Introduction
Glioblastoma is the most aggressive malignancy in
the human brain, accounting for 40% of all primary malignant brain
tumors. The survival rate is only 6–12 months following initial
diagnosis (1–3). The majority of glioblastomas appear
to be sporadic without any genetic predisposition; however, they
occur more frequently in males. Surgery is currently the preferred
approach to treating patients with glioblastoma; however, due to
the lack of the clear boundaries between cancerous and normal
tissues, it is difficult to completely remove the entire tumor
lesion. The residual glioblastoma cells commonly spread along the
ventricles following surgery. Radiotherapy is another choice for
the treatment of glioblastoma, but the tolerance threshold of
normal brain cells is lower than those of glioblastoma cells.
Chemotherapy and immunotherapy are also routinely used to treat
glioblastoma. The selection of these various treatments is
dependent on the tumor location and stage, but in the majority of
patients, a combined approach of surgery, radiation and
chemotherapy will improve the survival rate. In recent years, novel
approaches such as gene therapy or target therapy are emerging as
new forms of adjuvant treatment for glioblastoma. Thus, these new
approaches may provide useful insight into controlling glioblastoma
formation.
To this end, the first use of targeted gene therapy
for the treatment of glioblastoma was focused on targeting the
epidermal growth factor receptor (EGFR) and platelet-derived growth
factor receptor (PDGFR) (4–6).
EGFR and PDGFR are usually overexpressed in glioblastoma cells and
therefore the knockdown of their protein expression or inhibition
of their gene activity could effectively control glioblastoma cell
growth and improve the survival rate of the patients. Nevertheless,
published data thus far have shown that these approaches have
failed to achieve successful results (4–6),
potentially due to drug resistance. Furthermore, certain other
studies targeting the vascular endothelial growth factor (VEGF)
have found some promising data (7). VEGF is a growth factor and is
involved in vasculogenesis and angiogenesis. The overexpression of
VEGF plays a significant role in cancer progression, since solid
tumors cannot grow beyond a limited size without an adequate blood
supply (8). Therefore, anti-VEGF
therapy may become an important and effective approach in treating
certain types of cancer. For example, a previous study using
anti-VEGF therapy demonstrated the possibility of creating a
treatment time window for combined radiotherapy and chemotherapy
through antagonizing VEGF (9).
Therefore, in the present study we investigated the effects of VEGF
shRNA on the synergistic effects of combined chemotherapy and
radiotherapy treatment of the U251 glioblastoma cell line in
vitro. We evaluated VEGF shRNA in combination with liposomal
paclitaxel for a potential combination therapy for the treatment of
glioblastoma.
Materials and methods
Cell line and culture
The U251 glioma cell line was obtained from the
Shanghai Cell Bank of the Chinese Academy of Sciences (Shanghai,
China) and maintained in RPMI-1640 culture medium containing 10%
fetal bovine serum and 1% penicillin-streptomycin solution
(Hyclone, Logan, UT, USA) in a 5% CO2 humidified
incubator at 37°C.
Reagents
Liposomal paclitaxel (30 mg/bottle) was obtained
from the Institute of Pharmacology, Jiangsu Province, Nanjing Cisco
Pharmaceutical Limited (Nanjing, China) and dissolved in saline
into a 2 mg/ml stock solution prior to use.
VEGF shRNA oligonucleotides were custom-synthesized
by Shanghai GeneChem Co. (Shanghai, China), the plasmid extraction
kit was obtained from Invitrogen (Carlsbad, CA, USA), the plasmid
transfection kit was from Promega (Madison, WI, USA), the
VEGF-quantitative RT-PCR kit was from Da Hui Biological Agents
(Foshan, China), tetrazolium blue (MTT) was from Huamei
Biotechnology Ltd. (Luoyang, China), ethylenediaminetetraacetic
acid (EDTA) and Giemsa stain were from Shanghai Sheng Gong Ltd.
(Shanghai, China), dimethyl sulfoxide (DMSO) was from Shuang Liu
Gong Mao Ltd. (Shanghai, China), and propidium iodide (PI) staining
reagents were from BD company (San Jose, CA, USA).
