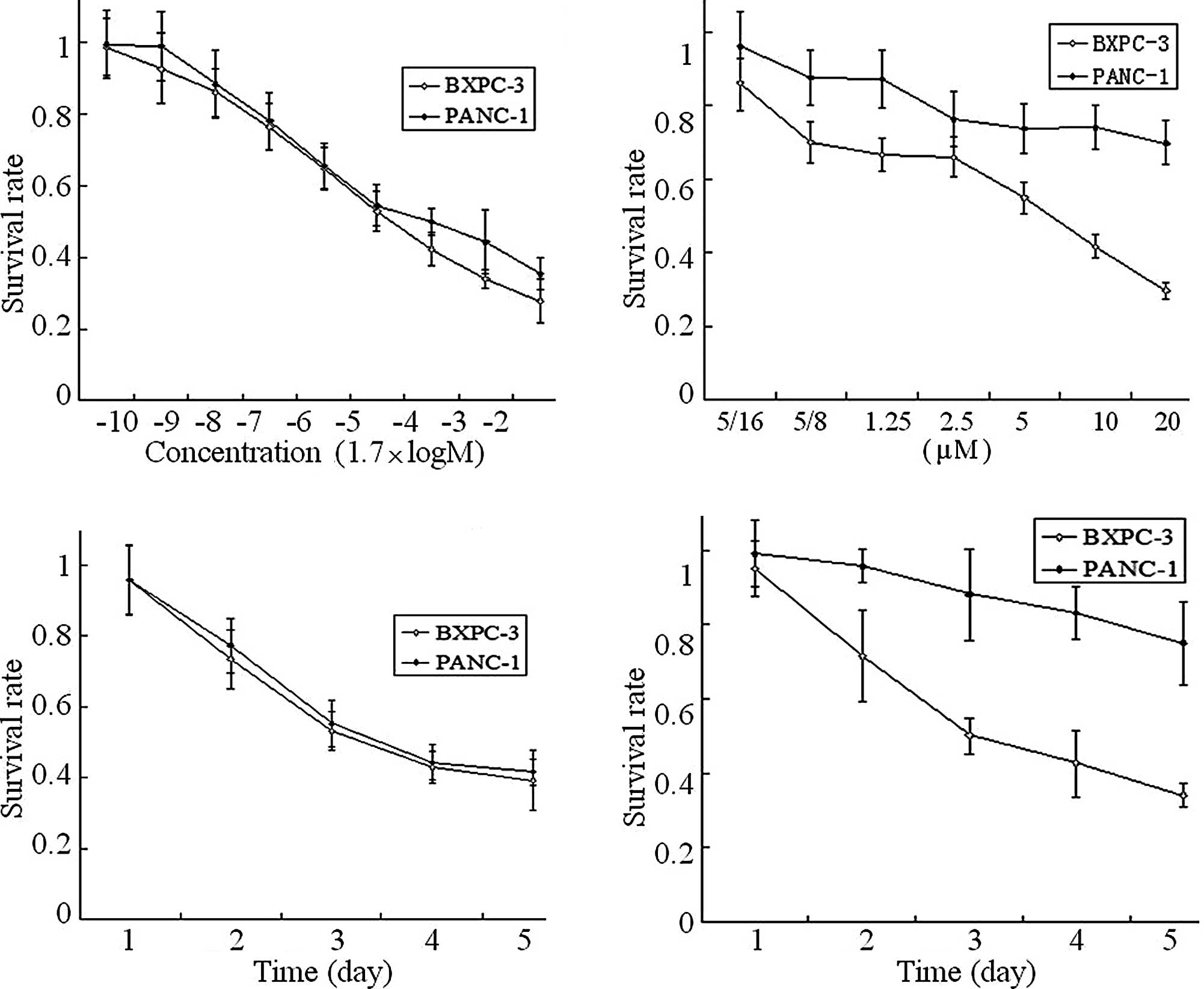
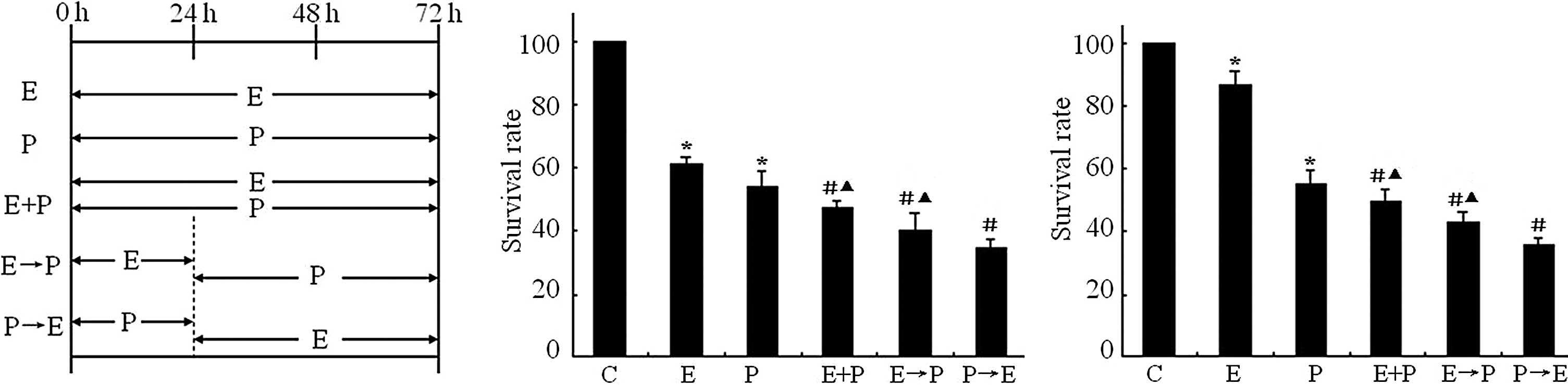
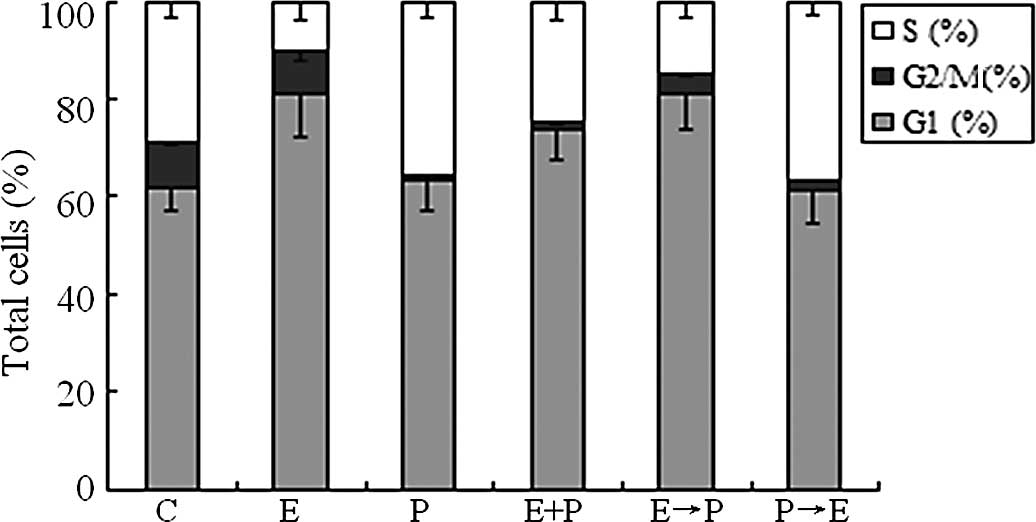
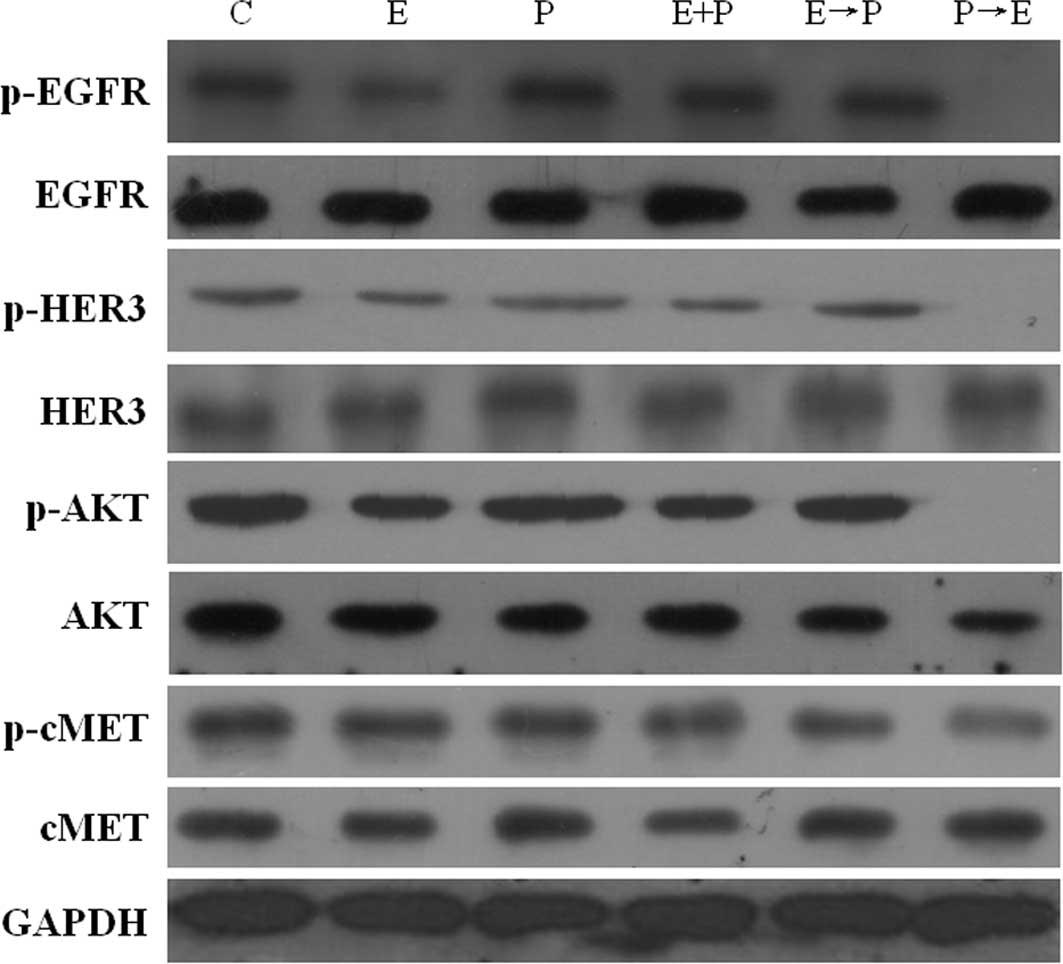
|
1.
|
Michaud DS: Epidemiology of pancreatic
cancer. Minerva Chir. 59:99–111. 2004.PubMed/NCBI
|
|
2.
|
Saidi RF, Remine SG and Jacobs MJ:
Interferon receptor alpha/beta is associated with improved survival
after adjuvant therapy in resected pancreatic cancer. HPB (Oxford).
