Introduction
Escin is the main active constituent of Aesculus
hippocastanum seed extracts, which is a triterpene saponin
mixture consisting of A, B, C and D escin. Accumulating
experimental evidence in previous studies suggests that escin
exerts potent anti-inflammatory and anti-edematous effects. Escin
inhibits acetic acid-induced increase in capillary permeability and
adhesion formation in animal models (1). Escin also attenuates hippocampal
injury after global cerebral ischemia in mice via regulating
certain inflammatory genes (2). A
recent study showed that escin has a potent protective effect on
LPS-induced acute lung injury by inhibiting the inflammatory
response (3).
According to Matsuda et al (4), the anti-inflammatory effects of escin
are mainly dependent on its anti-histaminic and anti-serotoninergic
activities. Another study (5)
reported that escin dose-dependently prevented the hypoxia-induced
activation of human endothelial cells, as evidenced by the
inhibition of hypoxia-increased phospholipase A2, an enzyme
responsible for the release of precursors of inflammatory
mediators. In addition, escin significantly inhibited nuclear
factor (NF)-κB activation and down-regulated the expression of
tumor necrosis factor (TNF)-α, alleviating brain edema in traumatic
brain injured rats (6). The
experiments listed above characterized that escin has potent
anti-inflammatory effects and its anti-inflammatory mechanisms are
similar to glucocorticoids (GCs). Furthermore, we recently found
that escin exerts synergistic anti-inflammatory effects with
glucocorticoids (7), and its
mechanism involves the up-regulation of the glucocorticoid receptor
(GR) (3).
In China, escin has been widely used clinically in
preventing inflammatory edema after trauma, such as fracture and
surgery (8–10). However, it is unclear whether escin
affects fracture healing, and whether escin has an inhibitory
effect on wound healing. In the present study, we investigated the
direct effects of escin on tibia fracture healing and abdominal
wound healing in rabbit and rat models, respectively.
Materials and methods
Chemicals and instruments
Sodium salt of escin (i.e., sodium escinate,
consisting of A, B, C and D and containing at least 65% A and B)
(batch no. 080902) was supplied by Shandong Luye Pharmaceutical
Co., Ltd. (Yantai, China). Osteocalcin was supplied by R&D
Systems (batch no. CK-E90207R). Alkaline phosphatase (ALP),
hydroxyproline, calcium and phosphate test kits were purchased from
the Institute of Nanjing Jiancheng Bioengineering (Nanjing, China).
All other chemicals and reagents used in this study were of
analytical grade.
Animals
New Zealand white rabbits weighing 3±0.5 kg were
provided by the Limited Liability Company of Luzhou (Anqiu,
Shandong, China), and the certicate number was G080710. Female
Sprague-Dawley rats weighing 250–300 g were provided by the
Experimental Animal Center of Luye Pharmaceutical Company. All
experimental procedures carried out in this study were performed in
accordance with the Guidelines for the Care and Use of Laboratory
Animals of Yantai University, and were approved by the ethics
committee. The animals were housed in a temperature-controlled room
(22±2°C) on a 12-h light/dark cycle with free access to food and
water.
Surgical procedure and experimental
design
Rabbits were anesthetized by subcutaneous
administration of urethane (1.6 g/kg), and placed in a lateral
position on the operation table. The right tibia was approached
through a 4-cm long skin incision. The fascia was cut, the muscles
separated and the posterior medial surface of the tibia was opened.
The periosteum was incised longitudinally and retracted. With a
stainless steel plate (26×6×1 mm) adhering to the side surface of
the tibia, 4 holes were drilled with a 1.2-mm drill. The periosteum
was protected and an osteotomy was performed at the mid-diaphysis
between the second and third holes. The steel plate and 4 stainless
steel self-taping screws (1.1×10 mm) were used as the inner
fixator, while 4 wooden splints were used as the external fixator.
The wound was irrigated with sterile normal saline and closed in
layers with interrupted sutures. Postoperative radiographs were
used to confirm the quality of the osteotomy. After the operation,
the rabbits were randomly divided into four groups with 6 animals
in each group. From the day of operation, animals in the model
group were injected with saline via the marginal ear vein once per
day for 10 days, while the animals in the other groups were
injected with escin (0.225, 0.45 or 0.9 mg/kg) via the marginal ear
vein, and the escin dosage was determmined according to the
therapeutic dose for inflammatory edema treatment in the
clinic.
Fifty rats were randomly divided into five groups:
control group, model group, escin 1.8 mg/kg group (escin
administered 6 h before abdominal incision, −6 h), escin 1.8 mg/kg
group (escin administered just after abdominal incision, 0 h) and
escin 1.8 mg/kg group (escin administrated 24 h after abdominal
incision, +24 h). The model and escin administration rats were
anesthetized intraperitoneally with chloral hydrate (400 mg/kg),
and placed on the operation table. After the abdominal skin was
shaved with a povidone-iodine scrub, a 4-cm long midline laparotomy
was performed. The abdominal fascia and skin were subsequently
closed by silk sutures. No surgery was performed in the control
group rats. Six days after the operation, animals in each group
were sacrificed. The abdominal incision wounds (2×1 cm portion of
the abdominal wall sample) were excised and stored at −80°C for
measuring the hydroxyproline levels.
