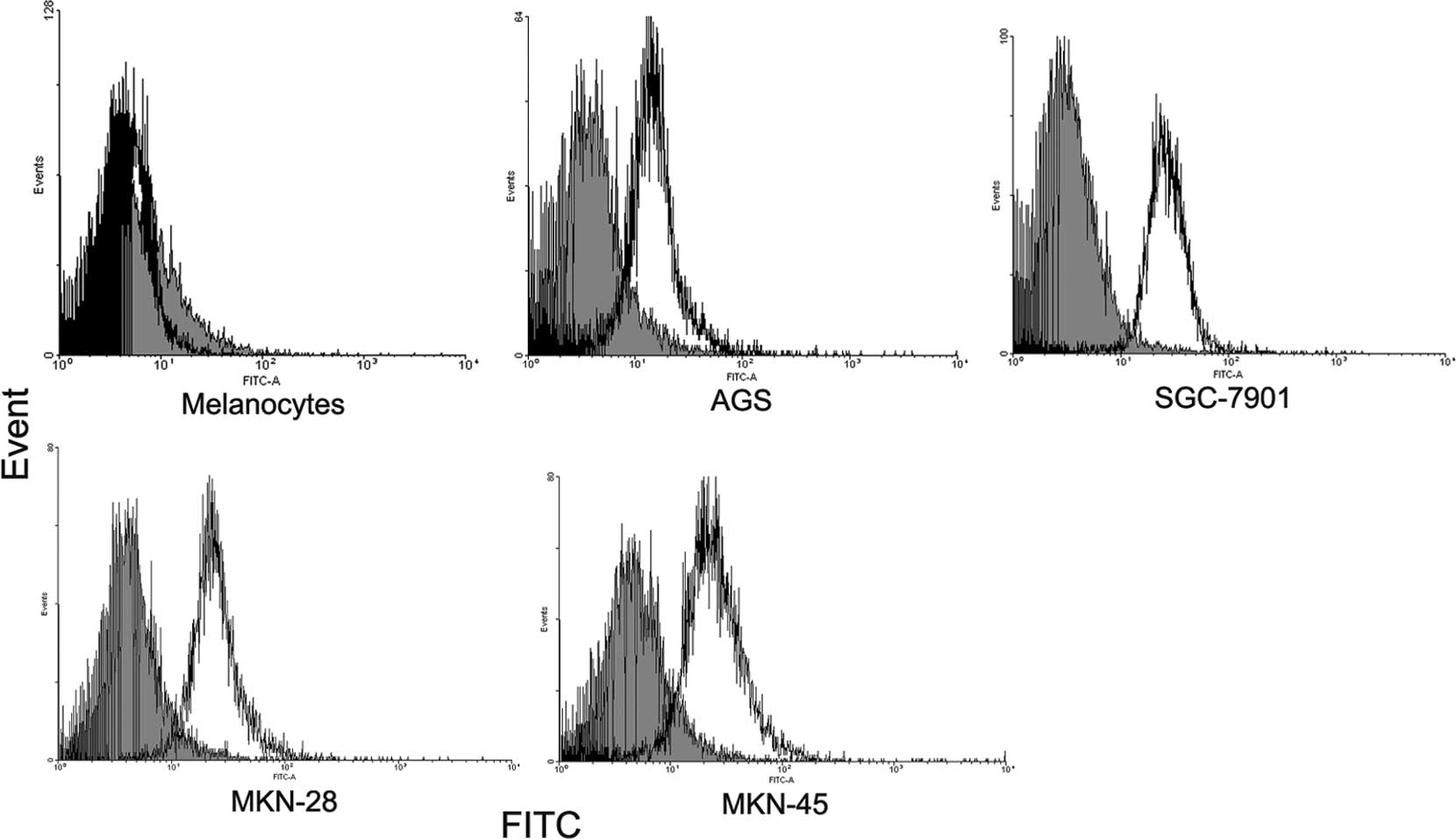
Staining with anti-FOXP3 resulted in a shift in the
fluorescence of the entire population compared to the isotype
control. By contrast, melanocytes did not express detectable FOXP3
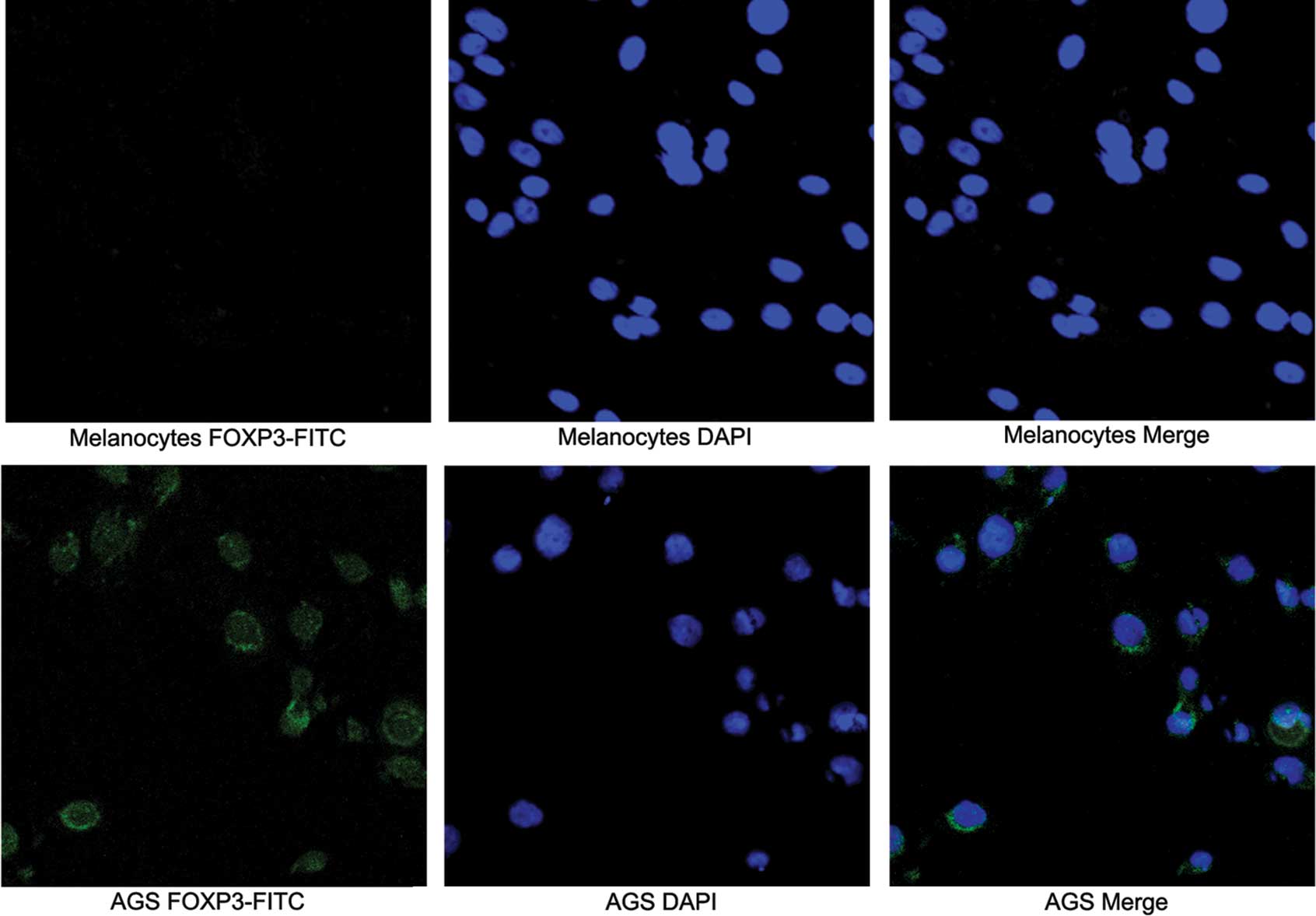
(Fig. 2). Subcellular distribution
of FOXP3 expression by confocal microscopy was used to examine the
distribution of FOXP3 in gastric cancer cell lines and melanocytes.
In these experiments, the nuclei were stained with DAPI to
facilitate analysis. Tumor cells exhibiting intense nuclear and
less intense cytoplasmic expression were observed in the AGS cell
line (Fig. 3).
