Introduction
Five to fifteen percent of women in their
reproductive age suffer from endometriosis, an estrogen-dependent,
chronic disease characterized by the presence of ectopic
endometrium in either the pelvic cavity or the uterus (1). Main symptoms are pelvic pain,
dysmenorrhea, dyspareunia and infertility (2). According to the localization of
endometriotic lesions, endometriosis can be divided into
endometriosis interna (presence of ectopic endometrium within the
myometrium) and endometriosis externa (presence of ectopic
endometrium within the pelvic cavity) (3). Formerly, endometriosis interna and
externa were perceived as one pathological entity characterized by
mucosal invasions and termed adenomyoma (4). In 1927, the theory of retrograde
menstruation was generated and served as a potential
pathophysiological explanation for the development of endometriosis
externa and, as a consequence, led to the separation of
endometriosis externa and interna (5). Nowadays, there is again increasing
evidence that endometriosis interna and externa may represent
different phenotypes of the same disease (3,4). The
tissue-injury and repair theory suggests that local
hyperestrogenism in response to microtraumatization at the
endometrial-myometrial interface leads to enhanced uterine
peristaltic activity within the endomyometrial junctional zone
(6). As a consequence, dislocated
basal endometrium either infiltrates the myometrium (adenomyosis,
endometriosis interna) or reaches via the ovarian tubes the
peritoneal cavity leading to endometriosis externa (6).
Since endometriosis significantly impairs quality of
life of severely affected women, there is a continuous medical need
for the development of new treatment paradigms. Currently, besides
laparoscopy, progestins, oral contraceptives, GnRH analogues,
danazol, as well as pain medication and other experimental
approaches, such as COX-2 inhibitors, aromatase inhibitors,
selective estrogen receptor modulators and GnRH antagonists, are
employed (3,7). Animal models that are used in early
stages of drug testing often rely on non-menstruating rodents with
induced endometriosis-like lesions. In homologous models, normal
endometrial tissue is surgically transplanted into the peritoneal
cavity of immunocompetent recipients and starts to grow in an
estrogen-dependent manner. In heterologous models, human
endometriotic lesions are transplanted into the peritoneal cavity
of immunodeficient mice (8,9). In
both models, drug candidates are analysed with regard to their
ability to influence the estrogen-dependent growth of the
endometrial or endometriotic transplants. Apart from rodents,
primates that spontaneously develop endometriosis or that have been
transplanted intraperitoneally with endometrium can be used to
study drug candidates (8).
However, primate studies are expensive and do not allow for high
throughput analysis in early stages of drug discovery. On the other
hand, the established endometriosis externa models in rodents are
under discussion. It remains to be established whether the use of
immunodeficient mice or whether the transplantation of normal
endometrium into the peritoneal cavity of a non-menstruating
species indeed completely reflects all pathophysiological aspects
of human endometriosis. Additional models, that complement the
established battery of endometriosis externa models, may therefore
be helpful.
Taking into account that human adenomyosis and
endometriosis are again perceived as two phenotypes of the same
disease (3,4), we used a previously described murine
endometriosis interna model (10)
and addressed the question of whether this model is suitable for
the characterization of drug candidates that effectively treat
human endometriosis. We analysed three different compounds in this
model: danazol, an androgenic progestin that was widely used in the
clinic in the 1980s (11),
cetrorelix, a GnRH antagonist that was successfully employed in
human experimental studies (11),
and the antiestrogen Faslodex (ICI182780), which we used as a tool
compound to examine the estrogen dependency of the disease.
Materials and methods
Chemicals
Danazol, Faslodex (ICI182780) and cetrorelix were
synthesized in the Chemistry Department of Bayer Pharma AG (Berlin,
Germany).
Animals
SHN mice were kindly provided by the Japanese RIKEN
BRC Institute, and the SHN breeding colony was maintained at
Taconic (Denmark). Mice were kept on a 14-h light/10-h dark cycle
and provided with food and water ad libitum. All animal
procedures were carried out according to German animal welfare law
with the permission of the District Government of Berlin.
