Introduction
Tumor cell vaccines are a promising emerging
treatment option to cure tumors, although novel immunotherapeutic
options for all types of tumors have been recently developed.
Apoptotic tumor cells can be recognized and eliminated by the power
of the immune response, which has resulted in the intense interest
in the development of tumor cell vaccines transfected with DNA
containing target genes, immune molecules or treated using
different biological methods (e.g., freeze-thawing, large amounts
of ultraviolet light, γ-irradiation or anticancer drugs). These
tumor cell vaccines are currently being evaluated as prophylactic
and therapeutic vaccines for tumors (1,2).
Mitoxantrone (MIT), a non-cell cycle-specific
anthraquinone anticancer drug, induces cell apoptosis by inhibiting
DNA synthesis. Treatment of tumor cells with MIT was found to
result in translocation of calreticulin from endoplasmic reticulum
to the cell surface along with cell apoptosis, which leads to the
increased immunogenicity of tumor cells. These MIT-treated
apoptotic B16F10 cells may be used as a type of tumor cell vaccine
to initiate an effective antitumor immunoresponse in mice (3). The drug-treating tumor cells
inoculated into mice are able to be effectively recognized by
dendritic cells (DCs) and stimulate the immune system to
effectively eliminate tumor cells that are resistant to
chemotherapeutic drugs (4).
However, certain tumor cells exhibiting a high degree of
malignancy, such as murine melanoma B16F10 cells, express
ATP-binding cassette (ABC) transporter proteins, such as multidrug
resistance 1 (MDR1 or ABCB1), the multidrug resistance protein 1,
(MRP1 or ABCC1), ABCB5 and breast cancer resistance protein 1
(ABCG2/BCRP1) (5–7). These multidrug-resistant proteins are
able to rapidly ‘pump out’ anticancer drug from tumor cells, so
that a few of the tumor cells that were treated with anticancer
drugs survive and generate tumors in vivo. In order to
assess the feasibility, safety and immunogenicity of tumor cell
vaccines, efforts are ongoing.
Verapamil (VP) and reserpine (RP) are known
inhibitors of efflux pumps and block the function of
multidrug-resistant proteins, resulting in the retention of
anticancer drugs in the tumor cells (8–10).
In the present study, B16F10 cells were pretreated with VP and RP
to inhibit the activity of the efflux of MIT in the cells, and then
B16F10 cells were treated with MIT to cause retention of MIT in the
cells. The treated tumor cells gradually underwent apoptosis in the
experimental mice and were phagocytosed by autologous DCs. This
finally induced mice to generate the initiation of naturally
occurring antitumor immunity that partly prevented tumor growth.
Here, we report the safety and immunogenicity of an apoptotic
B16F10 tumor cell vaccine in a controlled study of C57BL/6
mice.
Materials and methods
Animals and cells
Female C57BL/6 mice, 6–8 weeks of age, 18–20 g, were
obtained from the University of Yangzhou, China. All mice were
housed under pathogen-free conditions, and the experiments were
performed in compliance with the Guidelines of the Animal Research
Ethics Board of Southeast University. The B16F10 murine melanoma
cell line is syngeneic in C57Bl/6 mice and was a gift from
Professor Pingsheng Chen, Medical School, Southeast University; the
YAC-1 cell line (Moloney leukemia-induced T-cell lymphoma of A/Sn
mouse origin) was obtained from the Cellular Institute of China in
Shanghai. These cells were cultured at 37°C in a 5% CO2
atmosphere in RPMI-1640 supplemented with 10% fetal bovine serum
which contained 100 U/ml penicillin G sodium and 100 μg/ml
streptomycin sulfate.
Animal experiments
In the safety experiment, C57BL/6 mice were first
divided into three experimental groups and one control group. Each
mouse was subcutaneously (s.c.) immunized in the abdominal region
with 2×105, 5×105 or 1×106 B16F10
cells treated with MIT for 12 h, or 1×105 wild-type
B16F10 cells as control. C57BL/6 mice were secondly divided into
four experimental groups and one control group. Each mouse was s.c.
immunized in the abdominal region with 1×106 B16F10
cells treated with MIT in combination with RP and VP for 12 h, or
1×105 wild-type B16F10 cells as control.
In the antitumor effect experiment, C57BL/6 mice
were randomly divided into the experimental, the B16F10 cell and
the PBS control groups. Each mouse was immunized s.c. in the
abdominal region, respectively, with a vaccine of 5×105
B16F10 tumor cells treated with MIT in combination with RP and VP
for 12 h or 1×105 wild-type B16F10 cells inactivated
with mitomycin C or 100 μl PBS. The same immunization was repeated
twice at an interval of 2 weeks. Two weeks after the second
immunization, 1×105 B16F10 cells were injected into the
abdominal region of each mouse. Tumor growth was monitored twice a
week and tumor-free and surviving mice were observed for 60 days.
Ten mice/group were used routinely in each experiment (3,11).