Construction of VEGF shRNA and gene
transfection
To knock down VEGF protein expression in the U251
glioma cell line, we first selected specific VEGF sequences for
generating VEGF shRNA constructs. We searched GenBank and chose the
VEGF sequence of 5′-GGAGTACCCTGATGAGATC-3′ as the target for PCR
amplification. We then annealed the hairpin-designed sense and
antisense VEGF shRNA oligonucleotides together to form the
double-strand DNA for the construction of the VEGF shRNA vector. A
total of 5 μl each of sense and antisense single-stranded
oligonucleotides, 20 μl 5X buffer and 70 μl ddH2O were
mixed and incubated at 90°C for 4 min and 70°C for 10 min and then
slowly cooled down to room temperature and diluted into 100 ng/μl.
The newly-annealed double-strand DNA oligonucleotides were ligated
to the previously linearized pGCsiU6/Neo/GFP vector (Shanghai
GeneChem Co.) using T4 ligase (Fermentas China Co. Ltd., Shenzhen,
China), and then amplified and sequence-confirmed prior to use. For
gene transfection, we mixed 6 μl of the liposome with plasmid DNA
at a ratio of 3:1 and added this into 2.5 ml serum free medium to
transfer VEGF shRNA into the glioma cells. One hour later, the
growth medium was refreshed with regular RPMI-1640 and the cells
were cultured for up to 72 h. Cell growth and gene expression were
then measured.
Quantitative reverse
transcription-polymerase chain reaction (qRT-PCR)
qRT-PCR was used to detect gene expression. Briefly,
following gene transfection, the VEGF mRNA levels in U251 cells
were assessed using qRT-PCR. Total RNA and mRNA were extracted and
isolated according to a previously described protocol (10). mRNA was then subjected to reverse
transcription using random primers with M-MLV reverse transcriptase
(Promega) into cDNA. VEGF primers used for qRT-PCR were
5′-TGCCAGCAACACTACCAC-3′ (upstream) and 5′-GAGTCATCTCCAGCATCC-3′
(downstream). qRT-PCR was performed using a kit from Da Hui
Biological agents, which also contained a standard control for copy
numbers (i.e., 1×109 diluted into 4×104 to
4×107). SYBR-Green was used to detect the yield of these
copy numbers. PCR was initiated with a denaturation of 5 min at
95°C, followed by 35 cycles of 95°C for 30 sec, 59°C for 35 sec,
and a final elongation of 72°C for 10 min using an Applied
Biosystems PE7500 Sequence Detection System (Applied Biosystems,
Foster City, CA, USA). Gene expression was normalized and controls
were calculated from the standard curve.
Flow cytometry and MTT colorimetric
assay
U251 cells in logarithmic growth phases were
suspended and seeded at a density of 10,000 cells/well for culture
for 24, 48, 72 and 96 h, with or without drug treatment, radiation,
or gene transfection prior to collection into centrifuge tubes for
supernatant isolation. The isolated cells were then processed for
PI staining prior to flow cytometry to examine the cell cycle and
rates of apoptosis.
For the MTT assay, the U251 cells were treated with
liposomal paclitaxel at a concentration of 0.512, 2.56, 12.8 and 64
μg/ml, and 0.32, 1.6, 8, 40, 200 or 1,000 mg/ml for 48 h with or
without gene transfection and radiation treatment. A total of 5
μg/ml of MTT was then added to the cultures for an additional 4 h
and the reaction was stopped by adding 150 μl DMSO. The optical
density (OD) of the cell cultures was then read using an Agilent
8453 spectrophotometer (Agilent Technologies, Foster City, CA, USA)
with an absorption wavelength of 550 nm. The inhibition rate =
[blank control group OD550 − (experimental group
OD550 − background control group
OD550)]/control group OD550 × 100%.
Colony formation assay, cloning
efficiency test and morphological analysis
To test the effects of VEGF shRNA on the modulation
of sensitivity of U251 cells to liposomal paclitaxel and/or
radiation treatment, U251 cells in a logarithmic growth phase were
cultured in soft agar and treated with liposomal paclitaxel at a
concentration of 64 mg/ml and/or a 6 Gy dose of radiation for 48 h
in 8 groups, i.e., control group (A group), control + radiation
(B), control + paclitaxel (C), control + paclitaxel + radiation
(D), VEGF shRNA-transfected cells (E), VEGF shRNA-transfected cells
+ radiotherapy (F), VEGF shRNA-transfected cells + paclitaxel (G),
VEGF shRNA-transfected cells + paclitaxel + radiation (H). The
medium was then refreshed and the cells were cultured under normal
conditions for an additional 10 days prior to fixation and Giemsa
staining. The number of clones was counted in each well (50 cells
or more were considered a colony) and 3 wells were used for each
treatment. For total colony counts for each treatment group, the
average of the total of the 3 wells was used for calculation.