9:289–294. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Burris HA III, Moore MJ, Andersen J, et
al: Improvements in survival and clinical benefit with gemcitabine
as first-line therapy for patients with advanced pancreas cancer: a
randomized trial. J Clin Oncol. 15:2403–2413. 1997.PubMed/NCBI
|
|
4.
|
Moore MJ, Goldstein D, Hamm J, et al:
erlotinib plus gemcitabine compared with gemcitabine alone in
patients with advanced pancreatic cancer: a phase III trial of the
National Cancer Institute of Canada Clinical Trials Group. J Clin
Oncol. 25:1960–1966. 2007. View Article : Google Scholar
|
|
5.
|
Taylor EC, Kuhnt D, Shih C, et al: A
dideazatetrahydrofolate analog lacking a chiral center at C-6:
N-[4-[2-(2-amino-3,4-dihydro-4-oxo-7H-pyrrolo
[2,3-d]pyrimidin-5yl)ethyl [benzoyl]-L-glutamic acid is an
inhibitor of thymidylate synthase. J Med Chem. 35:4450–4454.
1992.PubMed/NCBI
|
|
6.
|
Chattopadhyay S, Moran RG and Goldman ID:
pemetrexed: biochemical and cellular pharmacology, mechanisms, and
clinical applications. Mol Cancer Ther. 6:404–417. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Miller KD, Picus J, Blanke C, John W,
Clark J, Shulman LN, Thornton D, Rowinsky E and Loehrer PJ Sr:
Phase II study of the multitargeted antifolate LY231514 (ALIMTA,
MTA, pemetrexed disodium) in patients with advanced pancreatic
cancer. Ann Oncol. 11:101–103. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Giovannetti E, Mey V, Danesi R, Mosca I
and Del Tacca M: Synergistic cytotoxicity and pharmacogenetics of
gemcitabine and pemetrexed combination in pancreatic cancer cell
lines. Clin Cancer Res. 10:2936–2943. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Li T, Ling YH, Goldman ID and Perez-Soler
R: Schedule-dependent cytotoxic synergism of pemetrexed and
erlotinib in human non-small cell lung cancer cells. Clin Cancer
Res. 13:3413–3422. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Solomon B and Bunn PA Jr: Clinical
activity of pemetrexed: a multitargeted antifolate anticancer
agent. Future Oncol. 1:733–746. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Lu YY, Jing DD, Xu M, Wu K and Wang XP:
Anti-tumor activity of erlotinib in the BxPC-3 pancreatic cancer
cell line. World J Gastroenterol. 14:5403–5411. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Giovannetti E, Lemos C, Tekle C, Smid K,
Nannizzi S, Rodriguez JA, Ricciardi S, Danesi R, Giaccone G and
Peters GJ: Mechanisms underlying the synergistic interaction of
erlotinib, an epidermal growth factor receptor tyrosine kinase
inhibitor, with the multitargeted antifolate pemetrexed in
non-small-cell lung cancer cells. Mol Pharmacol. 73:1290–1300.
2008. View Article : Google Scholar
|
|
13.
|
Davies AM, Ho C, Lara PN Jr, Mack P,
Gumerlock PH and Gandara DR: Pharmacodynamic separation of
epidermal growth factor receptor tyrosine kinase inhibitors and
chemotherapy in non-small-cell lung cancer. Clin Lung Cancer.
7:385–388. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Mahaffey CM, Davies AM, Lara PN Jr, Pryde
B, Holland W, Mack PC, Gumerlock PH and Gandara DR:
Schedule-dependent apoptosis in K-ras mutant non-small-cell lung
cancer cell lines treated with docetaxel and erlotinib: rationale
for pharmacodynamic separation. Clin Lung Cancer. 8:548–553. 2007.
View Article : Google Scholar
|
|
15.
|
Giovannetti E, Lemos C, Tekle C, et al:
Molecular mechanisms underlying the synergistic interaction of
erlotinib, an epidermal growth factor receptor tyrosine kinase
inhibitor, with the multitargeted antifolate pemetrexed in
non-small-cell lung cancer cells. Mol Pharmacol. 73:1290–1300.
