Introduction
Colorectal cancer (CRC) is one of the most common
malignant neoplasms worldwide. Despite surgical treatment and
chemotherapy, the prognosis of CRC remains poor, particularly for
patients with metastasis. Although it is believed that alterations
of oncogene K-ras, and tumor-suppressor genes, such as P53, APC and
DCC, are involved in the pathogenesis, progression and metastasis
of CRC, the molecular mechanisms remain unknown.
Osteopontin (OPN) is a secreted phosphorylated
glycoprotein which is constitutively expressed in osteoblasts,
osteoclasts, smooth muscle cells, epithelia of the kidney, lung,
stomach and breast, and plays a key role in calcium salt deposit
(1,2). It is also involved in inflammation
and the immunoreaction process for expression in activated T
lymphocytes and macrophages (3).
Recently, cumulative evidence suggests that OPN is overexpressed in
several types of carcinomas, such as breast, stomach, lung and
liver, and thus is associated with tumor invasion, progression and
metastasis (4–8). The mechanisms involved in the
up-regulation of OPN in cancers remain unclear, and it is presumed
that activation of OPN is controlled by complex regulation pathways
due to diverse regulatory sequences in the promoter regions.
As far as CRC is concerned, OPN was identified as a
leading marker among the screening of 12,000 genes, and has been
correlated with tumorigenesis, invasion and metastasis (8–11).
However, to our knowledge immunohistochemistry has only been
performed in a few studies, and whether or not OPN plays a role in
increasing tumor proliferation has not yet been determined.
Furthermore, the tumor-suppressor gene P53, which was suggested to
play a role in OPN activation by molecular studies, has not been
validated in clinical tissues to date. Thus, in the present study,
we examined the expression of OPN protein by immunohistochemistry
in CRC and determined its correlation with the proliferation index,
clinicopathological characteristics and P53.
Materials and methods
Tissue specimens
Tissue samples were obtained by surgical resection
from 77 patients (36 males and 41 females; age 27–87 years, mean
60) with CRC in our department between 2006 and 2009; none of whom
had received irradiation or chemotherapy prior to surgery. The
study was approved by the local ethics committees. The cases were
histologically confirmed by two experienced pathologists,
respectively. Normal colorectal tissues for each case were obtained
∼5 cm away from the tumors, which were also confirmed by
histology.
Antibodies
Rabbit polyclonal antibody against human OPN and
mouse monoclonal antibodies against Ki-67 and P53 (clone nos. MIB-1
and DO-7, respectively) were purchased from Maxin-Bio Corp.
(Fuzhou, China).
Immunohistochemistry
Immunohistochemistry was performed on
formalin-fixed, paraffin-embedded tissue sections using the
Envision peroxidase detection method. Sections (4-μm) were
deparaffinized in xylene, dehydrated through graded ethanols and
subsequently treated with 0.3% hydrogen peroxide in methanol for 30
min at room temperature to eliminate the endogenous peroxidase
activity. For antigen retrieval, the sections were microwaved in 10
mM citrate buffer (pH 6.0) for 10 min. The primary antibodies were
incubated overnight at 4°C after blocking non-specific binding with
10% normal goat serum in PBS for 30 min. For negative controls, PBS
was used as substitutes for the primary antibodies. Sections were
incubated with Envision peroxidase complex for 20 min. Sections
were counterstained with Mayer’s hematoxylin after immunostaining
prior to mounting.
Evaluation of OPN, Ki-67 and P53
immunostaining
All sections were evaluated without knowledge of the
clinical and pathological background of the patients. Unambiguous
yellow staining of the cytoplasm was regarded as OPN-positive, and
nuclear staining as Ki-67- and P53-positive. The expression of OPN
and P53 was determined in each case when positive cells were
>10%, since the positive pattern was often diffuse and similar
in staining intensity. For Ki-67, the index was classified into
three groups according to the positive cell ratio; the low index
group was indicated when positive cells were <50%, high index
when positive cells were >75% and intermediate index was
indicated between the high and low range.
