Introduction
Birth weight (BW) refers to the body weight within 1
h of birth. Full-term newborns can be divided into three categories
based on their BW: i) Low birth weight (LBW), with a BW of
<2,500 g; ii) normal birth weight (NBW), with a BW of
2,500–4,000 g; and iii) fetal macrosomia (FM) with a BW of ≥4,000
g. A number of factors, including pregnancy nutrition, various
growth factors, hormones, and the regulatory role of the placenta,
affect BW through various mechanisms, leading to abnormal fetal BW
(1). Over the years, a number of
investigators have found that abnormal BW is closely related to the
pathogenesis of numerous adult diseases (2–4). For
example, LBW and FM may lead to adult metabolic syndrome (obesity,
hypertension and diabetes) through various mechanisms (5). Therefore, it is necessary to clarify
the manner in which fetuses with abnormal BW differ from those with
normal BW and to explain the mechanisms involved.
Currently, studies on abnormal BW are focusing on
nutrition during pregnancy, a variety of growth factors, hormones,
and the regulatory role of the placenta (1). It is worth noting that there are
genes expressed in the placenta that control resource utilization
(6). These genes affect placental
function and thus play an important role in fetal growth and
development (7).
Human solute carrier family 38 member 4
(SLC38A4) gene is located on chromosome 12q13.11. The
encoded protein is known as the sodium-coupled neutral amino acid
transporter 4 (SNAT4), which is a neutral amino acid transporter
and a subtype of the amino acid transport system (known as system
A, including SNAT1, SNAT2 and SNAT4) (8). SNAT4 is a neutral amino acid
transporter in the placenta that plays a crucial role in fetal
growth and development (9). It has
been reported that SNAT4 is highly expressed in human placenta at
the early and late stages of development (9). As an important subtype of system A,
SNAT4 up-regulation leads to enhanced system A activity. In animal
studies, it has been found that the inhibition of system A activity
decreases the body weight of the rat fetus (10,11).
It has been suggested that SNAT4 and system A may be closely
related to fetal development. However, to our knowledge, no such
studies have been carried out in humans to date. Therefore, we
studied the expression of SLC38A4 mRNA and SNAT4 levels as
well as system A activity in the human placenta in order to
investigate the relevant mechanisms for abnormal fetal BW.
Materials and methods
Clinical information and specimen
collection
Maternal placental tissues were collected from
delivery patients in the Department of Obstetrics of Shengjing
Hospital of China Medical University from July 2009 to March 2010.
The collections were approved by the Ethics Committee of the
Shengjing Hospital, and written informed consent was obtained from
all patients prior to the study. The maternal age was 25–35 years
of age, gestational age was 38–42 weeks, maternal height was
155–170 cm, father height was 165–185 cm, all were primipara with
12.5–20 kg of maternal weight gain during pregnancy and without
intercurrent diseases and complications, including hypertension,
diabetes, heart diseases, other medical and surgical diseases, and
abnormal factors of placenta and umbilical cord. The categorization
of BW was carried out according to the guidelines outlined in
‘Gynecology and Obstetrics’. The placentas were divided into three
groups according to the fetal BW: i) LBW, BW <2,500 g, 10 cases;
ii) NBW, BW 2,500–4,000 g, 22 cases; iii) FM, BW ≥4,000 g, 20
cases. Two sheets of placental trophoblast tissue from the central
area were collected under sterile conditions. One tissue block was
flash-frozen in liquid nitrogen, then stored at −80°C until RNA and
protein extraction. The other placental tissue block was placed in
DMEM/Tyrode’s (1:3) culture medium for isolation and culture of
placental villous fragments to measure system A activity.
