Introduction
Intercellular electrical coupling occurs through
protein conduits and gap junctions that are formed by connexins. In
the heart, the most important connexins are connexins 40, 43 (Cx43)
and 45, while Cx43 is the main electrical coupling protein in the
ventricles (1). Cx43 has been
reported to contribute to the occurrence of ventricular arrhythmias
during myocardial ischemia (MI) (2,3).
Grippo et al (4) demonstrated that chronic mild stress
(CMS) rats exhibit an increased vulnerability to ventricular
arrhythmias induced by electrical stimulation. However, the
mechanism behind this remains unclear. Recent studies have revealed
that alterations in the amount of Cx43 and Cx43 phosphoralation
status are important in the genesis of ventricular arrhythmias
during acute MI (5,6). In the present study, we investigated
the expression of Cx43 and the incidences of ventricular
tachyarrhythmias [i.e., ventricular tachycardia (VT) and
ventricular fibrillation (VF)] during acute MI in CMS rats.
Materials and methods
Experimental animals
The experiment protocol conformed to the Guideline
for the Care and Use of Laboratory Animals published by the US
National Institutes of Health (NIH Publication, revised 1996) and
was approved by the Institutional Animal Care and Use Committee.
Male Sprague-Dawley (SD) rats were randomly assigned into the
following groups: MI group (control-MI, n=12), ligation of the left
anterior descending (LAD) coronary artery; sham operation (SO)
group (control-SO, n=12), without coronary ligation; CMS + SO group
(CMS-SO, n=12), without coronary ligation; CMS + MI group (CMS-MI,
n=12), ligation of the LAD coronary artery. The CMS model was
designed according to a previously described method (4).
VT was assessed and defined as ≥10 ventricular
ectopic beats with a cycle length <100 ms; VF was defined as
unidentifiable and low voltage QRS complexes; VF may be sustained
or may revert spontaneously to normal sinus rhythm (5).
Immunoblot analysis
Regions of pulverized frozen ischemia from left
ventricle samples were analyzed by quantitative immunoblotting
using a goat polyclonal anti-Cx43 antibody (sc-56698; Santa Cruz
Biotechnology, CA, USA) for total Cx43 protein and a
non-phosphorylated mouse monoclonal anti-Cx43 antibody (13–8300;
Zymed/Invitrogen, Carlsbad, CA, USA) for non-phosphorylated Cx43
content. The expressions of non-phosphorylated and total Cx43
protein were normalized to glyceraldehyde-3-phosphate dehydrogenase
(GAPDH) expression. The relative amounts of non-phosphorylated and
total Cx43 protein were expressed as a percentage of the SO
group.
Immunofluorescence
For immunofluorescence of Cx43, a polyclonal rabbit
anti-Cx43 antibody (Santa Cruz Biotechnology) was used. Frozen
hearts were sectioned into 6 μm-thick slices; following
permeabilization (0.25% Triton X-100), quenching and blocking (10%
goat serum), samples were incubated with the antibody [1:100
diluted in phosphate-buffered saline (PBS)] overnight at room
temperature. Primary antibody-bound Cx43 was visualized by
fluorescein isothiocyanate (FITC)-conjugated anti-rabbit IgG and
examined using a microscope (Leica, Germany).
Measurement of gap junctional
permeability
Gap junctional permeability values, which were
obtained from 5 samples in each group, were assessed using double
dye-loading (6). The ratio of
Lucifer yellow and rhodamine-dextran stained areas was calculated
using a computer-assisted image analysis system (Image-Pro Plus
3.0). Gap junctional permeability within the ischemic area was
expressed as the percentage of permeability measured within the
non-ischemic area of the SO group.
Statistical analysis
Statistical analysis was performed using SPSS 13.0
software (SPSS Inc., Chicago, IL, USA). All values were expressed
as the means ± SD or the percentage of incidence. The Student’s
t-test was used for comparisons between the two groups. Fisher’s
exact test or the Chi-square test were used for comparisons of the
incidences of ventricular tachyarrhythmias (i.e., VT and VF). A
P-value of <0.05 was considered to indicate a statistically
significant difference.