Endometriosis interna model using SHN
mice
To increase the incidence of endometriosis interna
in adult SHN mice, we grafted 1 male donor pituitary under the
kidney capsule of 8-week-old female mice as described previously
(10). Ten randomly chosen control
mice remained unoperated. Two weeks after pituitary grafting, 40
operated animals were randomly allocated into four groups. They
remained either untreated or were treated subcutaneously on 6 days
per week with danazol (25 mg/kg body weight, dissolved in sesame
oil), Faslodex (5 mg/kg body weight, dissolved in
ethanol/arachisoil, 10/90%, v/v) or cetrorelix (100 μg/mouse,
dissolved in water containing 5% mannitol). Treatment was performed
for 8 weeks. The experiment encompassed the following groups
(n=10): no pituitary grafting, no treatment; pituitary grafting, no
treatment; pituitary grafting, danazol treatment (25 mg/kg body
weight); pituitary grafting, Faslodex treatment (5 mg/kg body
weight); pituitary grafting, cetrorelix treatment (100 μg per
mouse).
Animals were sacrificed on day 73 after pituitary
grafting and relative uterine weights were determined. The uterine
horns were fixed overnight in 4% formalin in phosphate buffered
saline (PBS). Afterwards, the tissue was dehydrated and embedded in
paraffin. Sections (5-μm thick) were prepared and deparaffinized.
For antigen retrieval, sections were heated in citrate buffer
(S2031; Dako) at 900 W and boiled for 10 min at 140 W. Endogeneous
peroxidase was blocked by incubation with 3% hydrogen peroxide in
PBS at room temperature for 10 min. For actin staining, a mouse
monoclonal anti-human α-smooth muscle actin antibody (GTX18147,
dilution 1:150; Genetex) and a secondary
horseradish-peroxidase-coupled anti-mouse antibody were employed
according to the manufacturer’s instructions (Genetex). For nuclear
counterstain, sections were stained with hematoxylin, dehydrated
and embedded with Eukitt. Disease severity was assessed by an
investigator blinded to the experimental treatment the animals had
received. Four transverse uterine sections per animal were analysed
using a modification of a previously developed scoring system
(12). The adenomyosis score is a
six-ordered-level scoring system that reflects the degree of
myometrial infiltration by the endometrium: 0, no signs of
endometriosis interna; 1, the inner circular myometrial layer
looses its concentricity; 2, endometrial stroma and glands invade
the inner circular layer of the myometrium; 3, endometrial stroma
and glands are located between the inner circular and outer
longitudinal myometrial layer; 4, endometrial stroma and glands
infiltrate the outer myometrial layer; 5, endometrial stroma and
glands pass the outer myometrial layer and have direct contact with
the peritoneum.
Statistical analysis
For the statistical evaluation of the disease score,
the pituitary-grafted group was considered as reference against
which all other experimental groups were compared at the 5%
significance level. Primary endpoint was the disease score as a
measurement on an ordinal scale. Comparisons against the respective
reference group were made using Dunn’s procedure which controls for
the familywise error rate (13).
Results for relative uterine weights are depicted as
means ± standard deviation. The experimental groups were compared
to animals that did not receive a pituitary again using a
significance level of 5%. For data evaluation a log-normal
distribution is a suitable model. Therefore, Dunnett’s test
(14) was applied on logarithmized
data, keeping the familywise error rate under control. Since the
performed experiments were exploratory in nature, no across
variable α-adjustments were applied.