Changing rates for colony formation and colony formation inhibition
rates were calculated as follows: colony formation rate (%) =
colony/seeded cells × 100%; colony formation inhibition rate (%) =
(control colony number − plus drug group colony)/control group
colony × 100%.
A similar treatment of 8 groups of cells was plated
on a monolayer for morphological analyses. These cells were further
cultured under normal conditions for 24 or 48 h prior to being
observed under an inverted microscope.
Statistical analyses
The experimental data are summarized as the means ±
standard deviation (SD). Under the condition of homogeneity of
variance, the variance F-test was used to compare the means among
all the groups, and q for comparisons between any two tests
(Newman-Keuls method). Non-parametric test methods were used for
clone counting. SPSS 11.0 software (SPSS, Chicago, IL, USA) was
used for all statistical analyses and P<0.05 was considered to
indicate a statistically significant difference.
Results
Knockdown of VEGF mRNA expression using
VEGF shRNA
In this study, we first knocked down VEGF mRNA
expression in U251 glioma cells using a VEGF shRNA vector. Compared
to the control transfection, VEGF shRNA significantly inhibited
VEGF mRNA levels (P<0.001) (Table
I).
 | Table I.Knockdown of VEGF mRNA expression by
using VEGF shRNA. |
Table I.
Knockdown of VEGF mRNA expression by
using VEGF shRNA.
| VEGF copy number
pre- and post-transfection
|
|---|
| Control | 24 h | 48 h | 72 h |
|---|
| 1 | 4723.90 | 316.72 | 417.40 | 351.48 |
| 2 | 12361.20 | 451.65 | 746.38 | 750.98 |
| 3 | 46511.90 | 1817.73 | 1054.22 | 928.61 |
| 4 | 14063.20 | 357.57 | 852.76 | 440.27 |
| 5 | 10160.60 | 379.97 | 402.65 | 501.72 |
| Mean ± SD |
17564.16±7406.03 | 664.73±289.08 | 694.68±126.31 | 594.61±106.67 |
| P-value | 0.039
(F=6.074) |
Effects of VEGF knockdown on changes in
the cell cycle and apoptotic rate
The effects of VEGF knockdown on changes in the cell
cycle and apoptotic rate were determined in U251 cells. The flow
cytometric data showed that the control cells and VEGF
shRNA-transfected cells exhibited G0–G1 arrest and a reduced number
of cells in the G2 and M phases, but did not show a decrease in the
proportion of S phase cells within the first 48 h. By contrast,
after 48 h, VEGF shRNA-transfected cells showed apoptosis in a
time-dependent manner, i.e., the apoptotic rate of VEGF
shRNA-transfected cells was 1.5, 2.7 and 4.3% at 24, 48 and 72 h,
respectively, whereas that of the controls was 0.39, 0.55 and
0.30%, respectively (F=8.832, P=0.041).
Paclitaxel inhibition of U251 cell
viability
U251 cells were treated with various concentrations
of paclitaxel and we found that paclitaxel reduced U251 cell
viability, with an IC50 of 28.1 μg/ml following 48 h of
treatment. However, VEGF knockdown significantly sensitized U251
cells to paclitaxel treatment (IC50 of 28.1 μg/ml to an
IC50 of 0.02 μg/ml in the VEGF-transfected cells). These
data suggest that the knockdown of VEGF significantly synergized
the effect of paclitaxel in U251 glioma cells (F=8.533, P=0.009).