FOXP3 expression in gastric cancer
tissues
Immunohistochemistry was used to analyze the protein
expression of FOXP3 in the gastric cancer specimens. Staining was
carried out on a set of 39 tissue sections (Table I) and on a tissue array containing
71 cores (Table II) from normal
and cancerous gastric tissues representing gastric cancer of all
differentiation grades. Twenty-two of 39 (56.4%) tissue sections
displayed nuclear FOXP3 staining. Of these, 3 tissue samples
exhibited nuclear and cytoplasmic staining. Similarly, in the
tissue array, FOXP3 nuclear staining was observed in ∼28 of the 71
(39.4%) cores (Table II). Nine
tissues exhibited nuclear and cytoplasmic staining. Thus, FOXP3 was
expressed in the vast majority of specimens.
 | Table I.Association of FOXP3 with
clinicopathological grades and staining intensity in 39 gastric
cancer in tissue sections. |
Table I.
Association of FOXP3 with
clinicopathological grades and staining intensity in 39 gastric
cancer in tissue sections.
| FOXP3 expression | No. of tumor
specimens (n=39) | FOXP3 | P-value | FOXP3
immuno-histochemistry intensity score, n (%) | P-value |
|---|
|
|
|---|
| Positive, n (%) | Negative, n (%) | 0 | 1 | 2 | 3 |
|---|
| Gender | | | | | | | | | |
| Male | 31 | 19 (61) | 12 (39) | 0.2610F | 12 (39) | 10 (32) | 7 (23) | 2 (6) | 0.7923F |
| Female | 8 | 3 (38) | 5 (62) | | 5 (62) | 2 (25) | 1 (13) | 0 (0) | |
| Age (years) | | | | | | | | | |
| >60 | 16 | 10 (63) | 6 (37) | 0.5220P | 6 (37) | 6 (37) | 3 (19) | 1 (7) | 0.8464F |
| ≤60 | 23 | 12 (52) | 11 (48) | | 11 (48) | 6 (26) | 5 (22) | 1 (4) | |
| Grade | | | | | | | | | |
| Well
differentiated | 21 | 10 (48) | 11 (52) | 0.4287F | 11 (52) | 5 (24) | 4 (19) | 1 (5) | 0.5615F |
| Moderately
differentiated | 9 | 5 (56) | 4 (44) | | 4 (44) | 2 (22) | 2 (22) | 1 (12) | |
| Poorly
differentiated | 9 | 7 (78) | 2 (22) | | 2 (22) | 5 (78) | 2 (22) | 0 (0) | |
| Tumor | 39 | 22 (56) | 17 (44) | 0.0001Pa | 17 (44) | 12 (31) | 8 (21) | 2 (4) | 0.0006Fa |
| Normal | 16 | 0 (0) | 16 (100) | | 16 (100) | 0 (0) | 0 (0) | 0 (0) | |
 | Table II.Association of FOXP3 with
clinicopathological grades and staining intensity in 71 gastric
cancer tissue arrays. |
Table II.
Association of FOXP3 with
clinicopathological grades and staining intensity in 71 gastric
cancer tissue arrays.
| FOXP3
expression | No. of tumor
specimens (n=71) | FOXP3 | P-value | FOXP3
immuno-histochemistry intensity score, n (%) | P-value |
|---|
|
|
|---|
| Positive, n
(%) | Negative, n
(%) | 0 | 1 | 2 | 3 |
|---|
| Gender | | | | | | | | | |
| Male | 54 | 24 (44) | 30 (56) | 0.1238P | 30 (56) | 14 (26) | 7 (13) | 3 (5) | 0.5779F |
| Female | 17 | 4 (24) | 13 (76) | | 13 (76) | 3 (18) | 1 (6) | 0 (0) | |
| Age (years) | | | | | | | | | |
| >60 | 24 | 11 (46) | 13 (54) | 0.4306P | 13 (54) | 7 (29) | 3 (13) | 1 (4) | 0.8596F |
| ≤60 | 47 | 17 (36) | 30 (64) | | 30 (64) | 10 (21) | 5 (11) | 2 (4) | |
| Grade | | | | | | | | | |
| Well
differentiated | 37 | 11 (30) | 26 (70) | 0.0011Pa | 26 (70) | 6 (16) | 4 (11) | 1 (3) | 0.0057Fa |
| Moderately
differentiated | 21 | 6 (29) | 15 (71) | | 15 (71) | 3 (14) | 2 (10) | 1 (5) | |
| Poorly
differentiated | 13 | 11 (85) | 2 (15) | | 2 (15) | 8 (62) | 2 (15) | 1 (8) | |
| Tumor | 71 | 28 (39) | 43 (61) | 0.0023Pa | 43 (61) | 17 (24) | 8 (11) | 3 (4) | 0.0241Fa |
| Normal | 16 | 0 (0) | 16 (100) | | 16 (100) | 0 (0) | 0 (0) | 0 (0) | |
Notably, FOXP3 localized to the nucleus of most
gastric cancer cells in both the tissue sections and the tissue
array. To confirm the validity of the observed nuclear staining,
two different anti-human FOXP3-specific antibodies were used. Both
antibodies provided similar patterns (data not shown), thus
validating our observation of nuclear FOXP3 localization in gastric
cancer tissues.