Real-time PCR to measure SLC38A4
mRNA
Total RNA was extracted using the RNA extraction kit
and converted into cDNA using the cDNA reverse transcription kit
(Takara, China). Real-time PCR was performed on the ABI 7500 System
(Applied Biosystems, CA, USA) in a 20-μl SYBR-Green PCR reaction
containing 1X SYBR-Green PCR master mix (Takara), 10 ng cDNA and
100 nM forward and reverse primers synthesized specific to
SLC38A4 (5′-GAAATTCCAAATACC CTGCCCT-3′ and
5′-GCGGTGGGTGTAATCCATCA-3′) and GAPDH
(5′-GCACCGTCAAGGCTGAGAAC-3′ and 5′-TGG TGAAGACGCCAGTGGA-3′). The
reaction condition was 95°C for 10 min, followed by 40 cycles of
95°C for 15 sec, 60°C for 1 min and 72°C for 30 sec. Dissociation
curves were generated to ensure that a single and specific product
was amplified. Cycle threshold values (Ct) were analyzed using
SDS2.0 software (Applied Biosystems), and relative quantification
of SLC38A4 expression was determined using the comparative
Ct method with the GAPDH transcript as the internal control.
All experiments were repeated three times.
Western blot analysis to detect SNAT4
expression
Total protein was extracted from placental tissues
and the concentration was determined using the Bradford method.
Denatured protein was separated by electrophoresis and transferred
onto PVDF membranes (Millipore, USA). Membranes were subsequently
incubated with anti-SNAT4 antibody (Santa, USA) and GAPDH antibody
(Shanghai Kangcheng, China) as the primary antibody at 4°C
overnight, and followed by horseradish peroxidase-labeled secondary
antibody (Zhongshan, China) at room temperature for 2 h.
Luminescent assay was carried out on an ECL instrument (Gene Co.,
China).
Isotope incorporation method to detect
system A activity
System A activity was detected by
3H-Proline uptake. Placenta tissues were immersed in
DMEM/Tyrode’s (1:3) culture medium with/without Na+ at
37°C for 3 h. Tissues with similar 17-estradiol (based on
radioimmunoassay) concentrations were selected for subsequent
assays, with the addition of 3H-Proline (10.1 nmol/ml;
5.1 Ci/ml; PerkinElmer, USA) + ATP + Tyrode’s medium with/without
Na+, at 37°C for 2 h. The mixture was then rotated in
ice water for 10 min before adding 1 ml normal saline, washed three
times, placed in 37°C distilled water for 12 h and transferred into
0.3 mol/l NaOH for 24 h. System A activity was measured using
liquid scintillation assay.
Statistical analysis
SPSS 17.0 software was applied to analyze the data.
Data are shown as the means ± SD. One-way analysis of variance or
the Student’s unpaired two-tailed test were used for statistical
analysis. P<0.05 was considered statistically significant.
Results
Fetal BW, maternal age and gestational
age
As listed in Table
I, there were no significant differences in maternal and
gestational age among the FM, LBW and NBW groups (P>0.05). The
BW of the fetuses in the FM, LBW and NBW groups had statistically
significant differences (4097.1±69.21, 2433.3±60.72 vs.
3287.3±258.94; P<0.05).
 | Table I.Fetal birth weight (BW) and clinical
information. |
Table I.
Fetal birth weight (BW) and clinical
information.
