Introduction
Neuregulin 1 (NGR1) is a signaling protein which
plays an important role in the nervous system development. It is
mainly expressed in neurons, glial cells and organs, such as the
heart, liver, stomach, lung, kidney and spleen. Moreover, NGR1 is
implicated in numerous nervous system diseases. In models of
cerebral ischemia, exogenous NGR1 inhibits neuronal apoptosis and
reduces the volume of cerebral infarction (1–6).
Survivin is a well-known anti-apoptotic protein that inhibits the
activities of caspase-3 and caspase-7. Only recently has the role
of survivin in cerebral ischemia become appreciated (7). Aspirin has neuroprotective effects,
including the inhibition of apoptosis of ischemic brain cells
(8,9). However, the link between NGR1 and
survivin expression and aspirin in the treatment of cerebral
ischemia remains unclear. Here, we investigated the effect of
aspirin on the expression patterns of NGR1 and survivin after focal
cerebral ischemia/reperfusion in rats to further explore the
neuroprotective mechanism of aspirin.
Materials and methods
Animals and groups
A total of 80 healthy male Sprague Dawley rats
(weight 240–280 g) were provided by the Medical Animal Laboratory,
Shenyang Military General Hospital. Animals were fasted for 12 h
prior to surgery with water offered ad libitum. The rats
were randomly divided into a treatment group (aspirin, n=40) and a
control group (L-lysine, n=40). Each group was further divided into
five equal subgroups according to the time since reperfusion (6 h,
24 h, 3, 5 and 7 days, n=8 in each group). Aspirin powder was
provided by the Pharmaceutical Factory of Shenyang Pharmaceutical
University, and diluted with L-lysine before use.
Establishing the cerebral
ischemia/reperfusion (CI/RP) model
Cerebral ischemia was established with an occlusion
suture as previously described (10,11).
Two hours after the induction of cerebral ischemia, the suture was
pulled back slowly ∼2 mm for reperfusion. Aspirin powder (80 mg/kg
body weight) dissolved in 1.5 ml of 10% L-lysine saline solution
was injected intraperitoneally at the time of reperfusion and once
each morning thereafter. Brain samples were obtained at 6 h, 24 h,
3, 5 and 7 days after reperfusion. In the control group,
intraperitoneal infusion of 1.5 ml of 10% L-lysine solution was
administered at the same time points, and brain samples were
obtained as in the treatment group. No sham-operated group was set
since sham operation on cervical vessels does not cause cerebral
infarction in the supplied area. Supplementary rats were available
according to a protocol of randomization and grouping when rats
were excluded or died during the experiment. The evaluation of limb
function was carried out according to the Bederson scoring system
(12) every day after the CI/RP
surgery to assess the recovery of neurological function. The
scoring formula was as follows: 0, without any neurologic deficit;
1, dysfunction of left forelimb extension; 2, walking towards the
left; 3, circling towards the left; 4, were unable to walk or in a
coma. Rats with scores of 1–3 were assumed to be successful CI/RP
models and others were excluded from the study.
Immunohistochemical analysis
Immunohistochemical analysis was performed as
described previously (13). At
each experimental time point, after intraperitoneal anesthesia with
an overdose of chloral hydrate (10%), the thoracic cavity was
immediately opened to expose the heart. Perfusion then proceeded
rapidly with 200 ml isotonic saline and 200 ml 4% paraformaldehyde
in PBS injected into the left ventricle. The brain was extracted
after decapitation and fixed in 4% paraformaldehyde for 24 h. Brain
tissues were incised along the coronal plane, and then
conventionally dehydrated and embedded in paraffin. Serial sections
were cut at a thickness of 4 mm, and dried at 37°C in an incubator
overnight. Immunohistochemical staining was performed using S-P
immunohistochemical and diaminobenzidene (DAB) kits (Fujian Maixin
Biological, Fujian, China). NGR1 polyclonal antibody (sc-348) was
from Santa Cruz Company, USA. Positive cells showed as brown
particles in the cytoplasm or nucleus under a light microscope.
Five different high power fields (10×40) were randomly selected
blindly in positive cell areas (the temporal parietal cortex was
selected in the penumbra area and the temporal parietal subcortex
was selected in the infarct center), and the number of positive
cells in each visual field was counted (14).