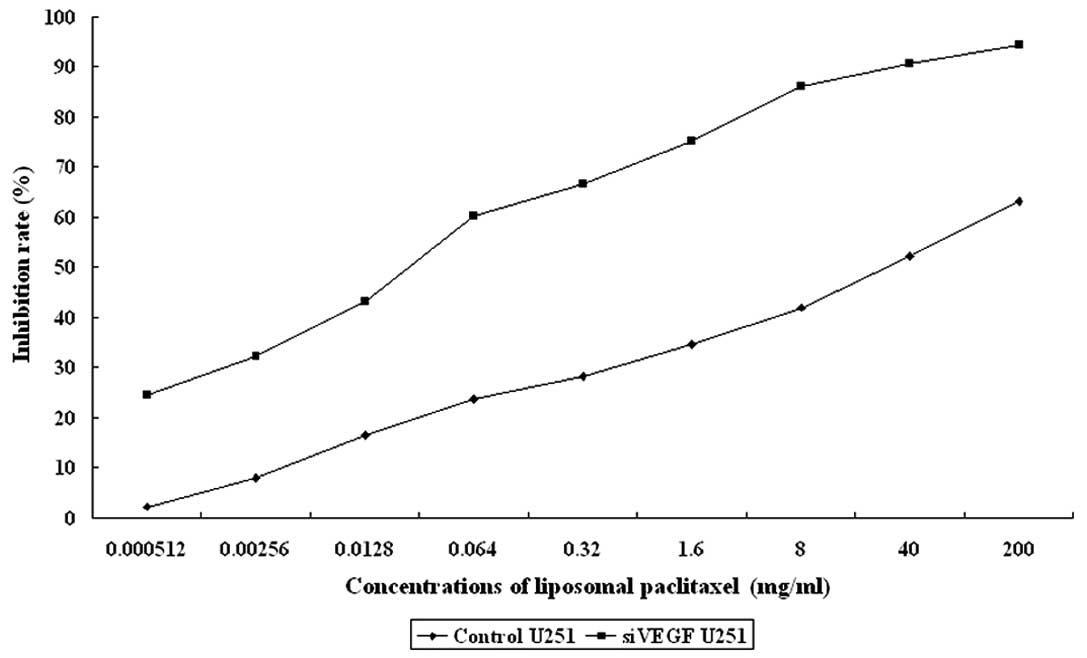
The effects of liposomal paclitaxel on U251 cell proliferation are
summarized in Table II and
Fig. 1.
 | Table II.Effect of VEGF knockdown on
modulation of U251 cell sensitivity to paclitaxel treatment. |
Table II.
Effect of VEGF knockdown on
modulation of U251 cell sensitivity to paclitaxel treatment.
| Drug
concentration | OD550
(x̄ ± SD)
| Inhibition rate (%)
|
|---|
| Control
transfection | VEGF shRNA
transfection | Control
transfection | VEGF shRNA
transfection |
|---|
| 0.000512 mg/ml | 1.65±0.11 | 1.23±0.12 | 2.17 | 24.46 |
| 0.00256 mg/ml | 1.55±0.10 | 1.10±0.13 | 7.90 | 32.18 |
| 0.0128 mg/ml | 1.41±0.15 | 0.92±0.11 | 16.50 | 43.18 |
| 0.064 mg/ml | 1.29±0.12 | 0.65±0.11 | 23.80 | 60.13 |
| 0.32 mg/ml | 1.21±0.15 | 0.54±0.16 | 28.20 | 66.61 |
| 1.6 mg/ml | 1.10±0.17 | 0.40±0.10 | 34.57 | 75.10 |
| 8 mg/ml | 0.98±0.26 | 0.22±0.12 | 41.96 | 86.19 |
| 40 mg/ml | 0.81±0.09 | 0.15±0.10 | 52.28 | 90.62 |
| 200 mg/ml | 0.62±0.14 | 0.09±0.05 | 63.14 | 94.32 |
| Control | 1.69±0.10 | 1.62±0.15 | P=0.009 |
Reduction of U251 cell colony-formation
with combined treatment with VEGF knockdown
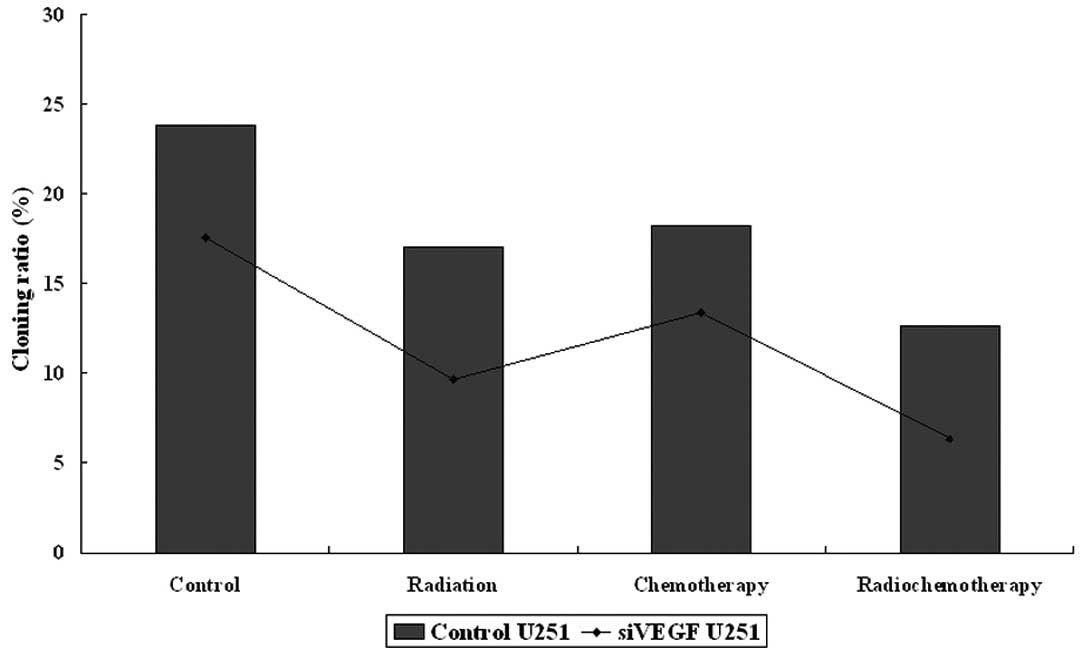
We also tested the combination of VEGF shRNA,
paclitaxel and radiation on changes in colony formation of U251
cells. We utilized a dose of paclitaxel (2.56 mg/ ml of liposomal
paclitaxel) to treat U251 glioma cells with or without VEGF shRNA
transfection for the colony formation assay. These data showed that
colony forming cells in the control group were (group A), 23.83%;
control + radiation (B), 17.0%; control + paclitaxel (C), 18.23%;
control + paclitaxel + radiation (D), 12.67%; VEGF
shRNA-transfected cells (E), 17.57%; radiation + VEGF
shRNA-transfection (F), 9.67%; VEGF shRNA-transfection + paclitaxel
(G), 13.4%; VEGF shRNA-transfection + paclitaxel + radiation (H),
6.33% (Fig. 2). These data
demonstrate that VEGF knockdown significantly sensitizes U251 cells
to paclitaxel and/or radiation treatment. For example, colony