2008. View Article : Google Scholar
|
|
16.
|
Li J, Li Y, Feng ZQ and Chen XG:
Anti-tumor activity of a novel EGFR tyrosine kinase inhibitor
against human NSCLC in vitro and in vivo. Cancer Lett. 279:213–220.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Lu YY, Jing DD, Xu M, Wu K and Wang XP:
Anti-tumor activity of erlotinib in the BxPC-3 pancreatic cancer
cell line. World J Gastroenterol. 14:5403–5411. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Sun SY, Rosenberg LM, Wang X, Zhou Z, Yue
P, Fu H and Khuri FR: Activation of Akt and eIF4E survival pathways
by rapamycin-mediated mammalian target of rapamycin inhibition.
Cancer Res. 65:7052–7058. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Roskoski R Jr: The ErbB/HER receptor
protein-tyrosine kinases and cancer. Biochem Biophys Res Commun.
319:1–11. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Tobita K, Kijima H, Dowaki S, et al:
Epidermal growth factor receptor expression in human pancreatic
cancer: Significance for liver metastasis. Int J Mol Med.
11:305–309. 2003.PubMed/NCBI
|
|
21.
|
Frolov A, Schuller K, Tzeng CW, Cannon EE,
Ku BC, Howard JH, Vickers SM, Heslin MJ, Buchsbaum DJ and Arnoletti
JP: ErbB3 expression and dimerization with EGFR influence
pancreatic cancer cell sensitivity to erlotinib. Cancer Biol Ther.
6:548–554. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Giovannetti E, Mey V, Nannizzi S,
Pasqualetti G, Del Tacca M and Danesi R: Pharmacogenetics of
anticancer drug sensitivity in pancreatic cancer. Mol Cancer Ther.
5:1387–1395. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Liles JS, Arnoletti JP, Tzeng CW, Howard
JH, Kossenkov AV, Kulesza P, Heslin MJ and Frolov A: ErbB3
expression promotes tumorigenesis in pancreatic adenocarcinoma.
Cancer Biol Ther. 10:555–563. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Yonezawa M, Wada K, Tatsuguchi A, Akamatsu
T, Gudis K, Seo T, Mitsui K, Nagata K, Tanaka S, Fujimori S and
Sakamoto C: Heregulin-induced VEGF expression via the ErbB3
signaling pathway in colon cancer. Digestion. 80:215–225. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Miller TW, Pérez-Torres M, Narasanna A, et
al: Loss of phosphatase and tensin homologue deleted on chromosome
10 engages ErbB3 and IGF-IR signaling to promote antiestrogen
resistance in breast cancer. Cancer Res. 69:4192–4201. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Chen K, Iribarren P, Gong W and Wang JM:
The essential role of phosphoinositide 3-kinases (PI3Ks) in
regulating pro-inflammatory responses and the progression of
cancer. Cell Mol Immunol. 2:241–252. 2005.PubMed/NCBI
|
|
27.
|
Buck E, Eyzaguirre A, Haley JD, Gibson NW,
Cagnoni P and Iwata KK: Inactivation of Akt by the epidermal growth
factor receptor inhibitor erlotinib is mediated by HER-3 in
pancreatic and colorectal tumor cell lines and contributes to
erlotinib sensitivity. Mol Cancer Ther. 5:2051–2059. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Engelman JA, Jänne PA, Mermel C, Pearlberg
J, Mukohara T, Fleet C, Cichowski K, Johnson BE and Cantley LC:
ErbB-3 mediates phosphoinositide 3-kinase activity in
gefitinib-sensitive non-small cell lung cancer cell lines. Proc
Natl Acad Sci USA. 102:3788–3793. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Engelman JA, Zejnullahu K, Mitsudomi T, et
al: MET amplification leads to gefitinib resistance in lung cancer
by activating ERBB3 signaling. Science. 316:1039–1043. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Turke AB, Zejnullahu K, Wu YL, et al:
Preexistence and clonal selection of MET amplification in EGFR
mutant NSCLC. Cancer Cell. 17:77–88. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Arteaga CL: HER3 and mutant EGFR meet MET.
Nat Med. 13:675–677. 2007. View Article : Google Scholar : PubMed/NCBI
|