Radiographic evaluation
Both anterior-posterior and lateral X-rays of the
right tibia were performed at 2, 4 and 6 weeks to study callus
formation and fracture healing using the VR X-ray shoot apparatus
(Fairfield, CT, USA).
Bone mineral density
With the rabbits under anesthetization induced by
urethane (0.8 g/kg), the right tibia was scanned by a dual-energy
X-ray absorptiometry (DEXA) scanner (Lunar DPX, USA) in a standard
position with the lateral surface of the bone facing the scanner
plate. At 2, 4 and 6 weeks, the bone mineral density (BMD) value of
a 15-mm long and 30-mm wide ‘ROI’ was measured centrally between
the second and the third screws, which included the proximal and
distal old bone and the callus.
Histological analysis
One rabbit selected from each group was sacrificed
at 4 and 8 weeks after operation, respectively. Retrievals
consisting of the 3-mm segmental defect and 7 mm of cortical bone
proximal and distal with the corresponding tibia, adjacent to the
defect, were fixed in 4% paraformaldehyde at 4°C for 24 h. After
fixation, bones were rinsed in phosphate-buffered saline (PBS). The
tissues were then decalcified in 5% HCl buffered formaldehyde at
4°C for 2 weeks, dehydrated in ascending concentrations of ethanol
and embedded in paraffin. During embedding, the positioning of the
tibia was standardized in an attempt to ensure that the same region
was evaluated in all specimens. Sections (∼4-μm) were obtained at
the middle of the specimens on a Polycut microtome (Leica, CM1950,
Germany) and stained with H&E. Pathological observation of the
tissues was performed under light microscopy.
Serum levels of osteocalcin, ALP, calcium