Statistical analyses
Statistical Package for the Social Sciences (SPSS)
12 software was used for statistical analyses. Data are represented
as the means ± standard deviation (SD). Variance analysis and the
t-test were used for tests of significance. A P-value <0.05 was
considered to denote statistical significance.
Results
After induction of CI/RP, neurological deficit was
observed in both the treatment and control groups, and the main
manifestations were as follows: adduction of the contralateral
forelimb; internal rotation of the shoulder; the left forelimb
could not straighten, or it was held close to the chest while
lifting the tail; sinistral circular motion; dumping; and decreased
muscle tension while walking. At 6 h after reperfusion,
neurological deficit scores were assessed again in the two groups,
compared and examined by t-test. No significant difference was
found between the two groups (P>0.05). Twenty-four hours after
CI/RP surgery, neurological function was restored gradually in both
the treatment and control groups, although a better recovery was
found in the treatment group. There were statistically significant
differences in Bederson scores between the two groups at each time
point after CI/RP (P<0.05; Table
I).
 | Table I.Bederson scores for the rats in the
two groups at different time points after CI/RP (mean ± SD). |
Table I.
Bederson scores for the rats in the
two groups at different time points after CI/RP (mean ± SD).
| Group | n | 6 h | 24 h | 3 days | 5 days | 7 days |
|---|
| Treatment | 40 | 1.97±0.09a | 1.47±0.11c | 1.22±0.08c | 0.85±0.15c | 0.59±0.12b |
| Control | 40 | 1.94±0.13 | 1.87±0.18 | 1.45±0.14 | 1.05±0.08 | 0.75±0.15 |
| t | | 0.89 | 5.36 | 4.03 | 3.33 | 2.36 |
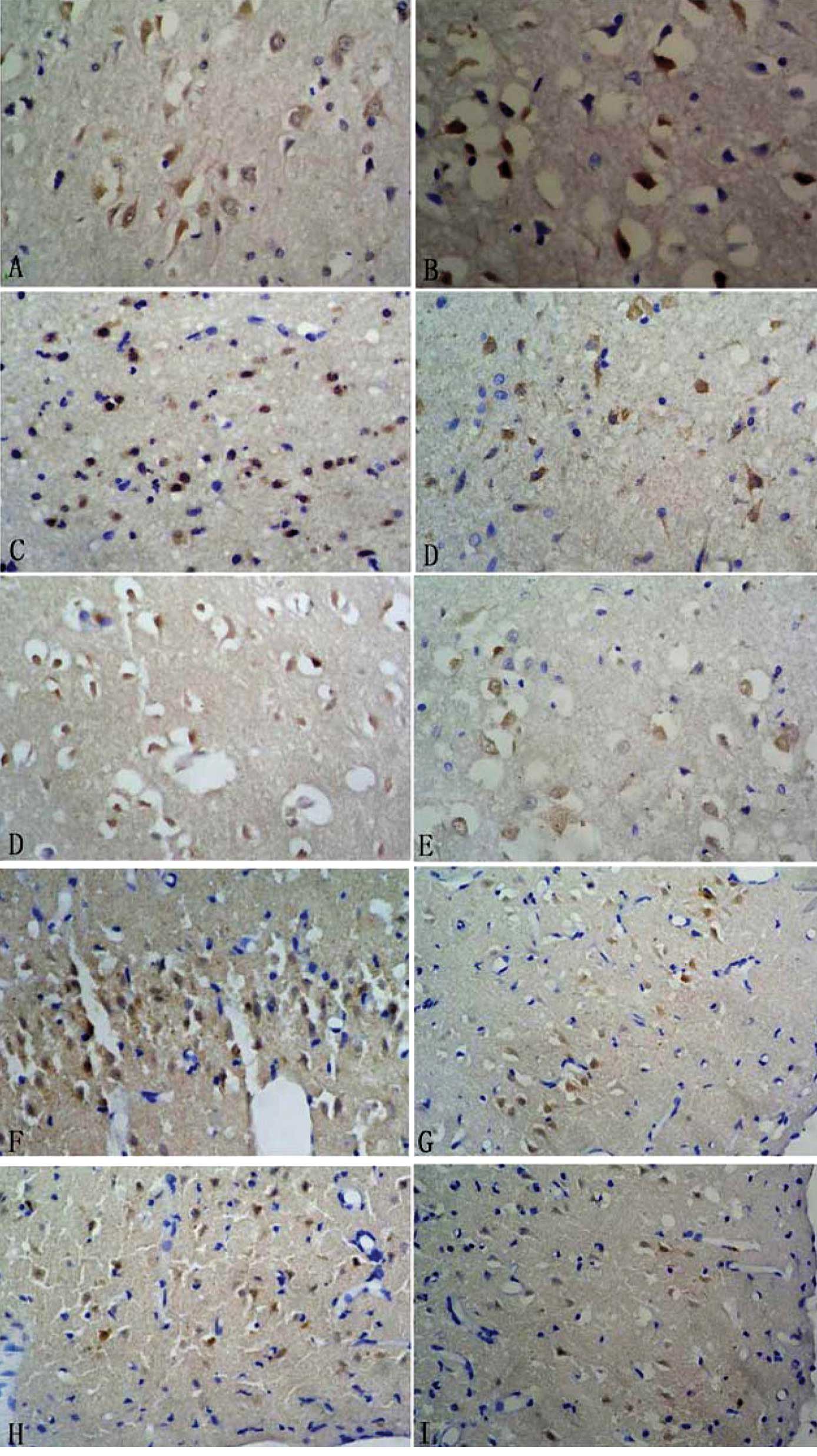
After CI/RP, NGR1 expression was detected by
immunohistochemistry in rat brain tissues in both the treatment and
control groups. NGR1 was mainly expressed in the nucleus and
cytoplasm of neurons in the infarct side. In the infarct center,
NGR1 was expressed strongly 6 h after CI/RP, and the number of
NGR1-positive cells was at a maximum at 6 h and decreased
gradually. In the peri-infarct area, the number of NGR1-positive
cells was small at 6 h, peaked at 3 days and then decreased
gradually. Compared to the control group, more NGR1-positive cells
were detected in the treatment group at each time point, with
significant differences between both time points (P<0.05;
Table II and Fig. 1).
 | Table II.NGR1 expression in different infarct
areas at different time points after CI/RP in the two groups (n=40;
cells/field). |
Table II.
NGR1 expression in different infarct
areas at different time points after CI/RP in the two groups (n=40;
cells/field).
| Time after CI/RP | Infarct center
| Peri-infarct area
|