Results
Incidences of VT and VF
The incidences of VT (7/12, 58.3%) and VF (5/12,
41.7%) in the CMS-MI group were significantly decreased compared to
those in the control-MI group (12/12, 100.0% and 11/12, 91.7%; both
P<0.05).
Changes in Cx43 protein expression
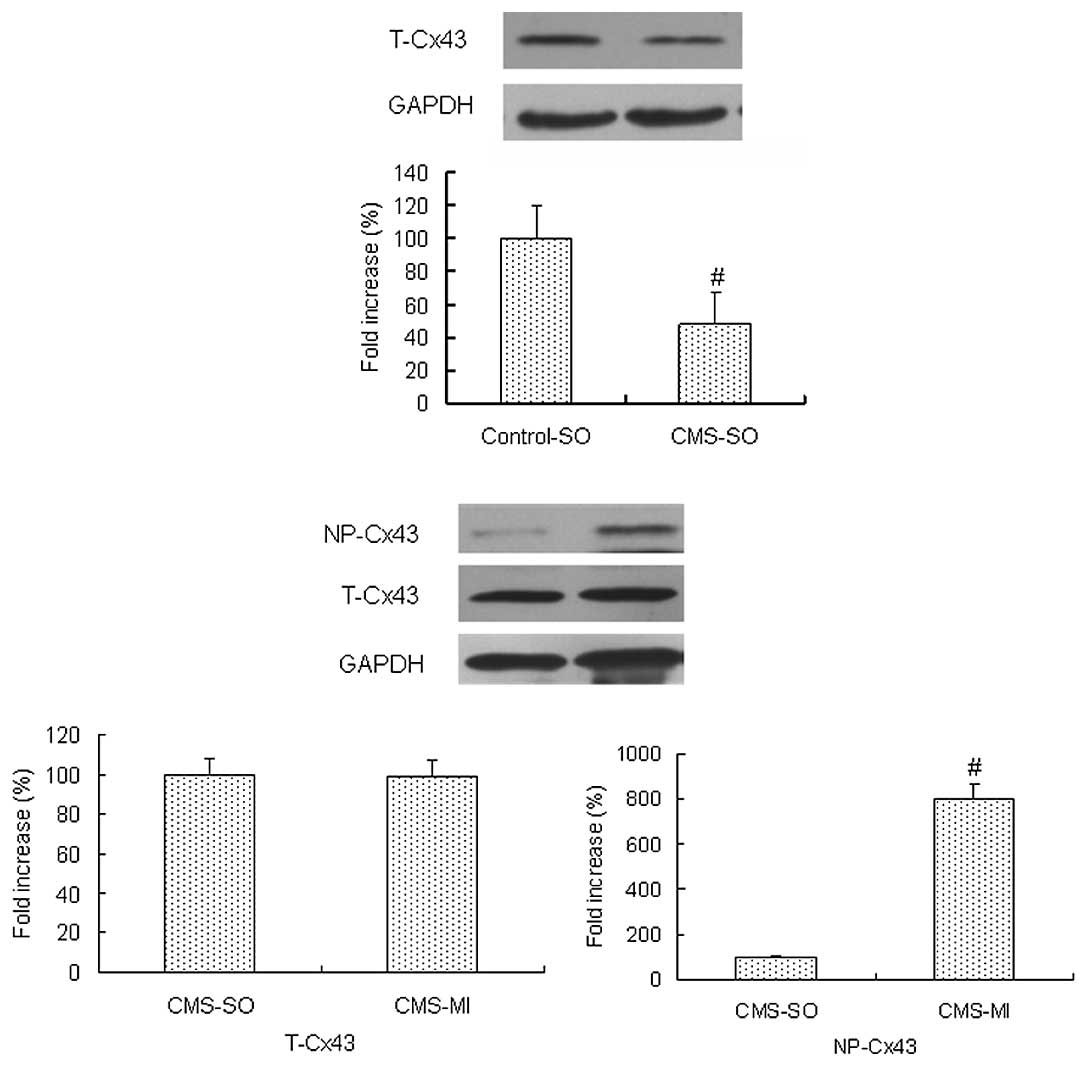
As shown in Fig.