Results
Adult SHN mice remained unoperated or received
pituitary isografts under the kidney capsule. Two weeks after
transplantation, animals remained either untreated or were treated
for 8 weeks with danazol, Faslodex or cetrorelix. Representative
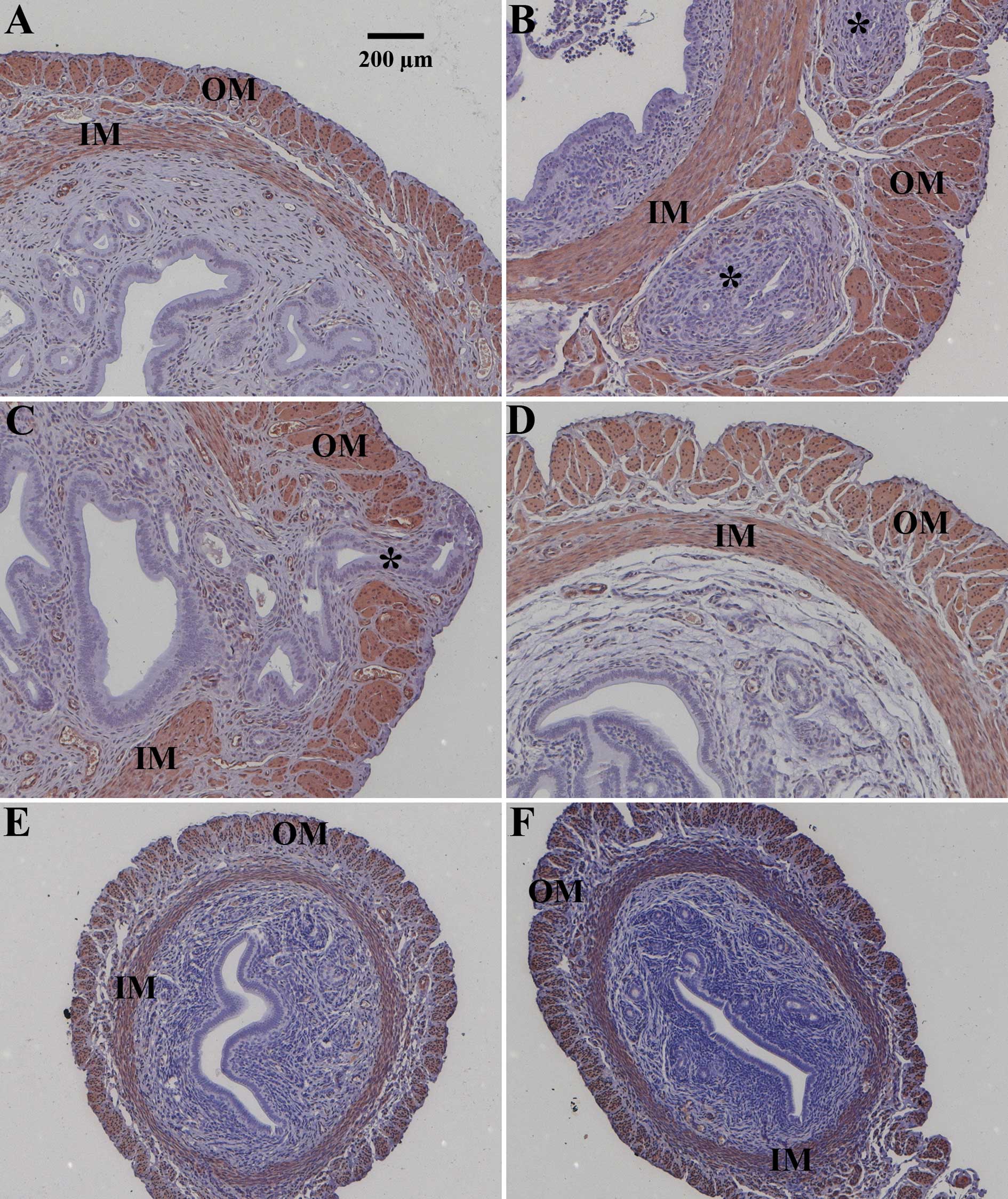
images of uteri from the different treatment groups are shown at
the same magnification (Fig. 1).