|---|
| Treatment group | Control group | t | Treatment group | Control group | t |
|---|
| 6 h | 43.4±4.7 | 40.30±5.6 | 2.68a | 16.7±2.6 | 15.3±2.2 | 2.59a |
| 24 h | 33.1±3.4 | 31.30±3.2 | 2.44a | 25.8±3.5 | 23.9±2.7 | 2.72a |
| 3 days | 25.4±2.2 | 23.11±1.9 | 4.57b | 31.4±4.5 | 27.6±4.1 | 3.95b |
| 5 days | 16.4±2.7 | 14.90±2.2 | 2.72a | 26.3±3.4 | 24.7±2.1 | 2.53a |
| 7 days | 6.9±2.1 | 5.80±1.7 | 2.57a | 19.3±2.6 | 17.8±2.4 | 2.68a |
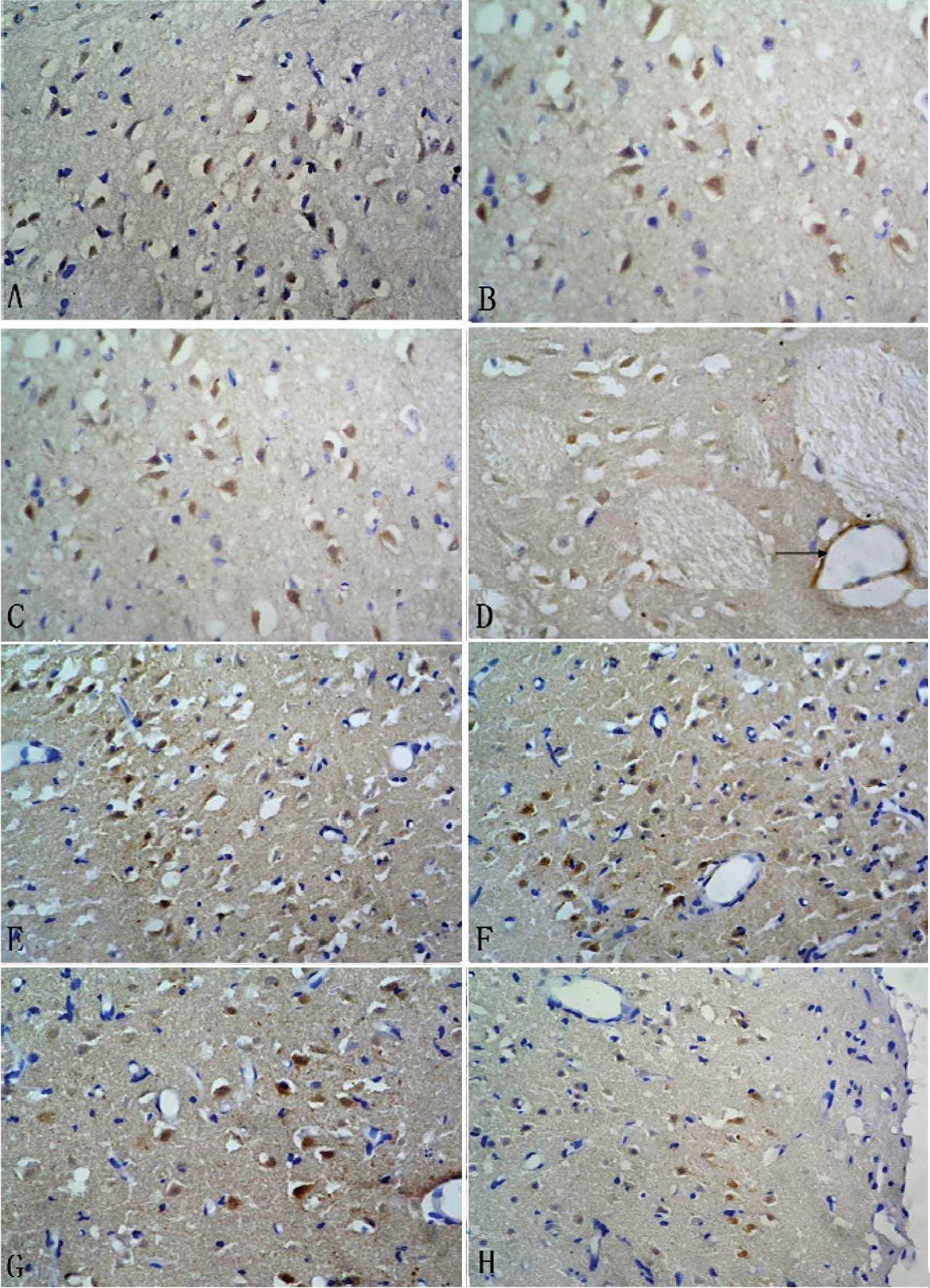
The expression pattern of survivin was similar to
that of NGR1 after CI/RP and, also, it was mainly expressed in the
nucleus and cytoplasm of neurons in the infarct side. In addition,
survivin expression was found in small vascular endothelial cells 3
days after CI/RP. In the infarct center, survivin was expressed
strongly 6 h after CI/RP, and the number of survivin-positive cells
was maximal at 6 h and decreased gradually. In the peri-infarct
area, the number of survivin-positive cells was small at 6 h,
peaked at 3 days and decreased gradually. Compared to the control
group, more survivin-positive cells were detected in the treatment
group at each time point, with significant differences between the
two time points (P<0.05; Table
III and Fig. 2).
 | Table III.Survivin expression in different
infarct areas at different time points after CI/RP in the two
groups (n=40; cells/field). |
Table III.