FOXP3 is associated with poorly
differentiated cancer and a high frequency of low intensity
staining
The effect of FOXP3 on overall cases for
histopathological grade was examined. Notably, many poorly
differentiated cancerous cases were FOXP3-positive, and the
intensity of FOXP3 staining was low. These results, however, were
statistically significant in the tissue array, but not in the tumor
sections, potentially due to the small sample size of tumor
sections. The proportion of stained cells was significantly
different among the histopathological grades in the tissue array.
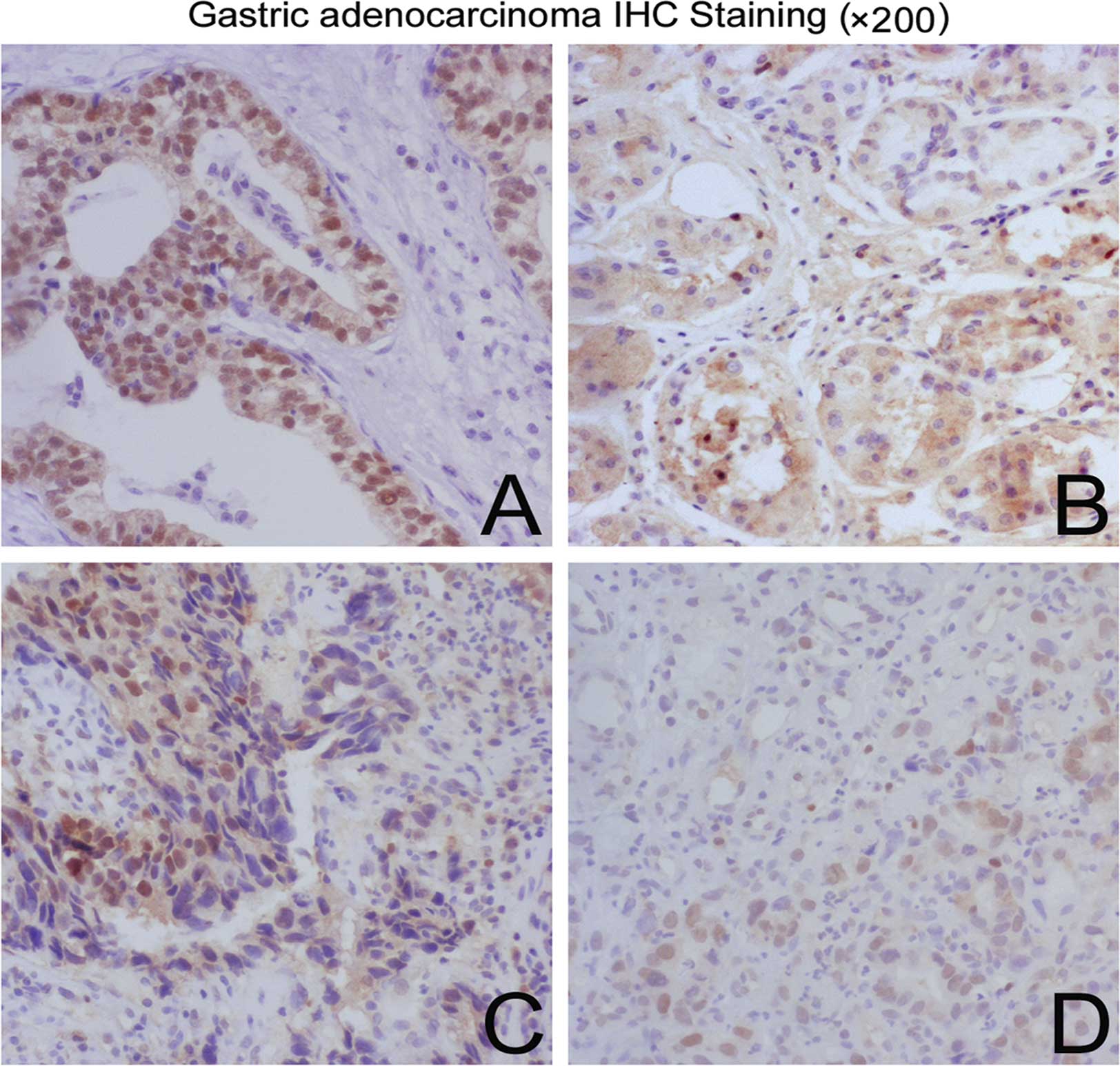
Of the 28 positive cancer cores in the tissue array, 11 of 37 (30%)
grade I, 6 of 21 (29%) grade II and 11 of 13 (85%) grade III
carcinoma cores (Fig. 4) were
positive for FOXP3 (P=0.0011, Pearson’s Chi-square test; Table II). This suggests that a high
frequency of nuclear FOXP3 may be a positive prognostic factor in
patients with poorly differentiated tumor tissues.