B16F10 cells (5×105) treated
with MIT alone or in combination with RP and VP were inoculated
into 24-well plates in RPMI-1640 medium
The drugs were applied at concentrations as follows:
2 μg/ml MIT, 2 μg/ml MIT + 1 μg/ml RP, 2 μg/ml MIT + 1 μg/ml VP and
2 μg/ml MIT in combination with 1 μg/ml RP and 1 μg/ml VP,
respectively. The samples at different time points (0, 12, 48, 96
and 144 h) were obtained to detect cell apoptosis with the Annexin
V-EGFP Apoptosis Detection kit (KeyGen Biotech. Co. Ltd., Nanjing,
China). The morphology of apoptotic cells was observed under a
fluorescence microscope (TE2000-E fluorescence inverted phase
contrast microscope; Nikon Corp.).
Preparation of DCs and analysis of
molecular expression
To prepare autologous DCs from mouse bone marrow,
the protocol was performed as described previously (12). The collected DCs were used for
incubation together with the treated B16F10 cells. For the analysis
of molecules of major histocompatibility complex (MHC) class II,
cluster of differentiation (CD)80 and CD11c on the DCs, the NKG2D
ligand on the B16F10 tumor cell vaccine and NKG2D on the
splenocytes, the experiment protocol was performed according to the
manufacturer’s protocol (eBioscience, USA) (13). Briefly, DCs, the B16F10 tumor cell
vaccine and splenocytes were stained with the rabbit anti-mouse MHC
class II-PE, CD80-APC and CD11c-FITC, rat anti-mouse NKG2D
ligand-PE and rat anti-mouse NKG2D-FITC monoclonal antibodies
(eBioscience), respectively, and subsequent steps were performed
according to the protocol provided in the kit (14).
RNA isolation and RT-PCR
The PCR sense primer sequence for the ABCB1 gene was
5′-CGAATGTCTGAGGACAAGCCAC-3′ and the anti-sense was
5′-CCATGAGGTCCTGGGCATG-3′. PCR sense primer sequence for the
β-actin gene was 5′-GGACTTCGAGCAAGAGATGG-3′ and the anti-sense was
5′-AGCACTGTGTTGGCGTACAG-3′. Total cellular RNA was extracted from
1×106 B16F10 cells by using the RNeasy Mini kit (Qiagen,
Valencia, CA, USA), according to the manufacturer’s instructions.
cDNA was synthesized with the reverse Super-Script Choice System
(Invitrogen, Carlsbad, CA, USA). cDNAs of ABCB1 and β-actin were
respectively amplified by PCR with the above-mentioned primers
(15).
Detection of side population cells in
B16F10 cells
B16F10 cells, 70% confluent in complete medium, were
transferred to 6-well plates for 24 h, and Hoechst 33342 (5 μg/ml)
was added to each well and maintained for 1 min at room
temperature. The cells were then washed with PBS three times and
subsequently observed under a light and fluorescence microscope,
respectively (16).
Effect of RP and VP on the exclusion rate
of MIT in B16F10 cells
B16F10 cells (70% confluent, 1×106) in
complete medium were transferred to 6-well plates for 24 h and then
1 μg/ml RP and 1 μg/ml VP were added to the wells for 30 min to
inhibit ABCB1 efflux pumps. Then, 2 μg/ml MIT was added to the
wells and cultured for another 12 h. Next, the cells were washed
with PBS three times and then fixed using 1% paraformaldehyde. The
exclusion rate of MIT was detected by fluorescence confocal
microscopy under 488-nm excitation light and 675-nm emission light
(17,18).
Assays of splenocyte cytotoxicity and
splenocyte proliferative response
Two weeks after the final immunization, 10 mice were
sacrificed to detect immune efficiency. Splenocytes
(5×106) were prepared from the C57BL/6 mice immunized
with the 5×105 B16F10 tumor cell vaccine treated with
MIT in combination with RP and VP. The harvested splenocytes were
labeled with 0.5 mM 5-(and 6)-carboxy-fluorescein diacetate
succinimidyl ester (CFSE; 20 μg/ml) at 37°C for 15 min. After the
incubation, the splenocytes were washed twice in PBS containing 5%
fetal bovine serum to sequester any free CFSE that had failed to
diffuse into the cells. Murine splenocytes (2×106) were
resuspended in 10% RPMI medium in 6-well plates and then incubated
with 2×104 B16F10 cells inactivated with mitomycin c
(100 μg/ml) for 72 h. The cells were then rinsed extensively with
complete medium and used in the proliferative assay (19,20).
ELISA for IFN-γ
The serum IFN-γ level was measured using a
commercially available enzyme linked-immunosorbent assay according
to the manufacturer’s protocol (eBioscience) (2,11).
Statistical analysis
For each group of mice, data were represented as the
mean value of each group and its associated standard deviation. The
Student’s t-test for two-group comparison and Bonferroni correction
for multiple comparisons were used to determine significant
differences. p<0.05 was considered statistically
significant.