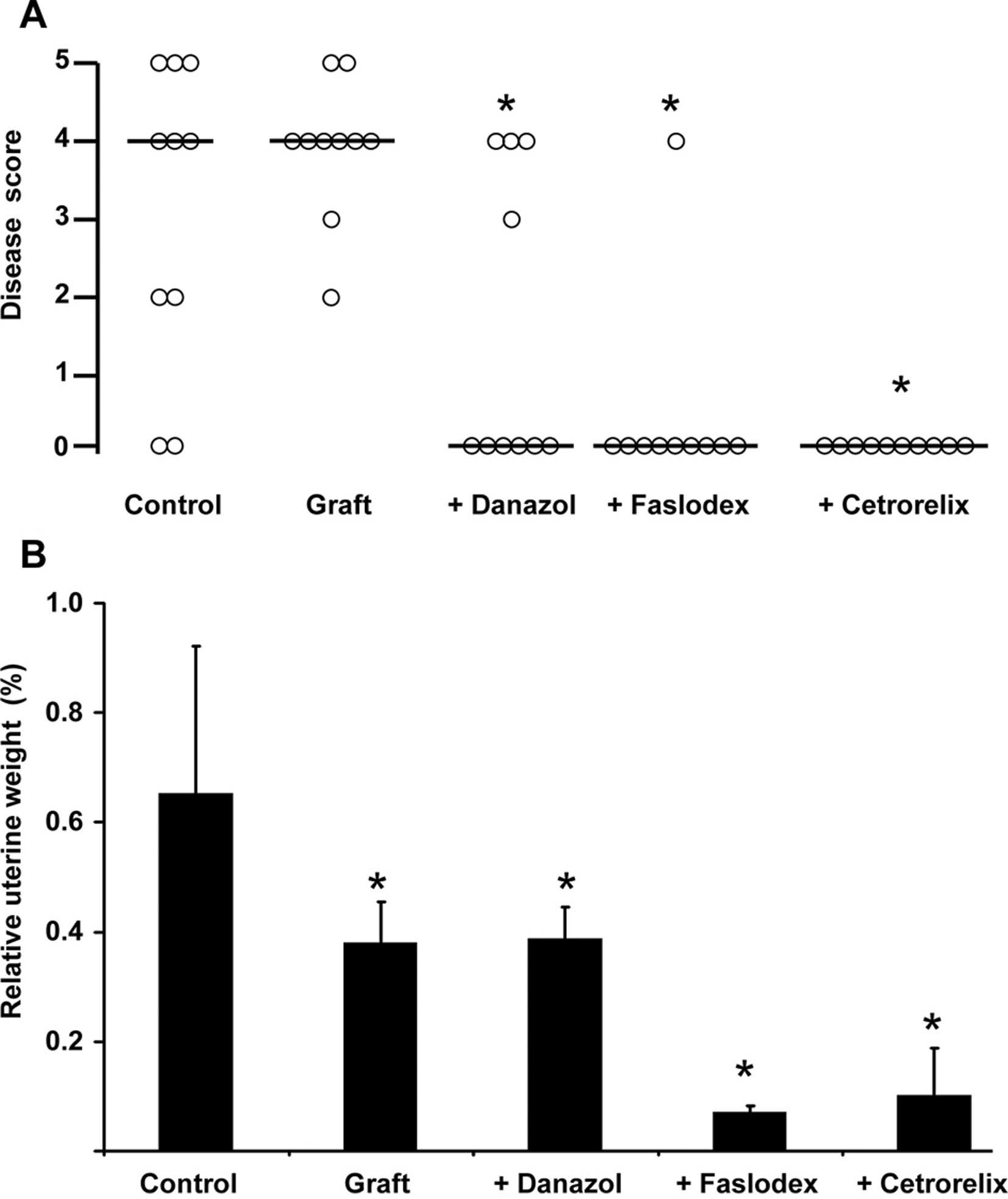
The results of the individual animals in the different treatment
groups were also evaluated using the scoring system described in
Materials and methods (Fig. 2A).
Briefly, a score of 0 was attributed to healthy animals, whereas
higher score numbers correlated with the invasion depth of
endometrial stroma and glands into the uterine smooth muscular
layers. The median score of each treatment group is depicted in
Fig. 2A as a horizontal bar.
Unoperated control animals were either healthy (Figs. 1A and 2A) or suffered from severe endometriosis
interna (Figs. 1B and 2A). Asterisks in Fig. 1 indicate endometrial glands and
stroma invading the inner (IM) and/or outer muscular (OM) layer of
the uterus. This heterogeneous disease activity in unoperated
animals (Fig. 1A vs. B; Fig. 2A) was described previously.