| Group | Case (n) | Fetal BW (g) | Maternal age
(years) | Gestational age
(weeks) |
|---|
| FM | 20 |
4,097.1±69.21a | 28.6±1.72 | 38.8±1.16 |
| NBW | 22 | 3,287.3±258.94 | 29.5±2.07 | 39.5±1.04 |
| LBW | 10 |
2,433.3±60.72a | 27.7±1.97 | 38.0±0.48 |
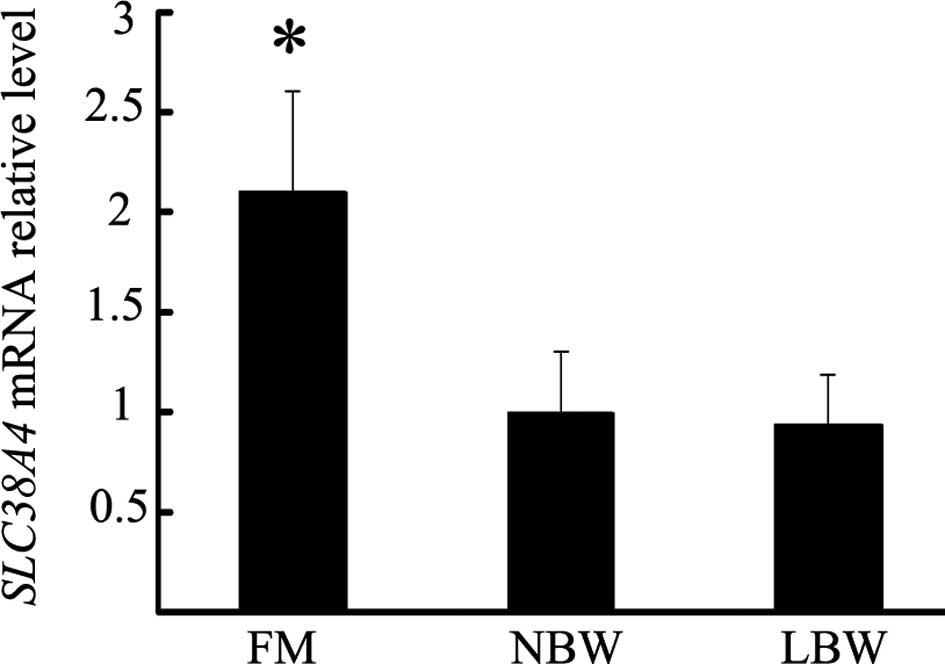
Placental SLC38A4 mRNA expression
As shown in Fig. 1,
the SLC38A4 mRNA expression in the placentas from the FM
group was significantly different from that in the placentas from
the NBW group (2.1±0.51 vs. 1.0±0.30; P<0.05). However there was
no significant difference in placental SLC38A4 mRNA
expression between the LBW and NBW group (0.9±0.24 vs. 1.0±0.30;
P>0.05)
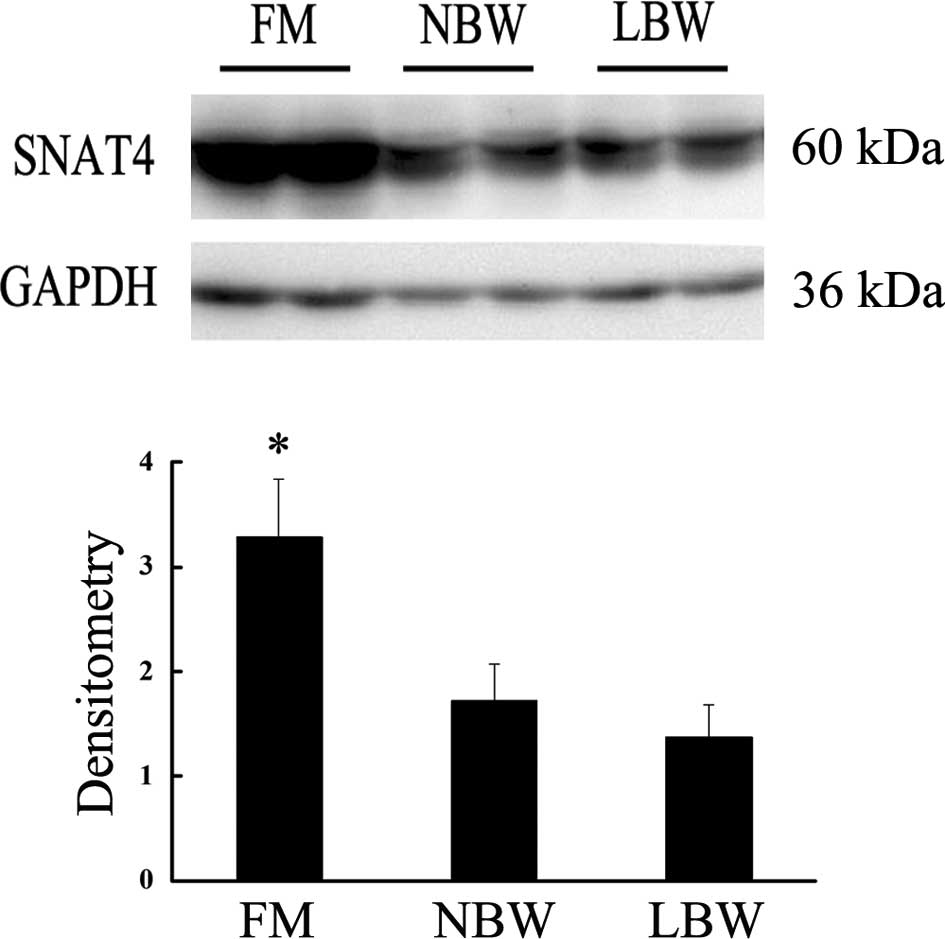
Placental SNAT4 expression
Western blot analysis and densitometry scanning
showed that placental SNAT4 expression in the FM group was
significantly higher compared to the NBW group. However, there was
no significant difference between the LBW and NBW groups (Fig. 2).