1A, the amount of total Cx43 in the CMS-SO group was
significantly decreased to ∼50% compared to that in the control-SO
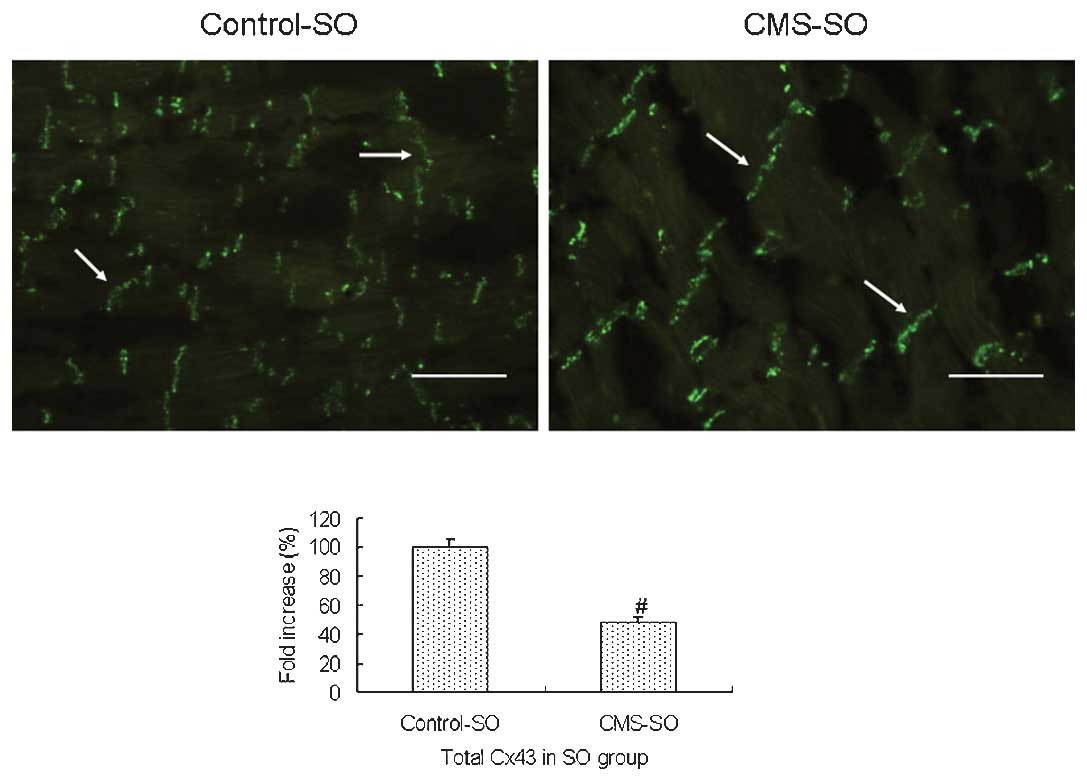
group (P<0.05). Immunofluorescence also revealed that the amount
of total Cx43 in the CMS-SO group was significantly decreased
compared to that in the control-SO group (P<0.05; Fig. 2).
As shown in Fig.
1B, the 30-min ischemia did not result in a significant change
in the amount of total Cx43 (total Cx43 = non-phosphorylated Cx43 +
phosphorylated Cx43) in the CMS rats (P>0.05). The amount of
non-phosphorylated Cx43 in the CMS-MI group was markedly increased
compared to that in the CMS-SO group (P<0.05), suggesting that
the relative amount of phosphorylated Cx43 was significantly
decreased in the CMS rats.