Statistical analysis
Statistical analysis was performed using SPSS
software with the χ2 test. All statistical significance
tests were two-tailed and significance was established at
P<0.05.
Results
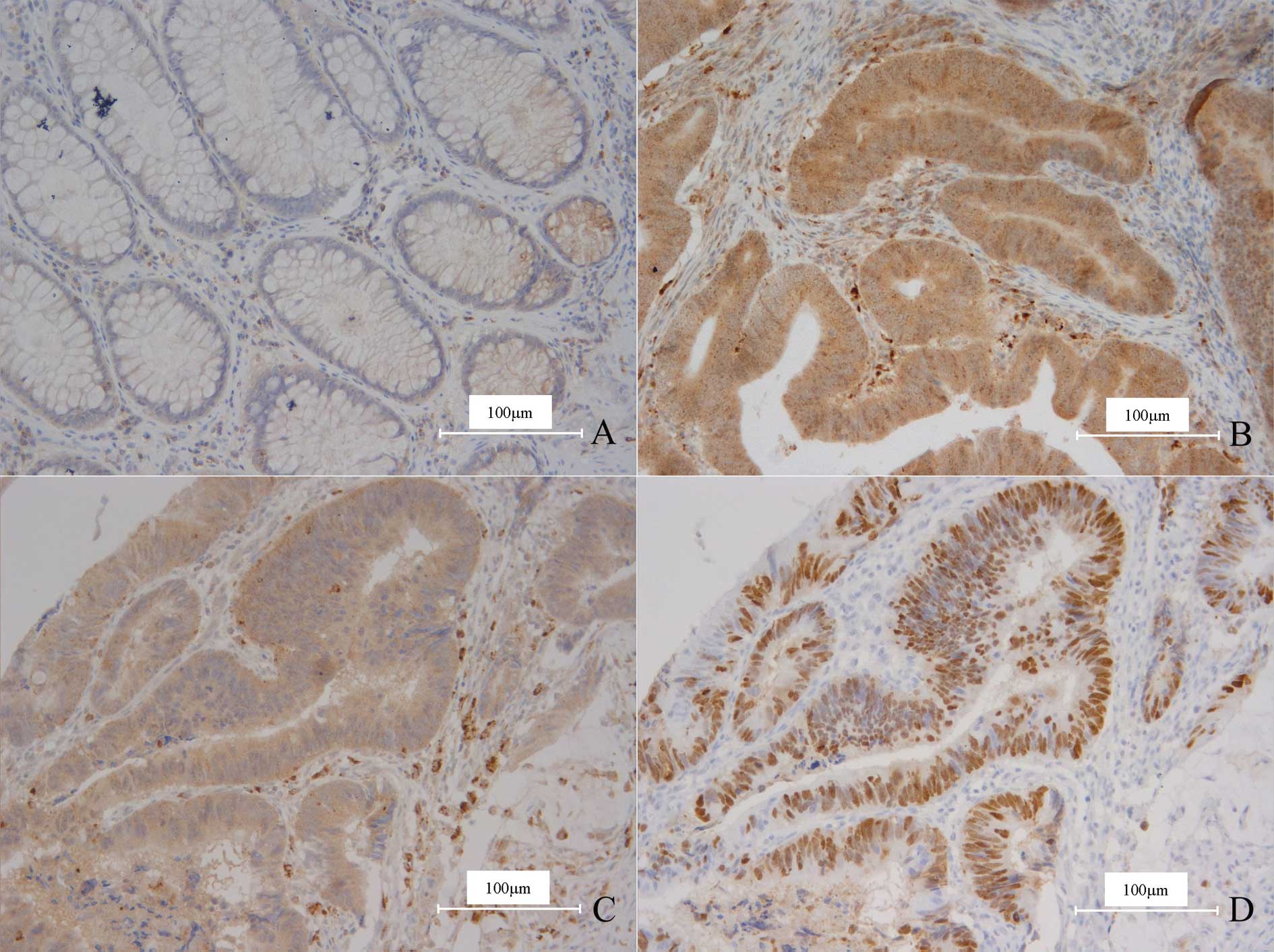
In either normal or carcinoma tissue, OPN was
expressed in smooth muscle cells and macrophages, which were thus
used as the inner positive control. In normal colorectal gland
epithelia, OPN only displayed weak staining in a few cells and the
ratios were much <10%, therefore all the cases were determined
as negative (Fig. 1A).