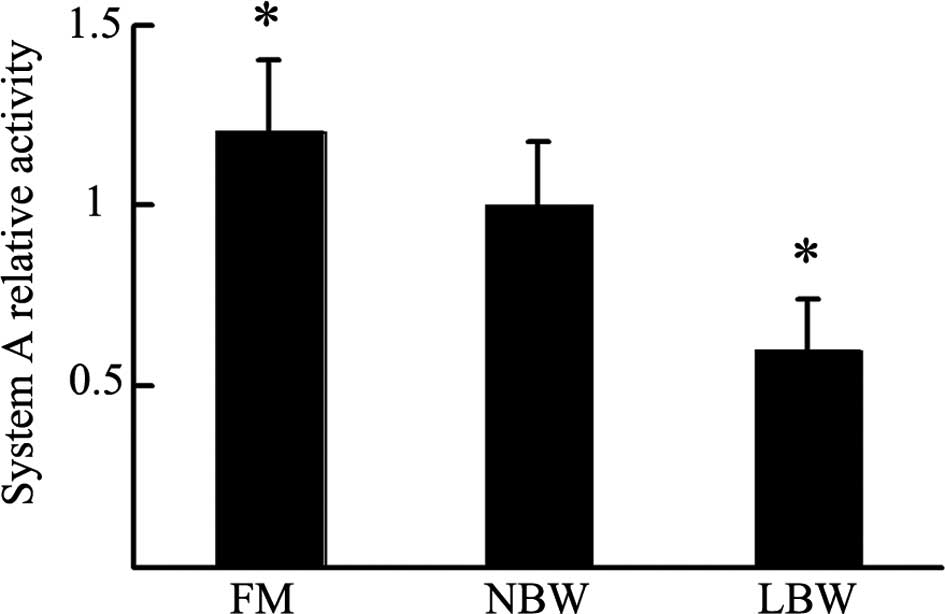
Placental system A activity
To further explore the difference among the three
groups, we used the isotope incorporation method to detect system A
activity. As shown in Fig. 3,
placental system A activity in the FM group was increased (1.2±0.20
vs. 1.0±0.18; P<0.05), while that in the LBW group was decreased
(0.6±0.14 vs. 1.0±0.18; P<0.05) compared to the NBW group.
Discussion
In this study, we first found that the levels of
SLC38A4 mRNA and SNAT4 in the placentas from the FM group
were much higher than those in placentas from the NBW group.
However, there was no significant difference in placental
SLC38A4 mRNA and SNAT4 expression between the LBW and NBW
groups. In addition, compared to the NBW group, placental system A
activity in the FM group was increased and that in the LBW group
was decreased. These results suggest that SLC38A4 and system
A have a strong association with fetal BW and that they may play a
key role in fetal growth and development.
Over the years, a large number of clinical studies
have shown that fetuses with abnormal BW are more susceptible to
adult diseases (fetal programming) (4,5,12).