Changes in gap junctional
permeability
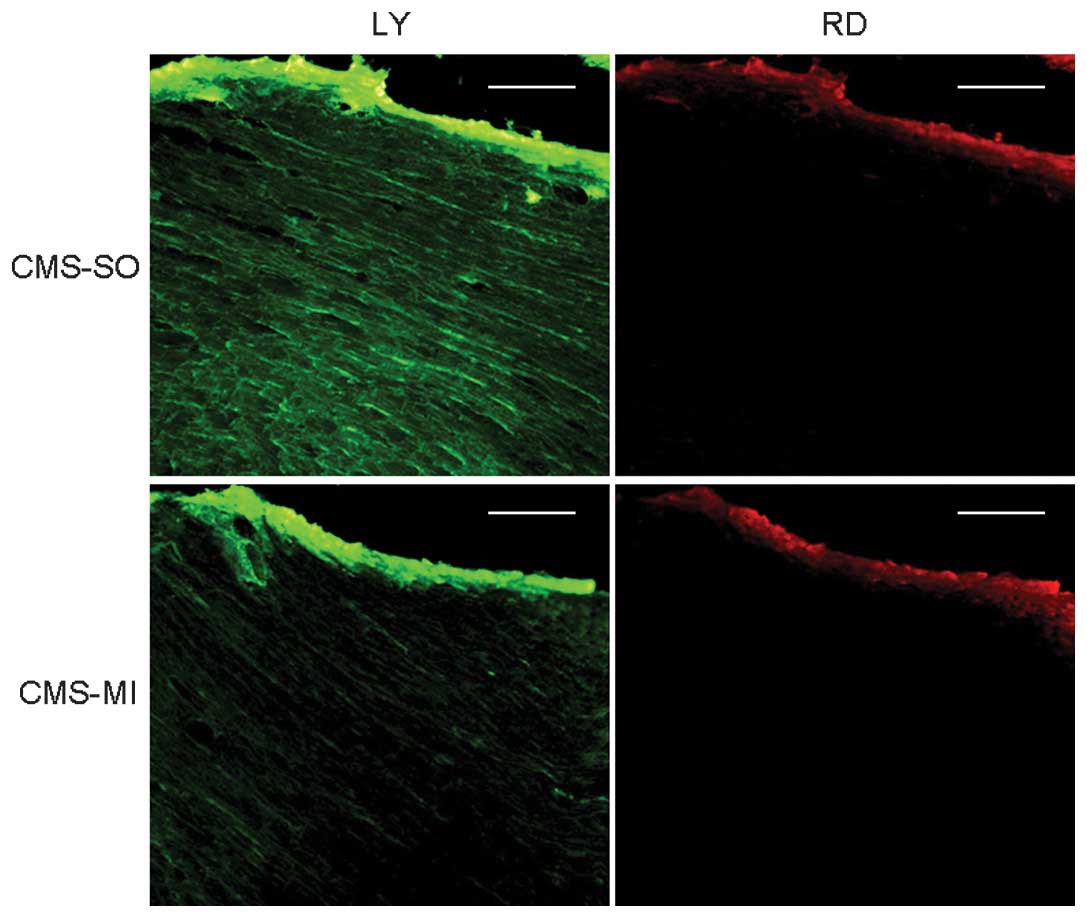
As shown in Fig. 3,
following a 30-min LAD ligation, the gap junctional permeability in
the CMS-MI group (50.4±4.9%) was significantly decreased compared
with the normal non-ischemic value in the CMS-SO group (100%).
Discussion
The prevalence of depression in patients with
coronary artery disease (such as myocardial infarction and heart
failure) is approximately 5 times that of the general population
(7). In the present study, we
found that CMS rats were susceptible to ischemia-induced
ventricular tachyarrhythmias. This indicates that depression may be
a cause of susceptibility to the occurrence of ventricular
arrhythmias.
Previous studies have demonstrated that reduced
expression of Cx43 results in a significant reduction in conduction
velocity during acute MI and accelerates the onset and increases
the incidence of ischemia-induced ventricular arrhythmias (2,3,8–10).
These results suggest that the changes in Cx43 expression may play
a significant role in the genesis of ventricular arrhythmias during
acute MI. Other studies have also shown that Cx43 underwent marked
dephosphorylation during the process of electrical uncoupling
induced by ischemia, which promoted the genesis of ischemia-induced
ventricular arrhythmias (6,11).
In the present study, the amount of total Cx43 in the CMS-SO group
was significantly decreased to approximately 50% compared to that
in the control-SO group. In addition, we found that ischemia did
result in Cx43 dephosphorylation and that the gap junctional
permeability, which could directly mediate the electrical coupling
and conduction (6) in the MI
group, was significantly decreased in the SO group in CMS rats,
indicating that the relative amount of phosphorylated Cx43 in CMS
rats was significantly lower than that in adult rats during MI
(5,10). These results suggest that the
increased susceptibility to ischemia-induced ventricular
tachyarrhythmias may be associated with a reduction in Cx43 protein
expression in depressed rats.