Results
Safety of the B16F10 tumor cell vaccine
and its antitumor efficacy in mice
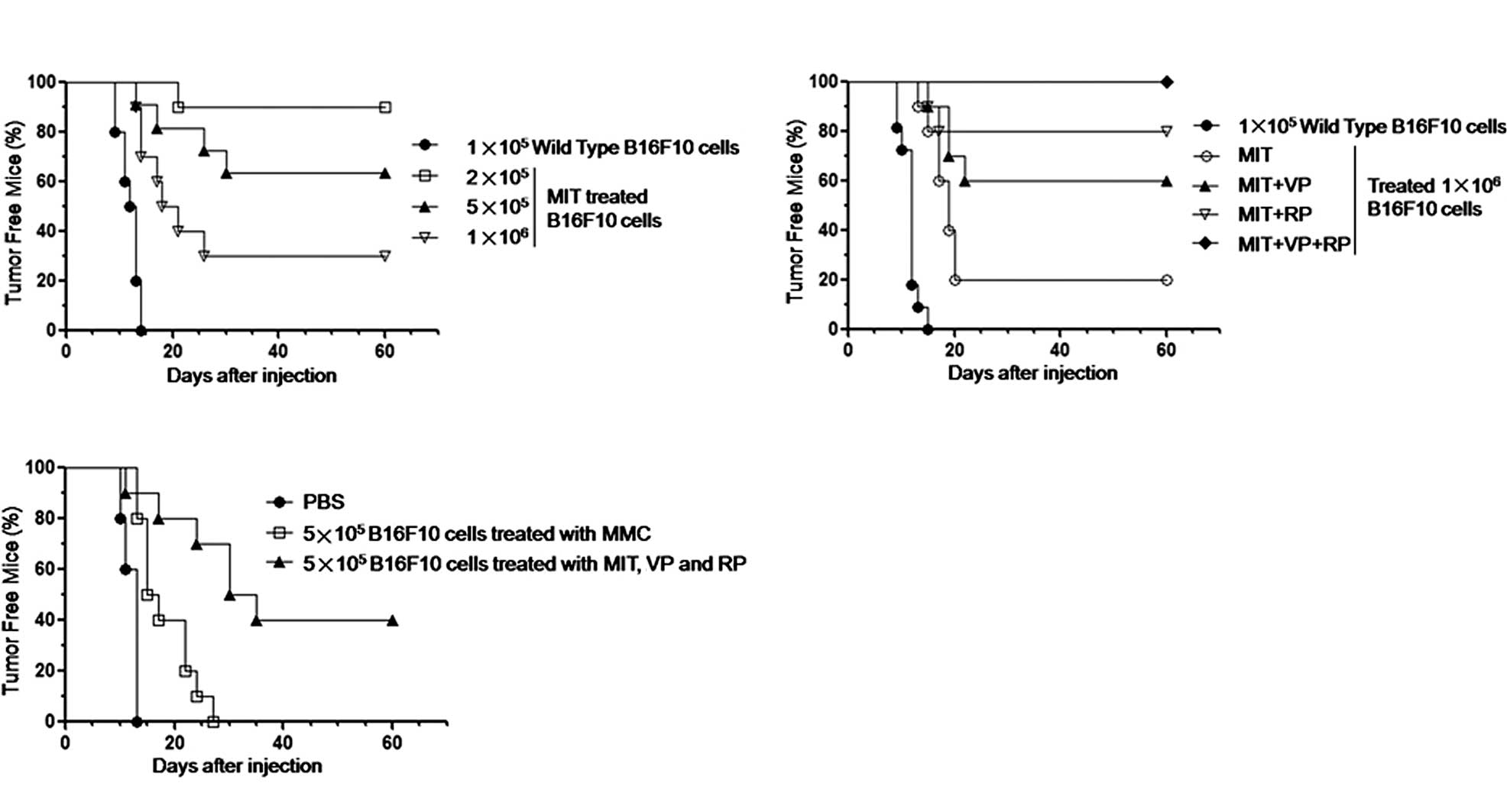
In the present study, we first assessed the safety
of the B16F10 tumor cell vaccine treated with MIT. After the B16F10
cells were treated with 2 μg/ml MIT for 12 h, the cells began to
undergo apoptosis and were then injected into the mice (10
mice/group). Measurable tumors were detected in the mice injected
with the MIT-treated 1×106 or 5×105 B16F10
cells on day 15 or on day 20 (in the 2×105 B16F10 cell
group) or on day 10 (in the 1×105 wild-type B16F10 cell
group), respectively. From the tumorigenesis data, 7 out of 10 mice
inoculated with the MIT-treated 1×106 tumor vaccine, 4
out of 10 mice inoculated with the MIT-treated 5×105
tumor vaccine, and 1 out of 10 mice inoculated with MIT-treated
2×105 B16F10 cells, respectively, formed tumors up until
60 days into the observation. The results indicated that the
MIT-treated 2×105 B16F10 cells still possessed
tumorigenic potential in C57BL/6 mice in spite of only 1 out of 10
mice developing tumors (Fig. 1A).
In the subsequent safety experiment, the B16F10 cells were
pretreated with 1 μg/ml RP or/and 1 μg/ml VP and then the cells
were re-treated with 2 μg/ml MIT. The B16F10 cell vaccine treated
with MIT + RP + VP was complete safety as all 10 mice did not
develop tumors until 60 days into the observation. However, in
regards to the mice treated with MIT + RP or MIT + VP, 2 out of 10
or 4 out of 10 mice, respectively, still developed tumors (Fig. 1B). Next, we evaluated the antitumor
efficacy in mice inoculated with the preparation of
5×105 B16F10 cells treated with MIT in combination with
RP and VP as a tumor cell vaccine. Fig. 1C shows that 6 out of 10 mice
immunized with the treated 1×106 B16F10 tumor cell
vaccine twice and challenged by 1×105 B16F10 cells
formed tumors on days 12, 16, 25, 30, 30 and 35. The remaining 4
mice did not form tumors throughout the 60-day observation.