Currently, it is believed that the factors that affect fetal BW
mainly include maternal factors, nutrition and placental factors
(1,13). However, the placental factors may
play an even greater role in BW, since placenta is the only bridge
between the fetus and the extracellular matrix. The underlying
mechanism could be of great clinical significance for both mother
and fetus. Studies on abnormal fetal BW are now focusing on gene
levels (14,15), as did our current study.
The rodent SLC38A4 gene has been proven to be
a mother-derived imprinted gene which promotes cell proliferation
and differentiation (16,17). The human SLC38A4 gene is
located on chromosome 12q13.11. The protein encoded by
SLC38A4 gene is known as SNAT4, a crucial component of
system A, which mainly transports neutral amino acid and consists
of three subtypes: SNAT1, SNAT2 and SNAT4 (formerly known as ATA1,
ATA2 and ATA3, respectively), which are encoded by three members of
the SLC38A gene family (SLC38A1, SLC38A2 and
SLC38A4, respectively) (8,18).
These three subtypes are expressed in both rat (15) and human placental tissue (9,20,21).
SLC38A4 is highly expressed in the liver and was once
believed to be a liver-specific gene. However, in 2006 Desforges
et al found that SLC38A4 was also expressed in human
placental tissue (9). Our results
confirm that SLC38A4 is expressed in the human placenta.
Desforges et al also found that the expression of SNAT4
differed at various stages of pregnancy, suggesting that the
SLC38A4 gene plays a diverse role at the different stages of
pregnancy (22). This study
focused mainly on a particular stage of pregnancy, i.e., late
pregnancy, when fetal growth and development have matured and the
effects of genes, proteins and other factors have stabilized, a
stage that was more favorable for the aims of our study. We found
that SNAT4 was highly expressed in the placentas from the FM group,
which suggests that it may play a crucial role in fetal
development. Similar studies have confirmed that SNAT4 is closely
related to fetal growth and development in rodents (7). The mechanism involved may be that the
overexpressed SNAT4 causes neutral amino acids to overload in the
placenta, which promotes endometrial cell proliferation,
differentiation and decidualization, enhances the invasive ability
of trophoblast cells upon blastocyst implantation, and
significantly affects normal development of the embryo, leading to
fetal macrosomia. By contrast, no difference in placental
SLC38A4 expression between the LBW and NBW groups was found.
Perhaps this could be due to the involvement of other factors, or
perhaps the sample size was not large enough for the difference to
be detected. In our experiment, the SLC38A4 mRNA levels were
consistent with SNAT4 expression. Namely, the regulation on
transcriptional levels played an essential role. However, we still
do not know through which pathway it affected fetal development.
Thus, further studies are required to explore the exact
mechanism.
System A, including SNAT1, SNAT2 and SNAT4, is a
Na+-dependent transporter that actively transports
small, zwitterionic, neutral amino acids with short, unbranched
side chains, such as alanine, serine and glutamine (23). Neutral amino acids account for the
majority of total amino acids, which play a crucial and are the
essential nutrients in protein formation and cell growth and
differentiation. Therefore, it is conceivable that the function of
system A is inseparable from fetal BW. In animal studies, it was
found that system A is associated with abnormal fetuses and its
inhibition decreases the BW of the fetuses. Reduced system A
activity has been demonstrated in microvillous membrane vesicles
isolated from placentas in which the fetus was growth-restricted
(24). Cramer et al
reported that the inhibition of system A in pregnant rats leads to
fetal growth restriction (10). It
has also been found that alterations in placental system A activity
are associated with fetal growth restriction in a rodent
intrauterine growth restriction model (11,25).
In this study, the placental system A activity was positively
correlated with the BW of fetuses in the three groups, i.e., the
transport activity of system A showed an increasing trend in the
LBW, NBW and FM groups, suggesting that placental system A may be
one of the significant factors in determining fetal BW. In
addition, Constancia et al found that enhanced system A
activity was partly due to up-regulation in the expression of SNAT4
(26), indicating that SNAT4 plays
a crucial role in determining the strength of system A activity. In
our study, the increased placental SNAT4 expression coincided with
enhanced system A activity in the FM group, which may be one of the
important mechanisms of macrosomia. However, in the LBW group,
although system A activity was decreased, the SNAT4 expression was
not. Thus, there may be other regulatory mechanisms requiring
investigation in the future. Our data also indicated that SNAT4 was
not responsible for the whole system A activity, as the role of the
other two subtypes (SNAT1 and SNAT2) cannot be ignored. One aspect
of this study was that we could only detect system A activity, but
not SNAT4 activity, to find its effect on fetal BW.