FOXP3 was detected in 28 of the 71 (39.4%) specimens
of the tissue array. Semi-quantitative staining was scored as 1+ in
17 (60.7%), 2+ in 8 (28.6%) and 3+ in 3 (10.7%) cases (examples
provided in Table II and Fig. 5). The staining scores varied
considerably between the subgroups. A significantly higher
frequency of low FOXP3 expression was noted in the poorly
differentiated gastric cancer tissues (Table II). As shown in Table II, a considerable linear trend of
gastric cancer differentiation was observed across FOXP3 staining
scores (nonzero correlation 4.86, P=0.003; general association
15.41, P=0.02; departure from linear regression 10.55,
0.1<P<0.05). Moreover, we found that FOXP3 was frequently
expressed in adenocarcinoma, while only seldom expressed in
mucinous cancers and signet-ring carcinomas. However, there were
fewer samples. The normal gastric tissues were also devoid of FOXP3
expression. In all patients, tumor-infiltrating lymphocytes were
FOXP3-positive (Fig. 5).
Discussion
Although the factors and molecular events associated
with the progression of gastric cancer are complex and are not well
established, FOXP3 has been shown to play an important role in
Tregs in gastric cancer invasion (18). In this study, we assessed the
expression and subcellular localization of FOXP3 in gastric cancer
cell lines and tissues. At the same time, we also identified the
relationship between the expression level of FOXP3 and tumor
differentiation grade.
Others researchers have previously noted nuclear and
cytoplasm localization of FOXP3 in pancreatic carcinoma, melanoma
and colon carcinoma cells by immunohistochemistry. In agreement
with previously published studies, expression of Foxp3 mRNA was
also revealed in various gastric cancer cell lines studied.
Relative expression values for tumor cells varied widely and were
significantly higher than those of melanocytes. At the same time,
we observed a higher frequency of expression of FOXP3 in the
whole-cell extracts of the gastric cancer cell lines (AGS,
SGC-7901, MKN-28 and MKN-45) compared to the melanocytes. Upon
analysis, we found that nuclear, and to a lesser extent,
cytoplasmic FOXP3 expression was more prevalent in the gastric
cancer cell lines. This observation may suggest a relationship
between expression and localization of FOXP3. To our knowledge,
this study provides the first evidence of nuclear and cytoplasmic
localization of FOXP3 in gastric cancer cell lines.
Notably, we found that gastric cancer cell lines
expressed FOXP3 mainly in the nucleus and cytoplasm, but in gastric
cancer tissues FOXP3 was mostly expressed in the nucleus. This
suggests that the microenvironment of tumors (such as cytokines,
immune cells, ligands and receptors of tumor cells) may induce a
change in the subcellular localization of FOXP3 from a cytoplasmic
to a nuclear expression pattern, which may result from
post-translational modifications (19). Therefore, the heterogeneous
subcellular localization of FOXP3 in gastric cancer cell lines and
tissues may reflect the presence of different post-translationally
modified forms of FOXP3. The functional relevance of this finding
requires further investigation. In particular, previous reports
have revealed the interaction of FOXP3 with the nuclear factor of
activated T cells, suggesting that FOXP3 plays an important role in
the formation of nuclear complexes that are important in the
regulation of the transcription of functional genes, that confer a
suppressive function to Tregs (20,21).