However, all 10 mice formed tumors in less than 14 days when
immunized with PBS or in less than 27 days when immunized with
5×105 B16F10 cells inactivated with mitomycin C twice
and challenged by 1×105 B16F10 cells. These findings
demonstrate that the B16F10 tumor cell vaccine treated with MIT in
combination with RP and VP is not only safe, but induces mice to
generate a powerful and efficacious antitumor immune response.
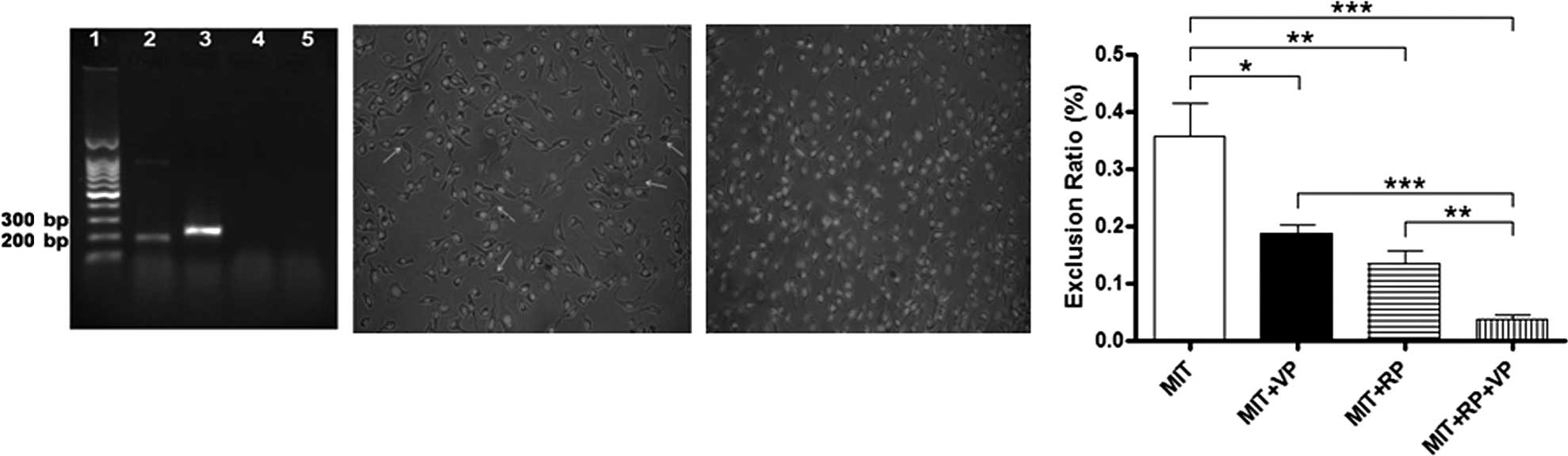
Detection of the ABCB1 gene, side
population cells and exclusion rate of MIT in B16F10 cells
From the tumorigenesis data, we found that the
MIT-treated B16F10 cells still exhibited tumorigenicity in mice.
The main reasons may be that the B16F10 cells are malignant cells
and have the ability to discharge MIT from the treated B16F10 cells
by ABC transporter proteins. Thus, a few of the B16F10 cells did
not undergo apoptosis after the MIT treatment. Since ABCB1 is one
of the ABC transporter proteins, we aimed to ascertain whether the
ABCB1 gene was present in the B16F10 cells. The RT-PCR result
demonstrated expression of the ABCB1 gene (Fig. 3A). It is also known that side
population (SP) cells, also termed ‘dull cells’, usually represent
only a small fraction of the whole cell population that are
identified by efflux of Hoechst dye through ABC transporter
proteins, and are present in virtually all malignant cells and a
part of normal tissues (21,22).
Thus, we further detected SP cells in the B16F10 cells. A small
fraction of SP cells (Fig. 2B,
arrows) existed in the B16F10 cells, nevertheless, the SP cells
were invisible in the B16F10 cells after the cells were treated
with RP and the VP (Fig. 2C). This
was due to the blockage of efflux pumps of ABCB1 by RP and the VP.
As a result, the SP cells were not observed under a fluorescence
microscope since the Hoechst 33342 stain was retained in the B16F10
cells, which made the SP cells were stained and did not be ‘dull
cells’. To further assess the function of the inhibition of ABCB1
efflux pumps by RP and the VP, we subsequently assessed the MIT
exclusion ratio in the B16F10 cells. As shown in Fig. 2D, the ability to exclude MIT was
statistically significantly decreased when the B16F10 cells were
concurrently treated with MIT, VP and RP in contrast to the ability
of MIT-treated (p<0.003), MIT + VP-treated (p<0.03) and MIT +
RP-treated B16F10 cells (p<0.05). The results suggest that RP
and VP block the function of ABCB1 efflux of MIT and assist MIT in
further inducing B16F10 cell apoptosis.