Mice of the SHN strain tend to develop endometriosis
interna spontaneously with increasing age (15), and pituitary grafting in these
animals is known to facilitate homogeneous disease development at
earlier time points. Pituitary grafting provoked endometriosis
interna in all animals (Figs. 1C
and 2A) leading to a median
disease score of 4 (Fig. 2A). As
an example, the most severe form of endometriosis interna
reflecting a score of 5 is depicted (Fig. 1C). Endometrial glands and stroma
passed through the outer muscular layer of the uterus (Fig. 1C). Sixty percent of the animals
treated with danazol after pituitary isografting were healthy; the
median disease score after danazol treatment was 0 (p<0.05 vs.
grafted animals) (Fig. 2A). An
example of successful danazol treatment is depicted in Fig. 1D. Faslodex (median disease score 0,
p<0.05 vs. grafted animals; Fig.
1E) and cetrorelix (median disease score 0, p<0.05 vs.
grafted animals; Fig. 1F)
successfully prevented endometriosis interna development after
pituitary isografting in SHN mice. Only 1 animal treated with
Faslodex still suffered from the disease (Fig. 2A). Relative uterine weights in the
unoperated control group showed a high standard deviation, since
the animals were in random cycle stages (Fig. 2B). Pituitary isografting resulted
in significantly lower uterine weights (Fig. 2B). This finding was in line with
previous reports demonstrating that pituitary isografting led to
increased progesterone levels and smaller uteri in
pituitary-grafted compared to unoperated animals (16). Progesterone inhibits the
proliferative activity of estradiol in the endometrium and
therefore reduces uterine weight. Consequently, additional
treatment with the androgenic progestin danazol also resulted in
lower uterine weights compared to unoperated control animals
(Fig. 2B). The ‘antiestrogenic’
drugs Faslodex (estrogen receptor antagonist) and cetrorelix (GnRH
receptor antagonist leading to inhibition of estradiol synthesis)
strongly reduced relative uterine weight (Fig. 2B) and size (Fig. 1E and F vs. Fig. 1A–D) by completely abolishing
estrogenic effects in the uterus. Faslodex-treated animals suffered
from ovarian cysts (data not shown), a phenotype that was also
observed in estrogen receptor α-deficient mice (17). Since Faslodex completely inhibited
estrogen receptor-mediated signaling and prevented the negative
feedback regulation of estradiol within the hypothalamic-gonadal
axis, ovaries were hyperstimulated and developed cysts.
In conclusion, our data provided evidence that
Faslodex and cetrorelix are fully effective for the treatment of
endometriosis interna in mice, whereas danazol showed efficacy in
the majority of treated animals.
Discussion
The aim of the present study was to evaluate whether
a murine endometriosis interna model could supplement established
rodent endometriosis externa models for drug research in human
endometriosis. For that purpose, we analysed three compounds that
have been employed either clinically or in experimental studies in
human endometriosis (11,18). The GnRH antagonist cetrorelix and
the estrogen receptor antagonist Faslodex, both interfering
negatively with estradiol-mediated signaling, completely suppressed
the disease. Danazol, an androgenic progestin, inhibited
endometriosis interna in the majority of animals as reported
previously (19).
To date, rodent endometriosis externa models are
widely used in drug research, but may have limitations and may not
mimick all aspects of human pathophysiology. For example, in
homologous rodent models, ‘healthy’ uterus is cut into fragments
and transplanted into the peritoneum (8,9),
whereas it has been suggested that the eutopic endometrium of women
suffering from endometriosis may already be abnormal (20). Nude mice lacking an intact immune
system are employed in the heterologous model which cannot mimick
the inflammatory response normally seen in human endometriotic
lesions (9). Although heterologous
rodent endometriosis externa models, such as human endometriosis,
are responsive towards drugs and manipulations that induce a
hypoestrogenic state, such as ovariectomy, GnRH agonists, aromatase
inhibitors, danazol and selective estrogen receptor modulators
(21), there may be difficulties
with the analysis of novel target families in which, for example,
the murine ligand does not bind to the receptor of the human
transplant. In some aspects, murine endometriosis interna models
may have some advantages compared to rodent endometriosis externa
models: i) the endometrium becomes invasive and, thus, does not
appear healthy any longer; ii) the mice have an intact immune
system; and iii) there is no problem with species selectivity of
certain receptor ligands.