formation in the control transfection group was 17.93±2.30%, while
colony formation in VEGF shRNA-transfected cells was 11.74±1.58%
(P=0.049). The groups with drug treatment alone and radiotherapy
alone showed no significant difference (P>0.05). Following VEGF
knockdown, colony formation in paclitaxel and radiation-treated
U251 cells was significantly reduced (from 17.57 to 6.33%,
P=0.001).
The changes in cell morphology following
treatment
In addition, we also observed changes in cell
morphology following these treatments. We found that cells in the
experimental groups showed some morphological changes when compared
to the control groups, and the transfected group showed more severe
cellular morphological changes than non-transfected cells by
microscopy (Fig. 3).
Discussion
To date, it is an enormous challenge to manage and
cure glioblastoma in neuro-oncological clinics, although surgery
and chemo- and radiation therapy are now available to treat this
deadly disease (11,12). This is due to the fact that the
majority of patients still die within 6–12 months of diagnosis. In
the current study, we explored the knockdown of VEGF expression as
a novel adjuvant treatment for glioblastoma. We first constructed a
VEGF shRNA expression vector to silence VEGF expression in a glioma
cell line. We found that compared to the vector-only control cells,
the VEGF shRNA-transfected glioma cells were much more sensitive to
various doses of liposomal paclitaxel, 6 Gy radiation or liposomal
paclitaxel plus radiation treatment. The tumor cells underwent
apoptosis, decreased colony formation in soft agar plates and
reduced cell viability following VEGF knockdown and the combined
chemo- and radiation treatment. Our study clearly demonstrates that
the silencing of VEGF expression synergistically sensitizes U251
glioma cells to liposomal paclitaxel, radiation and liposomal
paclitaxel plus radiation treatment. However, further studies are
required to evaluate the clinical efficacy of this treatment.