In tissue sections, nuclear FOXP3 was not present in
normal and para-tumor tissues. This observation was also confirmed
in the tissue array cores and was statistically significant (P=
0.0011), indicating that nuclear FOXP3 may play a role in gastric
cancer progression. Our statistical results revealed a linear
relationship between an increase in the worsening of
differentiation of gastric cancer tissues and intensity of FOXP3
expression. High frequency and intensity of staining of nuclear
FOXP3 expression may aid in the identification of gastric cancer of
poorly differentiated tumor potential. However, the results showed
no correlation between FOXP3 expression and gender or age, in the
tissue sections and array (Tables
I and II).
Five prior studies assessing FOXP3 in tissues and
cell lines have been reported. Hinz et al described for the
first time the expression and function of FOXP3 in pancreatic
ductal adenocarcinoma cells and tumors (13). They detected FOXP3 expression in
tumor cells of 24 out of 39 patients with pancreatic carcinoma.
Although they were unable to find a correlation between FOXP3
expression or subcellular localization and tumor stage or survival,
their findings indicate that pancreatic carcinoma cells share
growth suppressive effects with Tregs and suggest that mimicking
Treg function may represent a new mechanism of immune evasion in
pancreatic cancer. Ebert et al and Karanikas et al
also found that FOXP3 transcription factor was expressed in
melanoma and numerous types of tumor cells (14,15).
Evidence suggests that FOXP3 is related to tumor escape and could
be used as a potential tumor antigen. On the contrary, Zou et
al reported that functional somatic mutations and
down-regulation of the Foxp3 gene were commonly found in human
breast cancer samples. This also correlated with HER-2/ ErbB2 and
SKP-2 overexpression (22,23). Whether the presence of mutations
could account for the low expression levels of FOXP3 in breast
tumor cell lines remains to be investigated.
The statistical significance in the tissue sections
and the tissue array was similar, but the positive ratios of FOXP3
were different (tissue sections 56.4%; tissue microarray 39.4%).
During observation, we found that the expression of FOXP3 was
mostly focal in the tissue sections. Results of the FOXP3
immunohistochemistry intensity score system revealed that 62–78% of
the poorly differentiated cancer samples focally expressed FOXP3.
Therefore, we conclude that the discrepancy may be due to the small
square of tissue cores of the array, which could not include focal
expression of FOXP3. Although the percentages of FOXP3-positive
staining were different in the tissue sections and array, the
quantity of tissue array was sufficient to obtain significant
differences among the groups by statistical analysis.
The two principal findings of our study reveal that
FOXP3 is widely expressed in gastric cancer and a high intensity
and frequency is a prognostic marker in poorly differentiated
gastric cancer. This suggests the possibility that the intensity of
FOXP3 expression in poorly differentiated gastric cancer patients
may predict a worse prognosis and can be further studied to
determine its usefulness in guiding immune therapy strategies.
In conclusion, this is the first report of nuclear
localization of FOXP3 in gastric cancer cell lines and tissues.
This indicates that FOXP3 is widely expressed in tumor cells and
tissues. Although FOXP3 has been found to play an important role
during development and differentiation of Tregs, the molecular
mechanisms of FOXP3 and its function in cancer are yet unknown. The
inhibitory character of FOXP3 in Tregs has been demonstrated.
Further research is needed to determine whether FOXP3 plays an
inhibitory role in cancers. In addition, the correlation of nuclear
FOXP3 expression with tumor grade in gastric cancer tissues
warrants further study in order to understand the critical
molecular events associated with gastric cancer progression.
Acknowledgements
The authors thank Dr Lieping Chen and
Dr Yili Yang for the helpful comments and suggestions on the
manuscript, and the members of the Department of Pathology of
Xijing Hospital of Fourth Military Medical University for the
excellent technical support. This study was supported by the
program for Changjiang Scholars and Innovative Research Team in
University (PCSIRT) in China.
References
|
1.
|
Crew KD and Neugut AI: Epidemiology of
gastric cancer. World J Gastroenterol. 12:354–362. 2006.
|
|
2.
|
Wolf AM, Wolf D, Steurer M, Gastl G,
Gunsilius E and Grubeck-Loebenstein B: Increase of regulatory T
cells in the peripheral blood of cancer patients. Clin Cancer Res.