In this study, we used placentas from consenting
women collected immediately after their full-term babies were born.
None of the women had any intercurrent diseases and complications.
However, clinical studies have shown that in several pathological
pregnancy states, such as gestational hypertension and gestational
diabetes (27), the frequency of
fetuses with abnormal BW is greatly increased. Under these
conditions, the growth conditions of the fetuses have been changed,
and the demand for amino acids and other nutrients has also
changed. Therefore, in our future studies we plan to investigate
any changes in the amino acid transport activity at pathological
pregnancy states.
LBW and FM may lead to adult metabolic syndrome. We
found that system A, especially the SNAT4 subtype, was related to
abnormal fetal BW. This finding may aid in the understanding of the
modulatory mechanism of BW, and could lead to further studies of
fetal programming mechanisms focusing on adult metabolic syndrome.
Thus, it may play a facilitative role in preventing abnormal BW and
reducing the incidence of increasing and non-communicable diseases
that markedly affect quality of life.
Abbreviations:
|
SLC38A4
|
solute carrier family 38 member 4;
|
|
SNAT4
|
sodium-coupled neutral amino acid
transport protein 4;
|
|
BW
|
birth weight;
|
|
LBW
|
low birth weight;
|
|
NBW
|
normal birth weight;
|
|
FM
|
fetal macrosomia
|
Acknowledgements
This study was supported by the
Liaoning Educational Research Foundation (Grant no. LS2010164).
References
|
1.
|
S RastogiR RastogiD RastogiR RastogiG
SinghF ChiappelliEvaluating the impact of a pragmatic nutrition
awareness program for expectant mothers upon birth weight of the
newbornEvid Based Complement Alternat
Med2011185672201110.1093/ecam/neq03421607010
|
|
2.
|
VW JaddoeJC WittemanHypotheses on the
fetal origins of adult diseases: contributions of epidemiological
studiesEur J Epidemiol2191102200616518677
|
|
3.
|
KK OngDB DungerPerinatal growth failure:
the road to obesity, insulin resistance and cardiovascular disease
in adultsBest Pract Res Clin Endocrinol
Metab16191207200210.1053/beem.2002.019512064888
|
|
4.
|
Z HochbergR FeilM ConstanciaChild health,
developmental plasticity, and epigenetic programmingEndocr
Rev32159224201110.1210/er.2009-003920971919
|
|
5.
|
E OkenMW GillmanFetal origins of
obesityObes Res11496506200310.1038/oby.2003.69
|
|
6.
|
AR IslesAJ HollandImprinted genes and
mother-offspring interactionsEarly Hum
Dev817377200510.1016/j.earlhumdev.2004.10.00615707717
|
|
7.
|
E AngioliniA FowdenP CoanRegulation of
placental efficiency for nutrient transport by imprinted
genesPlacenta2798102200610.1016/j.placenta.2005.12.00816503350
|
|
8.
|
B MackenzieJD EricksonSodium-coupled
neutral amino acid (System N/A) transporters of the SLC38 gene
familyPflugers
Arch447784795200410.1007/s00424-003-1117-912845534
|
|
9.
|
M DesforgesHA LaceyJD GlazierSNAT4 isoform
of system A amino acid transporter is expressed in human placentaAm
J Physiol Cell
Physiol290305312200610.1152/ajpcell.00258.200516148032
|
|
10.
|
S CramerM BeveridgeM KilbergD
NovakPhysiological importance of system A-mediated amino acid
transport to rat fetal developmentAm J Physiol Cell
Physiol282153160200211742808
|
|
11.