VEGF is the main angiogenesis regulator that plays a
significant role in tumor development and progression. In the
present study, we showed that VEGF mRNA expression was decreased by
more than 60% following VEGF shRNA vector transfection into U251
glioma cells. Phenotypically, VEGF shRNA delayed tumor cell cycles
and increased apoptosis in U251 cells compared to the control
cells, which was supported by a previous study in experimental
mouse brain tumors (13). However,
our current study showed a lesser degree of apoptotic induction by
VEGF shRNA than that of the previous report (13). However, we did find the synergistic
effects of VEGF knockdown with radiation therapy in tumor cells, as
has been suggested in previous studies (14,15).
For example, Winkler et al (16) showed that anti-VEGF receptor
antibodies could open a time window with high oxygen levels in
tumor cells, during which time the radiation therapy could achieve
the best synergistic effect. Another study by Hovinga et al
(17) showed that the increase of
VEGF in tumor cells correlated with radiation therapy, and could
reflect the inborn response of self-protection, which would protect
tumor cells from apoptosis and induce new blood vessels. Our
current data indicate that VEGF shRNA increases the effect of
radiation in a glioma cell line in vitro. Furthermore, we
also found that VEGF shRNA enhances the efficiency of chemotherapy
in U251 cells. Treatment of glioma cells with paclitaxel and
radiotherapy alone showed similar results with limited effects,
whereas the addition of VEGF shRNA transfection showed the best
effects on glioma cells when compared to other double or single
treatments, suggesting that VEGF shRNA was able to sensitize U251
cells to radiotherapy and chemotherapy (18), possibly via different
mechanisms.
Furthermore, current chemotherapy treatment of
glioma lacks efficacy, which could be due to the blood-brain
barrier and less successful drug delivery methods (19). In the current study, we tested the
liposome-based drug delivery, which could provide a new avenue for
treatment. This is due to the fact that liposome-based drug
delivery is capable of enhancing permeation and retention rates
with nano-sized drug carriers (20). Liposomes could more easily cross
epithelial cells within the tumor from the blood circulation
(21,22), and cross the blood-brain barrier
through the enhanced permeability by VEGF shRNA transfection
(23). The liposome-based drug
delivery may also increase efficiency of the convection-enhanced
delivery system (24) to cross the
blood-brain barrier. Indeed, the results from our current study
showed that the IC50 of liposomal paclitaxel decreased
from 28.1 to 0.02 mg/ ml following VEGF shRNA transfection,
suggesting that the liposomal paclitaxel plus VEGF shRNA
transfection could be an effective adjuvant therapy, particularly
for glioblastoma and other types of cancer that are sensitive to
VEGF-targeted gene therapy.
In conclusion, the data from our current study
present a novel adjuvant treatment regime for glioblastoma with the
combination of chemo- and radiation therapy following VEGF shRNA
transfection. Our data demonstrate that the knockdown of VEGF
expression in U251 glioma cells inhibits tumor cell viability and
promotes tumor cell apoptosis, but significantly increases the
sensitivity of U251 cells to radiotherapy and chemotherapy.
Furthermore, liposomal paclitaxel, as a novel drug delivery method,
could enhance drug delivery in vivo, although the VEGF gene
interference and the practical application of liposomal paclitaxel
remains to be examined and explored in future studies.
Acknowledgements
We thank Medjaden Bioscience Limited,
Hong Kong, China, for editing the manuscript.
References
|
1.
|
A JemalT MurrayE WardCancer statisticsCA
Cancer J Clin5510302005
|
|
2.
|
A BehinK Hoang-XuanAF CarpentierPrimary
brain tumours in
adultsLancet361323331200310.1016/S0140-6736(03)12328-812559880
|
|
3.
|
MA MeyerMalignant gliomas in adultsN Engl
J Med35918502007
|
|
4.
|
FB FurnariT FentonRM BachooMalignant
astrocytic glioma: genetics, biology, and paths to treatmentGenes
Dev2126832710200710.1101/gad.159670717974913
|
|
5.
|
PC De Witt HamerSmall molecule kinase
inhibitors in glioblastoma: a systematic review of clinical
studiesNeuro Oncol12304316201020167819
|
|
6.
|
HW LoEGFR-targeted therapy in malignant
glioma: novel aspects and mechanisms of drug resistanceCurr Mol
Pharmacol33752201010.2174/187446721100301003720030624
|
|
7.