Thirty-eight cases (49.4%) of CRC demonstrated OPN
overexpression (Fig. 1B), and
compared to the normal tissues, the difference was significant
(χ2=50.448, P=0.000). The staining pattern of OPN was
quite diffuse and homogeneous; the intensity of the positive
carcinoma cells was almost identical wherever the cells were
located. In detail, the cells on the centers or borders of the
carcinoma nests, even in the invading fronts, displayed no
difference from the other cells.
Overexpression of OPN was not significantly
correlated with gender, histological differentiation, invasion
depth and TNM stages. However, it was associated with lymph node
metastasis (χ2=7.355, P=0.025), which indicated that OPN
expression was higher in carcinomas with metastases. It was also
correlated with Dukes’ stages, thus OPN expression increased with
advanced clinical stage (χ2=7.789, P=0.031). As far as
the proliferation or MIB-1 index of the carcinomas was concerned,
expression of OPN remained unchanged. The relationship between the
expression of OPN and clinicopathological parameters is summarized
in Table I.
 | Table I.Immunohistochemical expression of OPN
and its relationship with clinicopathological parameters of the CRC
patients. |
Table I.
Immunohistochemical expression of OPN
and its relationship with clinicopathological parameters of the CRC
patients.
| OPN expression, n (%)
| P-value |
|---|
| Positive | Negative |
|---|
| Gender | | | 0.573 |
| Male | 19 (24.7) | 17 (22.1) | |
| Female | 19 (24.7) | 22 (28.5) | |
| Degree of
differentiation | | | 0.982 |
| Well | 9 (11.7) | 11 (14.3) | |
| Moderate | 15 (19.5) | 22 (28.6) | |
| Poor | 14 (18.2) | 6 (7.7) | |
| Depth of
invasion | | | 0.854 |
| T1 | 1 (1.3) | 2 (2.5) | |
| T2 | 9 (11.7) | 9 (11.7) | |
| T3 | 28 (36.4) | 28 (36.4) | |
| Metastasis | | | 0.025 |
| N0 | 16 (20.8) | 28 (36.4) | |
| N1 | 15 (19.5) | 6 (7.8) | |
| N2 | 7 (9.1) | 5 (6.4) | |
| TNM stage | | | 0.111 |
| I | 6 (7.8) | 9 (11.7) | |
| II | 10 (13.0) | 19 (24.7) | |
| IIIA | 2 (2.6) | 1 (1.3) | |
| IIIB | 13 (16.9) | 5 (6.5) | |
| IIIC | 7 (9.1) | 5 (6.5) | |
| Dukes’ stage | | | 0.031 |
| A | 6 (7.8) | 9 (11.7) | |
| B | 10 (13.0) | 19 (24.7) | |
| C | 22 (28.6) | 11 (14.2) | |
| Ki-67 index | | | 0.770 |
| Low | 4 (5.2) | 6 (7.8) | |
| Moderate | 15 (19.5) | 16 (20.7) | |
| High | 19 (24.7) | 17 (22.1) | |
The expression of P53 was noted in 54 cases (70.1%),
and a statistical correlation between the expression of OPN and P53
was found (χ2=4.695, P=0.030; Fig. 1C and D; Table II).
 | Table II.Correlation between OPN and P53
immunostaining. |
Table II.