|
N JanssonJ PetterssonA
HaafizDown-regulation of placental transport of amino acidsprecedes
the development of intrauterine growth restriction in rats fed a
low protein dietJ Physiol576935946200616916910
|
|
12.
|
L MyattPlacental adaptive responses and
fetal programmingJ
Physiol5722530200610.1113/jphysiol.2006.10496816469781
|
|
13.
|
BA HaiderMY YakoobZA BhuttaEffect of
multiple micronutrient supplementation during pregnancy on maternal
and birth outcomesBMC Public
Health11S19201110.1186/1471-2458-11-S3-S1921501436
|
|
14.
|
RB JensenM ChellakootyS
VielwerthIntrauterine growth retardation and consequences for
endocrine and cardiovascular diseases in adult life: does
insulin-like growth factor-I play a role?Horm
Res60136148200310.1159/00007451514671411
|
|
15.
|
U HidenU LangG DesoyeFetoplacental
disturbances in gestational diabetes mellitusGynakol
Geburtshilfliche Rundsch49224229200920530933
|
|
16.
|
FJ RosarioN JanssonY KanaiMaternal protein
restriction in the rat inhibits placental insulin, mTOR, and STAT3
signaling and down-regulates placental amino acid
transportersEndocrinology15211191129201110.1210/en.2010-115321285325
|
|
17.
|
Y MizunoY SotomaruY KatsuzawaAsb4, Ata3,
and Dcn are novel imprinted genes identified by high-throughput
screening using RIKEN cDNA microarrayBiochem Biophys Res
Commun29014991505200210.1006/bbrc.2002.637011820791
|
|
18.
|
MA HedigerMF RomeroJB PengA RolfsH
TakanagaEA BrufordThe ABCs of solute carriers: physiological,
pathological and therapeutic implications of human membrane
transport proteinsPflugers
Arch447465468200410.1007/s00424-003-1192-y
|
|
19.
|
N HayN SonenbergUpstream and downstream of
mTORGenes Dev1819261945200410.1101/gad.1212704
|
|
20.
|
M DesforgesSL GreenwoodJD GlazierM
WestwoodCP SibleyThe contribution of SNAT1 to system A amino acid
transporter activity in human placental trophoblastBiochem Biophys
Res Commun398130134201010.1016/j.bbrc.2010.06.05120599747
|
|
21.
|
HN JonesT JanssonTL PowellIL-6 stimulates
system A amino acid transporter activity in trophoblast cells
through STAT3 and increased expression of SNAT2Am J Physiol Cell
Physiol29712281235200910.1152/ajpcell.00195.200919741197
|
|
22.
|
M DesforgesKJ MynettRL JonesThe SNAT4
isoform of the system A amino acid transporter is functional in
human placental microvillous plasma membraneJ
Physiol5876172200910.1113/jphysiol.2008.16133119015196
|
|
23.
|
LW JohnsonCH SmithNeutral amino acid
transport systems of microvillous membrane of human placentaAm J
Physiol25477378019883377068
|
|
24.
|
T JanssonK YlvenM WennergrenTL
PowellGlucose transport and system A activity in
syncytiotrophoblast microvillous and basal plasma membranes in
intrauterine growth
restrictionPlacenta23392399200210.1053/plac.2002.0826
|
|
25.
|
M ConstanciaM HembergerJ
HughesPlacental-specific IGF-II is a major modulator of placental
and fetal growthNature417945948200210.1038/nature0081912087403
|
|
26.
|
M ConstanciaE AngioliniI
SandoiviAdaptation of nutrient supply to fetal demand in the mouse
involves interaction between the Igf2 gene and placental
transporter systemsProc Natl Acad Sci
USA1021921919224200510.1073/pnas.050446810316365304
|
|
27.
|
A OrnoyPrenatal origin of obesity and
their complications: Gestational diabetes, maternal overweight and
the paradoxical effects of fetal growth restriction and
macrosomiaReprod
Toxicol32205212201110.1016/j.reprotox.2011.05.002
|