This study had several limitations, however. We
first observed that the incidence of ischemia-induced ventricular
tachyarrhythmias was markedly increased and that the Cx43 protein
expression in the ventricle was significantly decreased in
depressed rats; however, we did not have direct evidence
demonstrating the relationship between the alteration in Cx43
expression and the occurrence of VT/VF, which was also a limitation
of a previous study (10).
In conclusion, the present study suggests that the
incidence of ischemia-induced ventricular tachyarrhythmias is
markedly increased in depressed rats, which may be associated with
the reduction in Cx43 protein expression in the ventricle of
depressed rats.
Acknowledgements
This study was supported by grant no.
81070143 (to Z.L.) from the National Natural Science Foundation of
China, grant no. 4101024 from the Fundamental Research Funds for
the Central Universities (to Z.L.) and the Specialized Research
Fund for the Doctoral Program of Higher Education of China (grant
no. 20100141120072, to Z.L.).
References
|
1.
|
K BoenglerR SchulzG HeuschConnexin 43
signalling and
cardioprotectionHeart9217241727200610.1136/hrt.2005.06687816387816
|
|
2.
|
WT Smith IVWF FleetTA JohnsonCL EngleWE
CascioThe Ib phase of ventricular arrhythmias in ischemic in situ
porcine heart is related to changes in cell-to-cell electrical
coupling. Experimental Cardiology Group, University of North
CarolinaCirculation9230513060199510.1161/01.CIR.92.10.3051
|
|
3.
|
JE SaffitzRB SchuesslerKA YamadaMechanisms
of remodeling of gap junction distributions and the development of
anatomic substrates of arrhythmiasCardiovasc
Res42309317199910.1016/S0008-6363(99)00023-110533569
|
|
4.
|
AJ GrippoCM SantosRF JohnsonTG BeltzJB
MartinsRB FelderAK JohnsonIncreased susceptibility to ventricular
arrhythmias in a rodent model of experimental depressionAm J
Physiol Heart Circ
Physiol286H619H626200410.1152/ajpheart.00450.200314715499
|
|
5.
|
W WuZ LuLoss of anti-arrhythmic effect of
vagal nerve stimulation on ischemia-induced ventricular
tachyarrhythmia in aged ratsTohoku J Exp
Med2232733201110.1620/tjem.223.2721187697
|
|
6.
|
R PappM GoncziM KovacsG SeprenyiA VeghGap
junctional uncoupling plays a trigger role in the antiarrhythmic
effect of ischaemic preconditioningCardiovasc
Res74396405200710.1016/j.cardiores.2007.02.02117362896
|
|
7.
|
SJ SchleiferMM Macari-HinsonDA CoyleWR
SlaterM KahnR GorlinHD ZuckerThe nature and course of depression
following myocardial infarctionArch Intern
Med14917851789198910.1001/archinte.1989.003900800590142788396
|
|
8.
|
DL LernerKA YamadaRB SchuesslerJE
SaffitzAccelerated onset and increased incidence of ventricular
arrhythmias induced by ischemia in Cx43-deficient
miceCirculation101547552200010.1161/01.CIR.101.5.54710662753
|
|
9.
|
DE GutsteinGE MorleyH TamaddonD VaidyaMD
SchneiderJ ChenKR ChienH StuhlmannGI FishmanConduction slowing and
sudden arrhythmic death in mice with cardiac-restricted
inactivation of connexin43Circ
Res88333339200110.1161/01.RES.88.3.33311179202
|
|
10.
|
M AndoRG KatareY KakinumaD ZhangF
YamasakiK MuramotoT SatoEfferent vagal nerve stimulation protects
heart against ischemia-induced arrhythmias by preserving connexin43
proteinCirculation112164170200510.1161/CIRCULATIONAHA.104.52549315998674
|
|
11.
|
H JiangX HuZ LuH WenD ZhaoQ TangB
YangEffects of sympathetic nerve stimulation on ischemia-induced
ventricular arrhythmias by modulating connexin43 in ratsArch Med
Res39647654200810.1016/j.arcmed.2008.07.00518760192
|