Detection of apoptotic B16F10 cells
treated with MIT in combination with RP and VP as well as molecular
expression
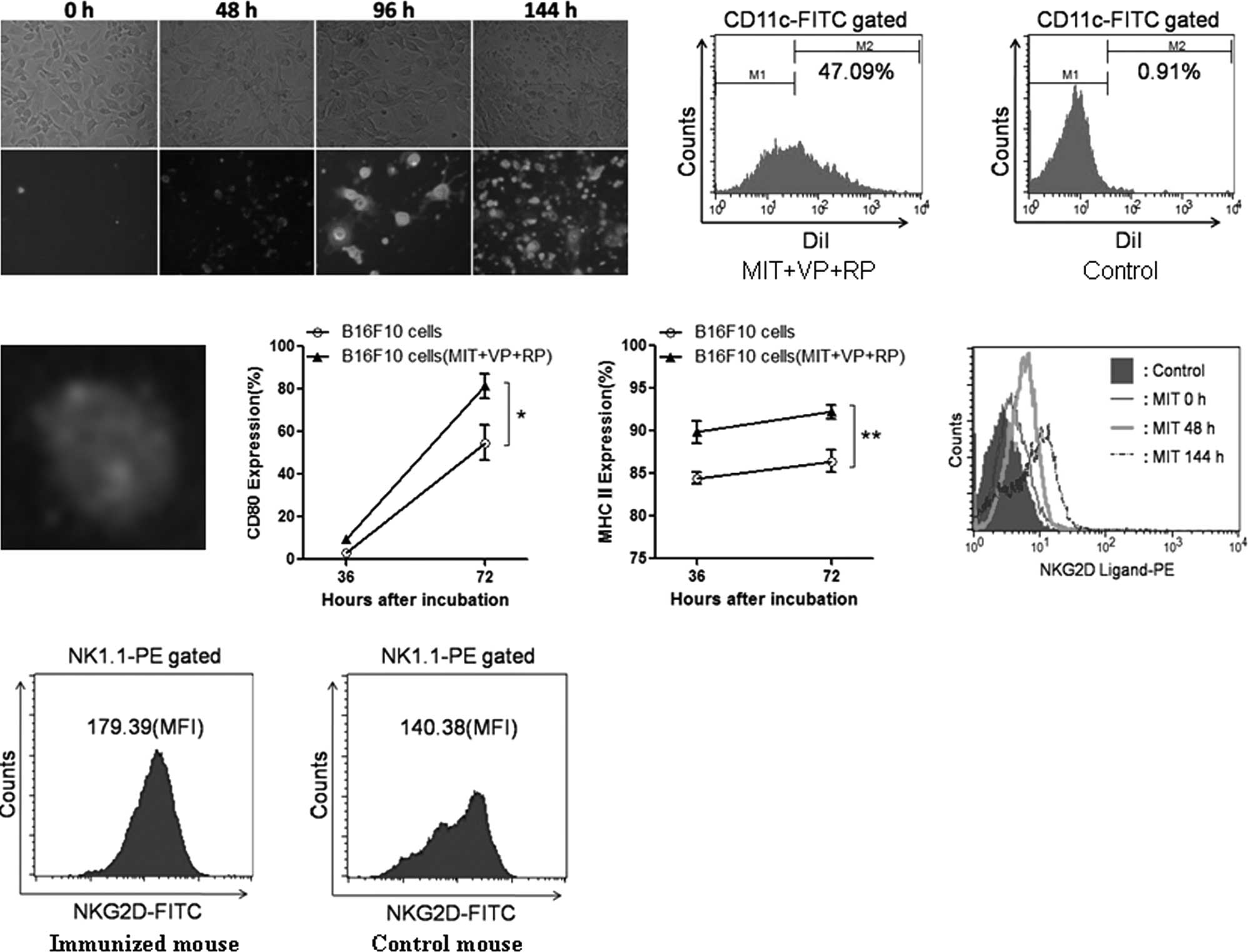
Fig. 3A shows that
B16F10 cells gradually experienced apoptosis 48 h after treatment
with MIT in combination with RP and VP. In viable apoptotic cells,
the cell membrane began to shrink and became a green color. In
non-viable apoptotic cells (after 96 h), the cell nucleus became a
red color, while the cell membrane remained green. When apoptotic
B16F10 cells were cultured with the autologous DCs from mouse bone
marrow for 72 h, the DCs ingested more apoptotic bodies (Fig. 3B) compared to the control DCs
(Fig. 3C). In order to visualize
the state of phagocytic apoptotic bodies, DiI-labeled apoptotic
B16F10 cells were incubated with the DCs stained for
FITC-conjugated CD11c. It was found that the apoptotic B16F10 cells
(red) were detected inside DCs (green) by fluorescence microscope
analysis (Fig. 3D). As the
capability of phagocytic apoptotic B16F10 cells was increased, the
molecular expression of CD80 (Fig.
3E) and MHC class II (Fig. 3F)
was statistically significantly increased on the surface of DCs,
respectively, compared to that of DCs incubated with wild-type
B16F10 cells (p<0.05 or <0.01). The increase in the molecular
expression suggested that DCs underwent a maturation process and
served as a potential trigger for the initiation of a immune
response in vivo.