Identical to endometriosis externa, endometriosis
interna is an estrogen-dependent disease and can be induced in
several non-menstruating species, such as mice, rabbits and guinea
pigs, after long-term estradiol treatment (22). Pituitary grafting is known to
stimulate an increase in prolactin, growth hormone and progesterone
(15). Ovariectomy of SHN mice,
prevented adenomyosis establishment (23), indirectly demonstrating the
estrogen-dependency of this model. In addition, the
estrogen-dependency of the SHN model was further substantiated by
our finding that Faslodex and cetrorelix suppressed endometriosis
interna.
Progestins are marketed drugs for the treatment of
endometriosis (24). However, as
reported previously (19) danazol,
an androgenic progestin, was only effective in the majority of
affected SHN mice. In the established homologous endometriosis
externa model, even weaker progestin responsivity was observed
(25). Wild-type mice were
ovariectomized and fragments of one uterine horn were transplanted
into the peritoneum. There was a strong increase in lesion volume
after estradiol treatment (from 25.8 mm3 in the
untreated group to 59.3 mm3 in the estradiol-treated
group) demonstrating estrogen dependency of the model. Compared to
the estradiol effects, the therapeutic progesterone effects,
although statistically significant, seemed to be minor; compared to
untreated animals, progesterone decreased lesion volume from 25.9
to 23.7 mm3 and, compared to estradiol-only treatment,
progesterone plus estradiol treatment diminished lesion volume from
66.8 to 59.3 mm3 (25).
Notably, a significant portion of endometriosis patients exhibits
progesterone resistance and does not respond to progestin treatment
(26). The employed endometriosis
interna model reflected the partial progestin responsivity seen in
the human situation. Further research is required to understand the
molecular mechanisms of treatment resistance towards progestins in
endometriosis.
Taken together, although still under debate, the
perception of endometriosis interna (adenomyosis) and externa as
two phenotypes of the same disease is growing (3,4). In
humans, endometriosis interna and externa respond to the same
medical treatment paradigms (27).
This may reflect that similar pathophysiological processes
stimulate the development of endometriosis interna and externa in
women. The results of our study suggest that it may be worthwhile
to exploit murine endometriosis interna models for drug research in
human endometriosis. Endometriosis interna models could represent a
valuable complement to the existing homologous and heterologous
endometriosis externa models.
Acknowledgements
The authors are very grateful to the
Japanese RIKEN BioResource Center which kindly provided the SHN
mouse strain. They also wish to thank Tanja Lehmann and Lam Cam
Quoc from Bayer Pharma AG, Berlin, Germany, for performing the
pituitary grafting in the mice. They thank Professor Daniel Medina
(Baylor College of Medicine, Houston, TX, USA) for the helpful
discussions and technical instructions regarding the pituitary
grafting.
References
|
1.
|
G LeyendeckerM HerbertzG KunzG
MallEndometriosis results from the dislocation of basal
endometriumHum
Reprod1727252736200210.1093/humrep/17.10.272512351554
|
|
2.
|
J KitawakiN KadoH IshiharaH KoshibaY
KitaokaH HonjoEndometriosis: the pathophysiology as an
estrogen-dependent diseaseJ Steroid Biochem Mol
Biol83149155200210.1016/S0960-0760(02)00260-112650711
|
|
3.
|
G HalisS MechsnerAD EbertDiagnosis and
treatment of deep infiltrating endometriosisDtsch Arztebl
Int1074464552010
|
|
4.
|
G BenagianoI BrosensS CarraraAdenomyosis:
new knowledge is generating new treatment strategiesWomens
Health5297311200919392615
|
|
5.
|
JA SampsonPeritoneal endometriosis due to
the menstrual dissemination of endometrial tissue into the
peritoneal cavityAm J Obstet Gynecol14422469192719969738
|
|
6.
|
G LeyendeckerL WildtG MallThe
pathophysiology of endometriosis and adenomyosis: tissue injury and
repairArch Gynecol
Obstet280529538200910.1007/s00404-009-1191-019644696
|
|
7.
|
L FedeleE SomiglianaG FrontinoL BenagliaP
ViganoNew drugs in development for the treatment of
endometriosisExpert Opin Investig
Drugs1711871202200810.1517/13543784.17.8.118718616415
|
|
8.
|
L StoryS KennedyAnimal studies in
endometriosis: a reviewILAR
J45132138200410.1093/ilar.45.2.13215111732
|
|
9.