Correlation between OPN and P53
immunostaining.
| OPN expression | P53
|
|---|
| Positive | Negative |
|---|
| Positive | 31 | 23 |
| Negative | 7 | 16 |
Discussion
OPN was initially proven to play a key role in
calcium salt deposit, inflammation and immunoreaction process, yet
further evidence suggests that OPN contributes to the
tumorigenesis, progression and metastasis of many types of
malignant neoplasms, such as breast, stomach, lung, prostate and
liver carcinoma.
Numerous studies have investigated the association
between OPN and CRC. Most strikingly, the screening of pooled
sample expression profiles among 12,000 human genes identified OPN
as a leading marker of colon cancer progression (9). In subsequent studies, overexpression
of OPN was confirmed in CRC by northern blotting, PCR or
immunohistochemistry. To date, an association between the
up-regulation of OPN and tumor progression has been demonstrated,
and it was determined as an independent prognostic factor in
multivariate regression analysis. Notably, the role of OPN in
metastasis has been intensively researched since its expression was
not only reserved, but was also increased in metastases (9,12,13).
In the present study, we further confirmed that OPN
is overexpressed in CRC and is associated with tumor stage and
lymph node metastasis by immunostaining, thereby relating it to
tumor progression and metastasis. The expression of OPN was
associated with Dukes’ stages, but not with TNM stages in our
results. The small number of samples in some stages, such as I and
IIIa, was rebuked resulting in some degree of statistical bias.
In previous studies, OPN was verified to be involved
in tumorigenesis, thus it seemed rational that OPN may promote
tumor proliferation. Ki-67 (MIB-1) recognizes all cells throughout
the cell cycle, except for G0 stage, and well reflects
cell proliferation. Ki-67 has been widely used in neoplasms to
estimate malignancy and prognosis. However, in our study the
possibility that OPN regulates CRC cell proliferation was not
confirmed. Therefore, it is speculated that other mechanisms exist
to explain the involvement of OPN in tumorigenesis, such as
facilitation of cell transformation or prevention of apoptosis.
It is believed that the involvement of OPN in
malignancy is due to its biological functions and several
activating downstream signal pathways. OPN mainly functions on
cell-matrix interactions through binding and activating relevant
ligands with certain surface structure domains. It has been widely
accepted that overexpressed OPN in tumors ligates with and
activates αvβ integrins or CD44 (14–17).
Data already indicate that αvβ integrins and CD44 may
contribute to carcinogenesis, progression or metastasis through
promoting cell transformation, migration, adhesion to extracellular
matrix, neovascularization and immune suppression. Thus, OPN
functions between tumor cells and mesenchyme mainly through such
exterior molecules. On the other hand, binding of OPN may also
activate several inner downstream signaling pathways, such as
phosphatidylinositol 3-kinase/protein kinase B, nuclear factor-κB
and urokinase plasminogen, which were also proven to be involved in
tumors (18–20).
Although the mechanisms regulating the expression of
OPN remain unknown, a variety of cisacting elements in the promoter
region, including TATA-box and CCAAT-box, suggests, that it is
controlled by complex regulatory pathways (21). The Wnt pathway is proven to be
activated in CRC, and immunostaining analysis also indicates that
increased OPN expression is significantly correlated with elevated
nuclear and cytoplasmic β-catenin staining, which is a central
component of the Wnt signaling pathway. It has been concluded that
over-expression of OPN is regulated by the Wnt pathway, at least in
part. However, OPN was found to be expressed more frequently than
β-catenin in CRC, so it was presumed that other pathways are
involved in the regulation of OPN (10). Tumor-suppressor gene P53, which
plays a critical role in cell-cycle regulation, apoptosis and
immunosurveillance, has a role in many types of tumors, including
CRC. Morimoto et al (22)
found that induction of OPN expression by P53 is conserved across
multiple species, and the endogenous OPN gene was induced in mouse
and rat embryo fibroblasts in a P53-dependent manner. OPN was also
induced in the A172 human glioblastoma cell line in P53-associated
activities. In addition, a potential P53 responsive element, ctGCT
TGCTT AGGCgAGCTC, located approximately 1 kb upstream of the first
exon sequence, which contains only three-mismatch nucleotides in
the non-critical position compared to the consensus P53-binding
sequence, was found in the OPN gene promoter and confirmed by
chromatin immunoprecipitation assay in the HCT116 human CRC cell
line (21). These results suggest
that the OPN gene is a direct target of transcription activation by
P53. In our immunohistochemical study, we demonstrated that
expression of P53 was significantly correlated with OPN, which is
an indirect proof of P53-regulated OPN expression.