Survivin expression in different
infarct areas at different time points after CI/RP in the two
groups (n=40; cells/field).
| After CI/RP | Infarct center
| Peri-infarct area
|
|---|
| Treatment group | Control group | t | Treatment group | Control group | t |
|---|
| 6 h | 41.2±3.9 | 38.8±4.7 | 2.49a | 17.4±2.8 | 15.8±3.7 | 2.18a |
| 24 h | 31.1±4.4 | 28.8±3.6 | 2.56a | 26.7±2.6 | 25.2±3.4 | 2.21a |
| 3 days | 21.8±3.7 | 18.6±4.5 | 3.47b | 33.4±3.7 | 30.7±2.5 | 3.82b |
| 5 days | 15.3±1.7 | 13.7±3.5 | 2.60a | 27.3±3.6 | 25.1±4.6 | 2.38a |
| 7 days | 8.9±3.4 | 7.3±2.1 | 2.53a | 20.3±1.6 | 19.2±2.6 | 2.28a |
Discussion
Emerging evidence suggests the potential
neuroprotective role of NGR1 in cerebral ischemia. Increased NGR1
expression has been found in neurons of ischemic penumbra, although
it is not expressed in astrocytes or microglia cells (15). In addition, injection of exogenous
NGR1 into the brain or carotid artery reduces significantly the
volume of the infarction and the number of TUNEL-positive and
caspase-3-positive cells (1–6).
Survivin is the strongest apoptosis inhibitory protein known, and
it directly or indirectly inhibits the caspase-dependent or
-independent apoptosis pathways (16).
Few reports have addressed the expression patterns
of NGR1 and survivin after cerebral ischemia with exogenous
stimulation (including administration of drugs). In the present
study, the expression patterns of NGR1 and survivin in brain
tissues after CI/RP alone, or in combination with aspirin
administration, were examined by immunohistochemistry. The results
demonstrated that NGR1 and survivin were mainly expressed in the
nucleus and cytoplasm of neurons in the infarct hemisphere after
CI/RP, transiting from the infarct center to the peri-infarct area.
In the infarct center, NGR1 and survivin were expressed most
significantly 6 h after CI/RP, and reached a peak after 3 days in
the peri-infarct area. With aspirin treatment, the number of
survivin- and NGR1-positive cells increased, but the time of peak
expression did not change. In the early stage of CI/RP (within 6 h
post-CI/RP), the infarct center was severely ischemic without
complete necrosis in most of the neurons, therefore NGR1 and
survivin expression remained high. However, the peri-infarct area
was only mildly ischemic, and so there were only a few NGR1- and
survivinpositive cells. As time passed (after 3 days), massive
necrosis of neurons was found in the infarct center, and the
expression of NGR1 and survivin subsided gradually. However, edema
and ischemia in the peri-infarct area became aggravated, and the
number of NGR1- and survivin-positive cells increased. At 7 days,
NGR1- and survivin-positive cells were fewer, which may be related
to the increased necrosis in the peri-infarct area and the
restoration of cerebral blood supply. Taken together, our data
suggest that ischemia is a strong stimulus to induce the expression
of endogenous NGR1 and survivin. NGR1 and survivin expression may
be further upregulated by exogenous aspirin. NGR1 and survivin may
play neuroprotective roles through the inhibition of apoptosis,
providing a mechanism by which aspirin exhibits its neuroprotective
effects.
Conway et al (7) reported that survivin was also
expressed in cerebral pia mater and small blood vessels. Our
experiment also confirmed that survivin is expressed in a portion
of the vascular endothelial cell infract center and peri-infarct
area, but NGR1 was not expressed in these areas. These data
indicate that survivin promotes angiogenesis (7). Indeed, survivin is upregulated by
VEGF and plays an anti-apoptosis role in the process of
angiogenesis (17). Plate et
al (18) reported that after
middle cerebral artery occlusion, the mRNA expression of vascular
endothelial growth factor (VEGF) was detected in gliocytes and
macrophages in the perivascular ischemic penumbra and reached a
peak after 2 days (18), which
appropriately coincides with post-ischemic angiogenesis in the
infarct center (19). Although
VEGF may facilitate the leakage of capillary blood and destroy the
blood-brain barrier to aggravate brain edema, its effect on
angiogenesis may be beneficial to long-term recuperation (20). Therefore, we propose that the
concomitant upregulation of VEGF and survivin may help maintain a
balance and promote maximal neuroprotective effects against
cerebral ischemia/reperfusion.