|
I Tirado-GonzalezG BarrientosN
TariverdianPC ArckMG GarciaBF KlappSM BloisEndometriosis research:
animal models for the study of a complex diseaseJ Reprod
Immunol86141147201010.1016/j.jri.2010.05.00120594597
|
|
10.
|
T MoriH NagasawaMechanism of development
of prolactin-induced adenomyosis in miceActa
Anat1164654198310.1159/0001457246858602
|
|
11.
|
AK RodgersT FalconeTreatment strategies
for endometriosisExpert Opin
Pharmacother9243255200810.1517/14656566.9.2.243
|
|
12.
|
T MoriM KyokuwaH NagasawaAnimal model of
uterine adenomyosis: induction of the lesion in rats by ectopic
pituitary isograftingLab Anim Sci48646819989517893
|
|
13.
|
OJ DunnMultiple comparisons using rank
sumsTechnometrics6241252196410.1080/00401706.1964.10490181
|
|
14.
|
CW DunnettA multiple comparison procedure
for comparing several treatments with a controlJ Am Statist
Assoc5010961121195510.1080/01621459.1955.10501294
|
|
15.
|
T SingtripopT MoriK ShiraishiMK ParkS
KawashimaAge-related changes in gonadotrophin, prolactin and growth
hormone levels with reference to the development of uterine
adenomyosis in female SHN miceIn Vivo714715019938364165
|
|
16.
|
R HusebyMJ SoaresF TalamantesEctopic
pituitary grafts in mice: hormone levels, effects on fertility, and
the development of adenomyosis uteri, prolactinomas, and mammary
carcinomasEndocrinology11614401448198510.1210/endo-116-4-14403971922
|
|
17.
|
JF CouseMM YatesVR WalkerK
KorachCharacterization of the hypothalamic pituitary-gonadal axis
in estrogen receptor (ER) null mice reveals hypergonadism and
endocrine sex reversal in females lacking ERα but not ERβMol
Endocrinol1710391053200312624116
|
|
18.
|
SW GuoDL OliveTwo unsuccessful clinical
trials on endometriosis and a few lessons learnedGynecol Obstet
Invest642435200710.1159/00009841317202821
|
|
19.
|
T SingtripopT MoriS SakamotoS SassaMK
ParkS KawashimaSuppression of the development of uterine
adenomyosis by danazol treatment in miceLife
Sci5111191125199210.1016/0024-3205(92)90513-O1518375
|
|
20.
|
LC GiudiceLC
KaoEndometriosisLancet36417891799200410.1016/S0140-6736(04)17403-515541453
|
|
21.
|
R GruemmerAnimal models in endometriosis
researchHum Reprod
Update12641649200610.1093/humupd/dml02616775193
|
|
22.
|
T MoriT SingtripopS KawashimaAnimal model
of uterine adenomyosis: is prolactin a potent inducer of
adenomyosis in mice?Am J Obstet
Gynecol165232234199110.1016/0002-9378(91)90258-S1853904
|
|
23.
|
T MoriH NagasawaS TakahashiThe induction
of adenomyosis in mice by intrauterine pituitary isograftsLife
Sci2912771282198110.1016/0024-3205(81)90234-47300555
|
|
24.
|
P VercelliniE SomiglianaP VaganoA AbbiatiG
BarbaraPG CrosignaniEndometriosis – current therapies and new
pharmacological developmentsDrugs696496752009
|
|
25.
|
Z FangS YangJP LydonF DeMayoM TamuraB
GuratesSE BulunIntact progesterone receptors are essential to
counteract the proliferative effect of estradiol in a genetically
engineered mouse model of endometriosisFertil
Steril82673678200410.1016/j.fertnstert.2004.01.048
|
|
26.
|
SE BulunYH ChengP YinG ImirH UtsunomiyaE
AttarJ InnesKJ JulieProgesterone resistance in endometriosis: link
to failure to metabolize estradiolMol Cell
Endocrinol24894103200610.1016/j.mce.2005.11.04116406281
|
|
27.
|
L FedeleS BianchiG FrontinoHormonal
treatment for adenomyosisBest Pract Res Clin Obstet
Gynaecol22333339200810.1016/j.bpobgyn.2007.07.00617765017
|