In addition, the expression of the NKG2D ligand in
MIT-treated apoptotic B16F10 cells (Fig. 3F) and the expression of NKG2D in
the NK1.1+ cells from the vaccinated mice (Fig. 3G) were statistically significantly
increased compared to those of wild-type B16F10 cells,
respectively. This data demonstrated that the apoptotic B16F10
tumor cells promoted DC maturation as well as enhanced the
expression of NKG2D and its ligand.
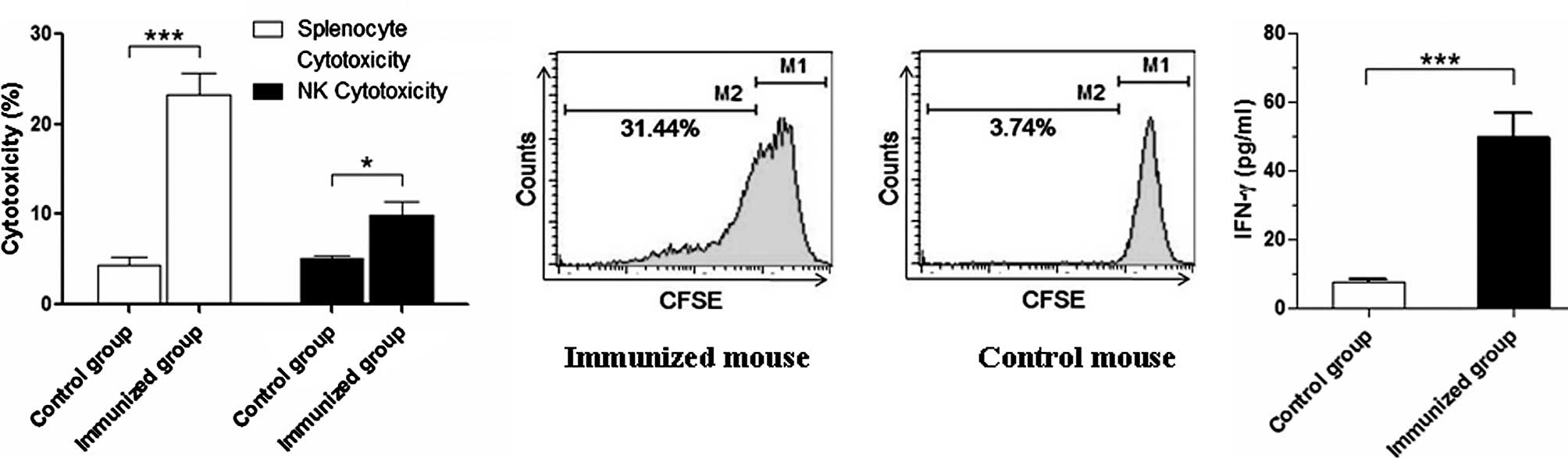
Immune efficacy induced by the B16F10
tumor cell vaccine treated with MIT in combination with RP and
VP
As shown in Fig.
1C, the B16F10 tumor cell vaccine treated with MIT in
combination with RP and VP induced the mice to elicit a powerful
prophylactic efficiency against B16F10 cell challenge. To
investigate the main mechanism of the antitumor effect induced by
the B16F10 tumor cell vaccine, we assessed the cytotoxicity of
splenocytes and NK cells and detected the splenocyte proliferative
response and the serum level of IFN-γ. Data showed that the
cytotoxic activity of splenocytes and NK cells was statistically
significantly increased (p<0.01 or <0.05), respectively,
compared to the control mice (Fig.
4A). This was also verified by the splenocyte proliferative
response and the serum level of IFN-γ (Fig. 4B and C). The results of the
immunological experiment suggest that the B16F10 tumor cell vaccine
enhanced the cellular immune function in vaccinated mice, which may
be a major mechanism of the antitumor efficacy induced by the
B16F10 tumor cell vaccine treated with MIT + RP and VP.
Discussion
Several studies have revealed that the use of
apoptotic cells in vaccines may serve as a potent source of
antigens for stimulating host immune responses in vivo
(3,23,24).
In the present study, the anthracycline drug MIT was used to induce
B16F10 cell apoptosis that was immunized into mice to assess the
safety of a vaccine of MIT-treated tumor cells. However, the result
was not satisfactory as these treated tumor cells retained
tumorigenic potential in the C57BL/6 murine model (Fig. 1A).
The goal of tumor vaccine development is its
safeness and effectiveness. Therefore, the reasons why MIT-treated
tumor cells possess tumorigenic potential were investigated. The
ABCB1 transporter protein (Fig.
2A) and a few SP cells (Fig.
2B) were found in the B16F10 cells. Approximately 0.3% MIT was
able to be discarded from the MIT-treated tumor cells through SP
cells via the ABCB1 transporter protein. Thus, a few B16F10 cells
did not undergo the apoptotic process and retained tumorigenic
potential in vivo. For this reasons, the B16F10 cells were
pretreated with RP and VP to block the function of efflux pumps,
and then were retreated with MIT. As a result, SP cells disappeared
in the B16F10 cells (Fig. 2C), MIT
was almost completely maintained in the treated tumor cells
(Fig. 2D) and there was nearly
complete apoptosis at 144 h (Fig.