Recent studies suggest that OPN and related
molecules are a potential target for anticancer therapy. In
addition, the plasma concentration of OPN in patients with tumor
metastasis is significantly increased in comparison to normal sera,
thus its prospects as a marker for evaluating tumor genesis and
metastasis are expected (22–24).
Accordingly, further research on OPN is warranted.
In summary, the present study provides
immunohistochemical evidence that OPN is overexpressed in CRC and
is related to tumor progression and lymph node metastasis
supposedly regulated by P53.
References
|
1.
|
Denhardt DT and Noda M: Osteopontin
expression and function: role in bone remodeling. J Cell Biochem
Suppl. 30–31:92–102. 1998.PubMed/NCBI
|
|
2.
|
Denhardt DT and Guo X: Osteopontin: a
protein with diverse functions. FASEB J. 7:1475–1482.
1993.PubMed/NCBI
|
|
3.
|
Lund SA, Giachelli CM and Scatena M: The
role of osteopontin in inflammatory processes. J Cell Commun
Signal. 3:311–322. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Patani N, Jouhra F, Jiang W and Mokbel K:
Osteopontin expression profiles predict pathological and clinical
outcome in breast cancer. Anticancer Res. 28:4105–4110.
2008.PubMed/NCBI
|
|
5.
|
Higashiyama M, Ito T, Tanaka E and Shimada
Y: Prognostic significance of osteopontin expression in human
gastric carcinoma. Ann Surg Oncol. 14:3419–3127. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Weber GF, Lett GS and Haubein NC:
Osteopontin is a marker for cancer aggressiveness and patient
survival. Br J Cancer. 103:861–869. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Korita PV, Wakai T, Shirai Y, Matsuda Y,
Sakata J, Cui X, Ajioka Y and Hatakeyama K: Overexpression of
osteopontin independently correlates with vascular invasion and
poor prognosis in patients with hepatocellular carcinoma. Hum
Pathol. 39:1777–1783. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Coppola D, Szabo M, Boulware D, Muraca P,
Alsarraj M, Chambers AF and Yeatman TJ: Correlation of osteopontin
protein expression and pathological stage across a wide variety of
tumor histologies. Clin Cancer Res. 10:184–190. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Agrawal D, Chen T, Irby R, Quackenbush J,
Chambers AF, Szabo M, Cantor A, Coppola D and Yeatman TJ:
Osteopontin identified as lead marker of colon cancer progression,
using pooled sample expression profiling. J Natl Cancer Inst.
94:513–521. 2002. View Article : Google Scholar
|
|
10.
|
Rohde F, Rimkus C, Friederichs J,
Rosenberg R, Marthen C, Doll D, Holzmann B, Siewert JR and Janssen
KP: Expression of osteopontin, a target gene of de-regulated Wnt
signaling, predicts survival in colon cancer. Int J Cancer.
21:1717–1723. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Allan AL, George R, Vantyghem SA, Lee MW,
Hodgson NC, Engel CJ, Holliday RL, Girvan DP, Scott LA, Postenka
CO, et al: Role of the integrin-binding protein osteopontin in
lymphatic metastasis of breast cancer. Am J Pathol. 169:233–246.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Carlinfante G, Vassiliou D, Svensson O,
Wendel M, Heinegård D and Andersson G: Differential expression of
osteopontin and bone sialoprotein in bone metastasis of breast and
prostate carcinoma. Clin Exp Metastasis. 20:437–444. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Irby RB, McCarthy SM and Yeatman TJ:
Osteopontin regulates multiple functions contributing to human
colon cancer development and progression. Clin Exp Metastasis.