9:606–612. 2003.PubMed/NCBI
|
|
3.
|
McMillan DC, Fyffe GD, Wotherspoon HA,
Cooke TG and McArdle CS: Prospective study of circulating
T-lymphocyte subpopulations and disease progression in colorectal
cancer. Dis Colon Rectum. 40:1068–1071. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Santos LB, Yamada FT and Scheinberg MA:
Monocyte and lymphocyte interaction in patients with advanced
cancer. Evidence for deficient IL-1 production. Cancer.
56:1553–1558. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Barbieri C, Fujisawa MM, Yasuda CL, et al:
Effect of surgical treatment on the cellular immune response of
gastric cancer patients. Braz J Med Biol Res. 36:339–345. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Parsonnet J, Hansen S, Rodriguez L, et al:
Helicobacter pylori infection and gastric lymphoma. N Engl J
Med. 330:1267–1271. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Ando T, Goto Y, Maeda O, Watanabe O,
Ishiguro K and Goto H: Causal role of Helicobacter pylori
infection in gastric cancer. World J Gastroenterol. 12:181–186.
2006.
|
|
8.
|
Aromaa A, Kosunen TU, Knekt P, et al:
Circulating anti-Helicobacter pylori immunoglobulin A
antibodies and low serum pepsinogen I level are associated with
increased risk of gastric cancer. Am J Epidemiol. 144:142–149.
1996.
|
|
9.
|
Rad R, Brenner L, Bauer S, et al:
CD25+/Foxp3+ T cells regulate gastric
inflammation and Helicobacter pylori colonization in vivo.
Gastroenterology. 131:525–537. 2006.
|
|
10.
|
Hori S and Sakaguchi S: Foxp3: a critical
regulator of the development and function of regulatory T cells.
Microbes Infect. 6:745–751. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Yagi H, Nomura T, Nakamura K, et al:
Crucial role of FOXP3 in the development and function of human
CD25+CD4+ regulatory T cells. Int Immunol.
16:1643–1656. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Allan SE, Passerini L, Bacchetta R, et al:
The role of 2 FOXP3 isoforms in the generation of human
CD4+ Tregs. J Clin Invest. 115:3276–3284. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Hinz S, Pagerols-Raluy L, Oberg HH, et al:
Foxp3 expression in pancreatic carcinoma cells as a novel mechanism
of immune evasion in cancer. Cancer Res. 67:8344–8350. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Ebert LM, Tan BS, Browning J, et al: The
regulatory T cell-associated transcription factor FoxP3 is
expressed by tumor cells. Cancer Res. 68:3001–3009. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Karanikas V, Speletas M, Zamanakou M, et
al: Foxp3 expression in human cancer cells. J Transl Med. 6:192008.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Iwasa S, Jin X, Okada K, Mitsumata M and
Ooi A: Increased expression of seprase, a membrane-type serine
protease, is associated with lymph node metastasis in human
colorectal cancer. Cancer Lett. 199:91–98. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Ariga N, Sato E, Ohuchi N, Nagura H and
Ohtani H: Stromal expression of fibroblast activation
protein/seprase, a cell membrane serine proteinase and gelatinase,
is associated with longer survival in patients with invasive ductal
carcinoma of breast. Int J Cancer. 95:67–72. 2001. View Article : Google Scholar
|
|
18.
|
Lundgren A, Stromberg E, Sjoling A, et al:
Mucosal FOXP3-expressing CD4+ CD25high regulatory T
cells in Helicobacter pylori-infected patients. Infect
Immun. 73:523–531. 2005.PubMed/NCBI
|
|
19.
|
Chen C, Rowell EA, Thomas RM, Hancock WW
and Wells AD: Transcriptional regulation by Foxp3 is associated
with direct promoter occupancy and modulation of histone
acetylation. J Biol Chem. 281:36828–36834. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Marson A, Kretschmer K, Frampton GM, et
al: Foxp3 occupancy and regulation of key target genes during
T-cell stimulation. Nature. 445:931–935. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Wu Y, Borde M, Heissmeyer V, et al: FOXP3
controls regulatory T cell function through cooperation with NFAT.
Cell. 126:375–387. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Zuo T, Wang L, Morrison C, et al: FOXP3 is
an X-linked breast cancer suppressor gene and an important
repressor of the HER-2/ErbB2 oncogene. Cell. 129:1275–1286. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Zuo T, Liu R, Zhang H, et al: FOXP3 is a
novel transcriptional repressor for the breast cancer oncogene
SKP2. J Clin Invest. 117:3765–3773. 2007.PubMed/NCBI
|