3A). The apoptotic B16F10 cells were utilized as a tumor
vaccine to vaccinate the mice twice and then B16F10 cell challenge.
The B16F10 tumor cell vaccine treated with MIT in combination with
RP and VP was not only completely safe (Fig. 1B), but induced an obvious
prophylactic effect against B16F10 cell attack in the murine model
(Fig. 1C).
Subsequently, the possible mechanism of antitumor
efficacy induced by the preparation of the B16F10 tumor cell
vaccine in mice was investigated. It is known that B16F10 tumor
cells exhibit low immunogenicity and do not easily elicit an
antitumor immune response in murine models. However, apoptotic
B16F10 tumor cells induced a strong immune response in the
tumor-bearing mice (3,25). For this reason, we induced B16F10
cell apoptosis by using the anthraquinone anticancer drug MIT, in
combination with RP and VP (Fig.
3A). When the autologous DCs from mouse bone marrow were
concurrently incubated with the apoptotic B16F10 cells for 3 days,
the immature DCs developed into mature DCs, resulting in the
increased molecular expression of CD80 and MHC class II on the cell
surface of DCs, as well as enhancing the ability of phagocytic and
present apoptotic B16F10 cells (Fig.
3B–D). DCs capture apoptotic tumor cells, process them and
present the relevant antigen epitope in the context of both class
II and I MHC to prime lymphocytes, which are specialized functions
of DCs (25,26). Therefore, the apoptotic B16F10
cells may act as a tumor cell vaccine that may elicit lymphocyte
activation via the above-mentioned mature DCs in vivo. In
addition, the NKG2D receptor (Fig.
3H) and NKG2D ligand (Fig. 3G)
were highly expressed in the NK and apoptotic tumor cells,
respectively. The NKG2D immunoreceptor interacting with the NKG2D
ligand serves as one of the most potent activating receptor and
ligand for effector NK cells, playing an important role in the
immunosurveillance of tumors (27). Although we did not detect the
translocation of calreticulin from endoplasmic reticulum to the
cell surface, this mechanism has been confirmed by other
researchers (3,4,28,29).
Accordingly, we assumed that the treatment of a tumor cell vaccine
with MIT would cause cell apoptosis resulting in a calreticulin
coating on the surface of apoptotic cells for recognition and
uptake by DCs. The presented apoptotic cells by DCs may include a
predominant antigen for eliciting lymphocytes in immunized mice to
generate strong immune responses (23,30).
Consequently, the cytotoxicity of splenocytes and NK cells as well
as the splenocyte proliferative response and the serum IFN-γ level
were markedly enhanced compared to the control mouse group
(Fig. 4).
In conclusion, this study demonstrated that the
B16F10 tumor cell vaccine treated with MIP in combination with RP
and VP was safe and efficient. The apoptotic tumor cells
facilitated DC maturation, leading to the activation of lymphocytes
in the vaccinated mice. The immune efficacy induced by the treated
tumor cell vaccine exhibited powerful prevention against B16F10
cell challenge in the murine model. These data provide knowledge
that may be useful for developing an effective B16F10 tumor cell
vaccine for the treatment of melanoma patients in clinical
trials.
Acknowledgements
The authors wish to extend gratitude
to Dr Lili Chu and Dr Fengshu Zhao for the expert administrative
assistance. This study was partly supported by the National Natural
Science Foundation of China (no. 81071769) and in part by the 973
Program of China (no. 2011CB933500).
References
|
1.
|
Saito H, Frleta D, Dubsky P and Palucka
AK: Dendritic cell-based vaccination against cancer. Hematol Oncol
Clin North Am. 20:689–710. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Dou J, Chu LL, Zhao FS, et al: Study of
immunotherapy of murine myeloma by an IL-21-based tumor vaccine in
Balb/c mice. Cancer Biol Ther. 6:1871–1879. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Chunyu C, Han Yu, Yushan R and Yanlin W:
Mitoxantrone-mediated apoptotic B16-F1 cells induce specific
anti-tumor immune response. Cell Mol Immunol. 6:469–475. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Obeid M, Tesniere A, Ghiringhelli F, et
al: Calreticulin exposure dictates the immunogenicity of cancer
cell death. Nat Med. 13:54–61. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Mimeault M, Hauke R, Mehta PP, et al:
Recent advances in cancer stem/progenitor cell research:
therapeutic implications for overcoming resistance to the most
aggressive cancers. J Cell Mol Med. 11:981–1011. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
An Y and Ongkeko WM: ABCG2: the key to
chemoresistance in cancer stem cells? Expert Opin Drug Metab
Toxicol. 5:1529–1542. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Schinkel AH and Jonker JW: Mammalian drug
efflux transporters of the ATP binding cassette (ABC) family: an
overview. Advanced Drug Delivery Rev. 55:3–29. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Van Veen HW, Venema K, Bolhuis H, et al:
Multidrug resistance mediated by a bacterial homolog of the human
multidrug transporter MDR1. Proc Natl Acad Sci USA. 93:10668–10672.