21:515–523. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Wai PY and Kuo PC: Osteopontin: regulation
in tumor metastasis. Cancer Metastasis Rev. 27:103–118. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Leali D, Moroni E, Bussolino F and Presta
M: Osteopontin overexpression inhibits in vitro
re-endothelialization via integrin engagement. J Biol Chem.
282:19676–19684. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Anborgh PH, Mutrie JC, Tuck AB and
Chambers AF: Role of the metastasis-promoting protein osteopontin
in the tumour micro-environment. J Cell Mol Med. 14:2037–2044.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Hsu KH, Tsai HW, Lin PW, Hsu YS, Shan YS
and Lu PJ: Osteopontin expression is an independent adverse
prognostic factor in resectable gastrointestinal stromal tumor and
its interaction with CD44 promotes tumor proliferation. Ann Surg
Oncol. 17:3043–3052. 2010. View Article : Google Scholar
|
|
18.
|
Dai J, Peng L, Fan K, Wang H, Wei R, Ji G,
Cai J, Lu B, Li B, Zhang D, Kang Y, Tan M, Qian W and Guo Y:
Osteopontin induces angiogenesis through activation of PI3K/AKT and
ERK1/2 in endothelial cells. Oncogene. 28:3412–3422. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Fong YC, Liu SC, Huang CY, Li TM, Hsu SF,
Kao ST, Tsai FJ, Chen WC, Chen CY and Tang CH: Osteopontin
increases lung cancer cells migration via activation of the
alphavbeta3 integrin/FAK/Akt and NF-kappaB-dependent pathway. Lung
Cancer. 64:263–270. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Tuck AB, Hota C and Chambers AF:
Osteopontin (OPN)-induced increase in human mammary epithelial cell
invasiveness is urokinase (uPA)-dependent. Breast Cancer Res Treat.
70:197–204. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Hijiya N, Setoguchi M, Matsuura K, Higuchi
Y, Akizuki S and Yamamoto S: Cloning and characterization of the
human osteopontin gene and its promoter. Biochem J. 303:255–262.
1994.PubMed/NCBI
|
|
22.
|
Morimoto I, Sasaki Y, Ishida S, Imai K and
Tokino T: Identification of the osteopontin gene as a direct target
of TP53. Genes Chromosomes Cancer. 33:270–278. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Cristaudo A, Foddis R, Bonotti A, Simonini
S, Vivaldi A, Guglielmi G, Ambrosino N, Canessa PA, Chella A,
Lucchi M, Mussi A and Mutti L: Comparison between plasma and serum
osteopontin levels: usefulness in diagnosis of epithelial malignant
pleural mesothelioma. Int J Biol Markers. 25:164–170. 2010.
|
|
24.
|
Sreekanthreddy P, Srinivasan H, Kumar DM,
Nijaguna MB, Sridevi S, Vrinda M, Arivazhagan A, Balasubramaniam A,
Hegde AS, Chandramouli BA, et al: Identification of potential serum
biomarkers of glioblastoma: serum osteopontin levels correlate with
poor prognosis. Cancer Epidemiol Biomarkers Prev. 19:1409–1422.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Isa S, Kawaguchi T, Teramukai S, Minato K,
Ohsaki Y, Shibata K, Yonei T, Hayashibara K, Fukushima M, Kawahara
M, Furuse K and Mack PC: Serum osteopontin levels are highly
prognostic for survival in advanced non-small cell lung cancer:
results from JMTO LC 0004. J Thorac Oncol. 4:1104–1110. 2009.
View Article : Google Scholar : PubMed/NCBI
|