References
|
1.
|
Guo WP, Wang J, Li RX and Peng YW:
Neuroprotective effects of neuregulin-1 in rat models of focal
cerebral ischemia. Brain Res. 1087:180–185. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Li Q, Li Z, Mei Y and Guo Y: Neuregulin
attenuated cerebral ischemia-reperfusion injury via inhibiting
apoptosis and upregulating aquaporin-4. Neurosci Lett. 443:155–159.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Li Q, Zhang R, Guo YL and Mei YW: Effect
of neuregulin on apoptosis and expressions of STAT3 and GFAP in
rats following cerebral ischemic reperfusion. J Mol Neurosci.
37:67–73. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Shyu WC, Lin SZ, Chiang MF, Yang HI,
Thajeb P and Li H: Neuregulin-1 reduces ischemia-induced brain
damage in rats. Neurobiol Aging. 25:935–944. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Xu Z, Croslan DR, Harris AE, Ford GD and
Ford BD: Extended therapeutic window and functional recovery after
intraarterial administration of neuregulin-1 after focal ischemic
stroke. J Cereb Blood Flow Metab. 26:527–535. 2006. View Article : Google Scholar
|
|
6.
|
Xu Z, Jiang J, Ford G and Ford BD:
Neuregulin-1 is neuroprotective and attenuates inflammatory
responses induced by ischemic stroke. Biochem Biophys Res Commun.
322:440–446. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Conway EM, Zwerts F, Van Eygen V, et al:
Survivin-dependent angiogenesis in ischemic brain: molecular
mechanisms of hypoxia-induced up-regulation. Am J Pathol.
163:935–946. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Legos JJ, Mangoni AA, Read SJ, et al:
Programmable microchip monitoring of post-stroke pyrexia: effects
of aspirin and paracetamol on temperature and infarct size in the
rat. J Neurosci Methods. 113:159–166. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Zheng Z, Schwab S, Grau A and Berger C:
Neuroprotection by early and delayed treatment of acute stroke with
high dose aspirin. Brain Res. 1186:275–280. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Arumugam TV, Phillips TM, Cheng A, Morrell
CH, Mattson MP and Wan R: Age and energy intake interact to modify
cell stress pathways and stroke outcome. Ann Neurol. 67:41–52.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Fan Y, Shen F, Frenzel T, et al:
Endothelial progenitor cell transplantation improves long-term
stroke outcome in mice. Ann Neurol. 67:488–497. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Bederson JB, Pitts LH, Tsuji M, Nishimura
MC, Davis RL and Bartkowski H: Rat middle cerebral artery
occlusion: evaluation of the model and development of a neurologic
examination. Stroke. 17:472–476. 1986. View Article : Google Scholar
|
|
13.
|
Leinonen V, Koivisto AM, Savolainen S, et
al: Amyloid and tau proteins in cortical brain biopsy and
Alzheimer's disease. Ann Neurol. 68:446–453. 2010.
|
|
14.
|
Perier C, Bove J, Dehay B, et al:
Apoptosis-inducing factor deficiency sensitizes dopaminergic
neurons to parkinsonian neurotoxins. Ann Neurol. 68:184–192.
2010.PubMed/NCBI
|
|
15.
|
Parker MW, Chen Y, Hallenbeck JM and Ford
BD: Neuregulin expression after focal stroke in the rat. Neurosci
Lett. 334:169–172. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Shin S, Sung BJ, Cho YS, et al: An
anti-apoptotic protein human survivin is a direct inhibitor of
caspase-3 and -7. Biochemistry. 40:1117–1123. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
O'Connor DS, Schechner JS, Adida C, et al:
Control of apoptosis during angiogenesis by survivin expression in
endothelial cells. Am J Pathol. 156:393–398. 2000.
|
|
18.
|
Plate KH, Beck H, Danner S, Allegrini PR
and Wiessner C: Cell type specific upregulation of vascular
endothelial growth factor in an MCA-occlusion model of cerebral
infarct. J Neuropathol Exp Neurol. 58:654–666. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Marti HJ, Bernaudin M, Bellail A, et al:
Hypoxia-induced vascular endothelial growth factor expression
precedes neovascularization after cerebral ischemia. Am J Pathol.
156:965–976. 2000. View Article : Google Scholar
|
|
20.
|
Zhang ZG, Zhang L, Jiang Q, et al: VEGF
enhances angiogenesis and promotes blood-brain barrier leakage in
the ischemic brain. J Clin Invest. 106:829–838. 2000. View Article : Google Scholar : PubMed/NCBI
|