1996.PubMed/NCBI
|
|
9.
|
Droi S, Eytan GD and Assaraf YG:
Potentiation of anticancer-drug cytotoxicity by
multidrug-resistance chemosensitizers involves alterations in
membrane fluidity leading to increased membrane permeability. Eur J
Biochem. 228:1020–1029. 1995. View Article : Google Scholar
|
|
10.
|
Spengler G, Viveiros M, Martins M, et al:
Demonstration of the activity of P-glycoprotein by a semi-automated
fluorometric method. Anticancer Res. 29:2173–2177. 2009.PubMed/NCBI
|
|
11.
|
Fengshu Z, Jun D, XiangFeng H, et al:
Enhancing therapy of B16F10 melanoma efficacy through tumor vaccine
expressing GPI-anchored IL-21 and secreting GM-CSF in mouse model.
Vaccine. 28:2846–2852. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Hyesung K, Hyemi P, Jungsun P, et al:
Dendritic cell vaccine in addition to FOLFIRI regimen improve
antitumor effects through the inhibition of immunosuppressive cells
in murine colorectal cancer model. Vaccine. 28:7787–7796. 2010.
View Article : Google Scholar
|
|
13.
|
Groh V, Steinle A, Spies T, et al:
Recognition of stress-induced MHC molecules by intestinal
epithelial gammadelta T cells. Science. 279:1737–1740. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Jun D, Yongfang W, Jing W, et al:
Antitumor efficacy induced by human ovarian cancer cells secreting
IL-21 alone or combination with GM-CSF cytokines in nude mice
model. Immunobiology. 214:483–492. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Jun D, Quan T, Fengshu Z, et al:
Comparison of immune responses induced in mice by vaccination with
DNA vaccine constructs expressing mycobacterial antigen 85A &
interleukin-21 and Bacillus Galmette-Guérin. Immunol Invest.
37:113–127. 2008.PubMed/NCBI
|
|
16.
|
Dou J, Wen P, Hu W, et al: Identifying
tumor stem-like cells in mouse melanoma cell lines by analyzing the
characteristics of side population cells. Cell Biol Int.
33:807–815. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Karla PK, Earla R, Boddu SH, et al:
Molecular expression and functional evidence of a drug efflux pump
(BCRP) in human corneal epithelial cells. Curr Eye Res. 34:1–9.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Yuan JH, He ZM, Yu YH, et al: Expression
establishment and functional analysis of breast cancer resistance
protein with doxycycline induced tet regulating system in mouse
fibroblast cell line PA317. Ai Zheng. 23:1127–1133. 2004.
|
|
19.
|
Lecoeur H, Février M, Garcia S, Rivière Y
and Gougeon ML: A novel flow cytometric assay for quantitation and
multiparametric characterization of cell-mediated cytotoxicity. J
Immunol Methods. 253:177–187. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Fengshu Z, Jun D, Wang J, et al:
Investigation on the anti-tumor efficacy by expression of
GPI-anchored mIL-21 on the surface of B16F10 cells in C57BL/6 mice.
Immunobiology. 215:89–100. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Goodell MA, Brose K, Paradis G, et al:
Isolation and functional properties of murine hematopoietic stem
cells that are replicating in vivo. J Exp Med. 183:1797–1806. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Jun D and Ning G: Emerging strategies for
the identification and targeting of cancer stem cells. Tumor Bio.
31:243–253. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Frank MO, Kaufman J, Tian S, et al:
Harnessing naturally occurring tumor immunity: a clinical vaccine
trial in prostate cancer. PLoS One. 5:e123672010. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Taniguchi K, Nishiura H, Ota Y, et al:
Roles of the ribosomal protein S19 dimer and chemically induced
apoptotic cells as a tumor vaccine in syngeneic mouse
transplantation models. J Immunother. 34:16–27. 2011. View Article : Google Scholar
|
|
25.
|
Banchereau J and Steinman R: Dendritic
cells and the control of immunity. Nature. 392:245–152. 1998.
View Article : Google Scholar
|
|
26.
|
Gardai SJ, McPhillips KA, Frasch SC, et
al: Cell-surface calreticulin initiates clearance of viable or
apoptotic cells through trans-activation of LRP on the phagocyte.
Cell. 123:321–334. 2005. View Article : Google Scholar
|
|
27.
|
Hu W, Wang J, Dou J, et al: Augmenting
therapy of ovarian cancer efficacy by secreting IL-21 human
umbilical cord blood stem cells in nude mice. Cell Transplant. Nov
5–2010.(E-pub ahead of print).
|
|
28.
|
Panaretakis T, Joza N, Modjtahedi N, et
al: The co-translocation of ERp57 and calreticulin determines the
immunogenicity of cell death. Cell Death Differ. 15:1499–1509.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Davide B, Stefano G, Giovanna C, et al:
Post-apoptotic tumors are more palatable to dendritic cells and
enhance their antigen cross-presentation activity. Vaccine.
26:6422–6432. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Palucka AK, Ueno H, Fay JW, et al: Taming
cancer by inducing immunity via dendritic cells. Immunol Rev.
220:129–150. 2007. View Article : Google Scholar : PubMed/NCBI
|