|
M MacheinLS de MiguelAngiogenesis in
gliomasRecent Results Cancer
Res171193215200910.1007/978-3-540-31206-2_12
|
|
8.
|
RK JainE di TomasoDG DudaAngiogenesis in
brain tumoursNat Rev Neurosci8610622200710.1038/nrn2175
|
|
9.
|
MI LinWC SessaAntiangiogenic therapy:
creating a unique ‘window’ of opportunityCancer Cell65295312004
|
|
10.
|
HM SaidC HagemannA StaabExpression
patterns of the hypoxia-related genes osteopontin, CA9,
erythropoietin, VEGF and HIF-1alpha in human glioma in vitro and in
vivoRadiother
Oncol83398405200710.1016/j.radonc.2007.05.00317524506
|
|
11.
|
V DamianoD MelisiC BiancoCooperative
antitumor effect of multitargeted kinase inhibitor ZD6474 and
ionizing radiation in glioblastomaClin Cancer
Res1156395644200510.1158/1078-0432.CCR-05-017416061883
|
|
12.
|
A AbdollahiKE LipsonA SckellCombined
therapy with direct and indirect angiogenesis inhibition results in
enhanced anti-angiogenic and antitumor effectsCancer
Res6388908898200314695206
|
|
13.
|
WS KamounCD LeyCT FarrarEdema control by
cediranib, a vascular endothelial growth factor receptor-targeted
kinase inhibitor, prolongs survival despite persistent brain tumor
growth in miceJ Clin
Oncol2725422552200910.1200/JCO.2008.19.9356
|
|
14.
|
G TabatabaiB FrankA WickSynergistic
antiglioma activity of radiotherapy and enzastaurinAnn
Neurol61153161200710.1002/ana.2105717212356
|
|
15.
|
PR WachsbergerR BurdC CardiVEGF trap in
combination with radiotherapy improves tumor control in u87
glioblastomaInt J Radiat Oncol Biol
Phys6715261537200710.1016/j.ijrobp.2006.11.01117234361
|
|
16.
|
F WinklerSV KozinRT TongKinetics of
vascular normalization by VEGFR2 blockade governs brain tumor
response to radiation: role of oxygenation, angiopoietin-1, and
matrix metalloproteinasesCancer Cell65535632004
|
|
17.
|
KE HovingaLJ StalpersC van
BreeRadiation-enhanced vascular endothelial growth factor (VEGF)
secretion in glioblastoma multiforme cell lines - a clue to
radioresistance?J
Neurooncol7499103200510.1007/s11060-004-4204-716193379
|
|
18.
|
G FountzilasG KarkavelasA
Kalogera-FountzilaPost-operative combined radiation and
chemotherapy with temozolomide and irinotecan in patients with
high-grade astrocytic tumors. A phase II study with biomarker
evaluationAnticancer Res2646754686200617214326
|
|
19.
|
S KesariD SchiffJW HensonPhase II study of
temozolomide, thalidomide, and celecoxib for newly diagnosed
glioblastoma in adultsNeuro
Oncol10300308200810.1215/15228517-2008-00518403492
|
|
20.
|
Y MatsumuraA MaedaA new concept for
macromolecular therapies in cancer chemotherapy: mechanisms of
tumortropic accumulation of proteins and the antitumor agents
smancsCancer Res466387639219862946403
|
|
21.
|
KJ HarringtonS MohammadtaghiPS
UsterEffective targeting of solid tumors in patients with locally
advanced cancers by radiolabeled pegylated liposomesClin Cancer
Res7243254200111234875
|
|
22.
|
CO NobleDB KirpotinME HayesDevelopment of
ligand targeted liposomes for cancer therapyExpert Opin Ther
Targets8335353200410.1517/14728222.8.4.33515268628
|
|
23.
|
H AokiK KakinumaK MoritaTherapeutic
efficacy of targeting chemotherapy using local hyperthermia and
thermosensitive liposome: evaluation of drug distribution in a rat
glioma modelInt J
Hypertherm20595605200510.1080/0265673041000170318615370816
|
|
24.
|
C MamotJB NguyenM PourdehnadExtensive
distribution of liposomes in rodent brains and brain tumors
following convection-enhanced deliveryJ
Neurooncol6819200410.1023/B:NEON.0000024743.56415.4b15174514
|