and phosphate
On day 3, and weeks 1, 2 and 4 post-surgery, blood
was obtained via the ear vein and centrifuged at 10,000 × g for 10
min. The plasma was then analyzed for osteocalcin, ALP, calcium and
phosphate concentration using the corresponding kit.
Measuring hydroxyproline
The abdominal wall samples were homogenized with
ice-cold saline for a 10% (w/v) homogenate. The hydroxyproline
contents were determined according to the method of Bergman and
Loxley (11) as mg per 100 mg of
tissue.
Statistical analysis
One-way ANOVA was used to analyze the significant
differences within the different groups; the comparison between two
groups was determined by the Student's unpaired t-test, using SPSS
11.5 statistical software. p<0.05 was accepted as indicative of
a statistical significant difference among the groups. All data in
this study were expressed as the mean values ± SD.
Results
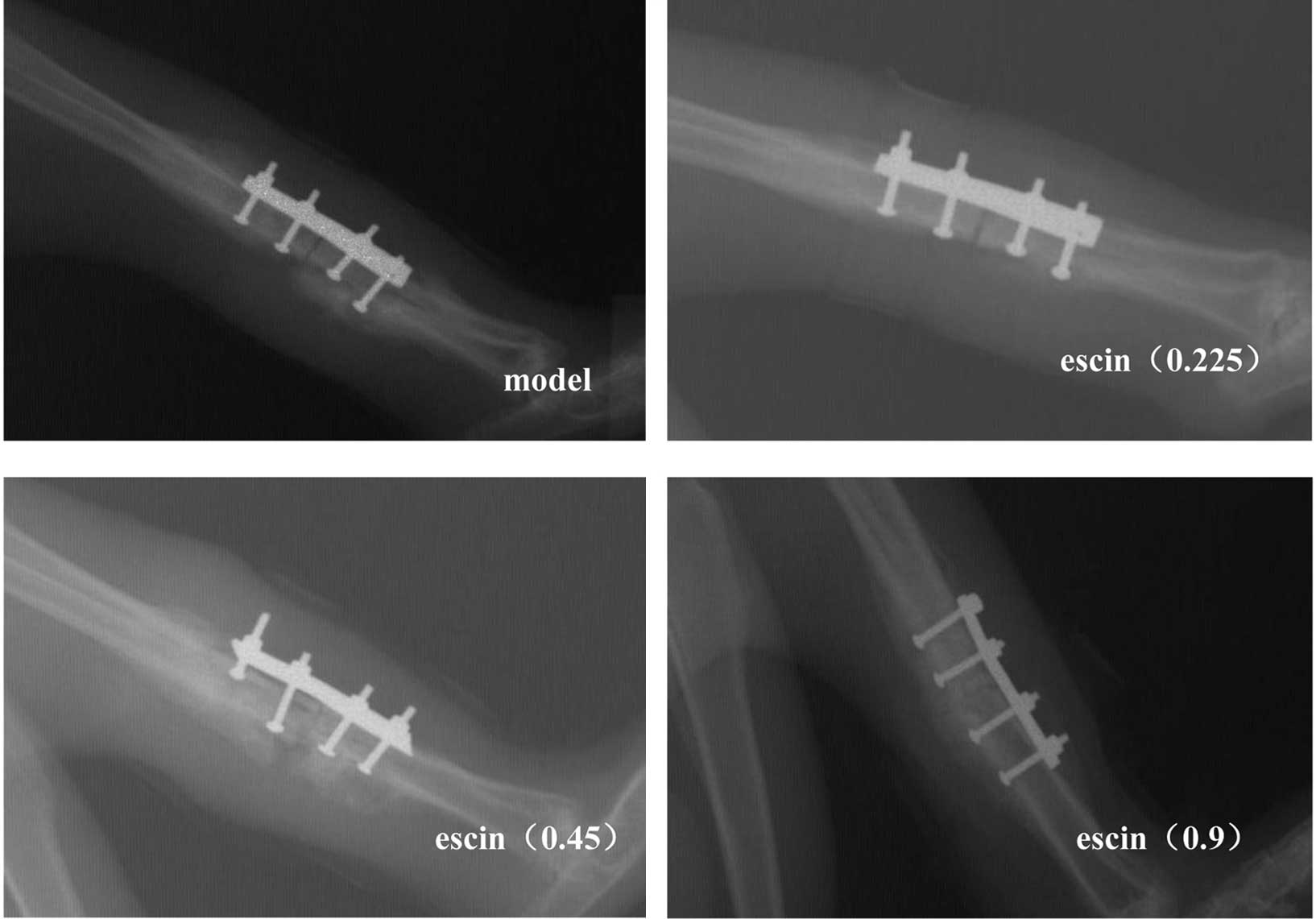
Effects of escin on the healing of
fracture
Two weeks post-operation, the fracture gap remained
visible and the callus was difficult to be recognized in all
groups. By contrast, at 4 weeks the callus size was markedly larger
and the fracture gap became less obvious, while there was no
significant difference among all groups. After 6 weeks, calluses in
all groups were apparent (Fig. 1).
However, no significant difference was found between the
escin-treated groups and the model group.
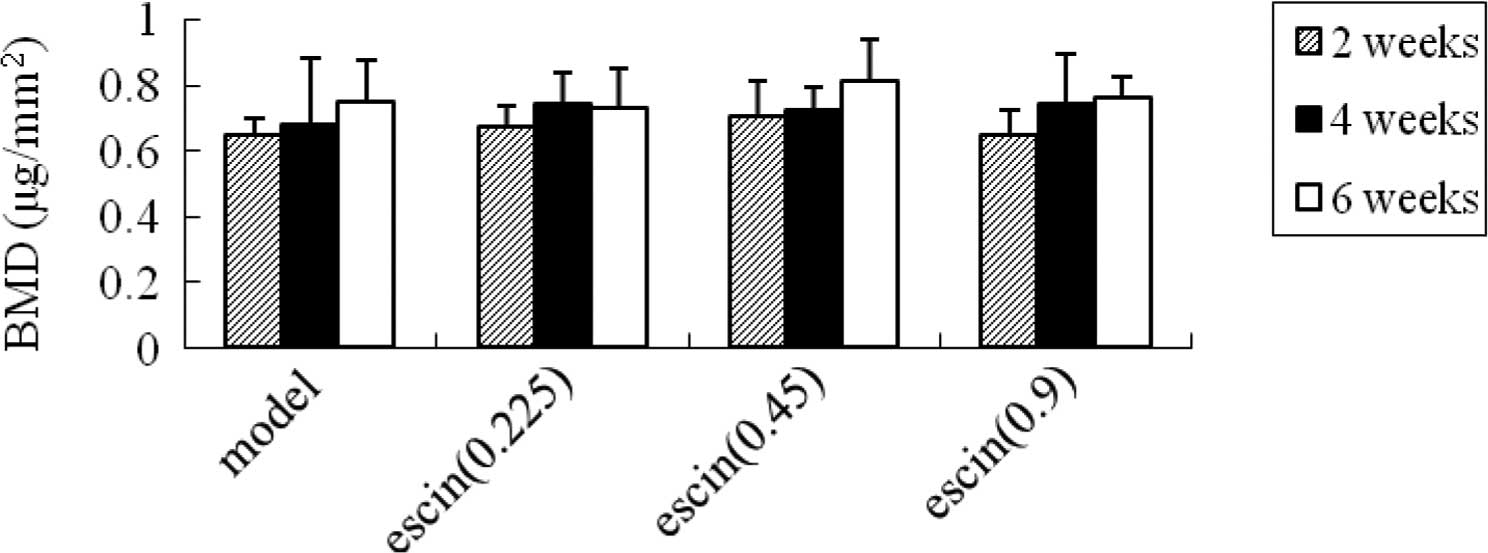
Effects of escin on the bone mineral
density after fracture
At weeks 2, 4 and 6, no significant differences were
found between the model group and the escin-treated groups
(Fig. 2).
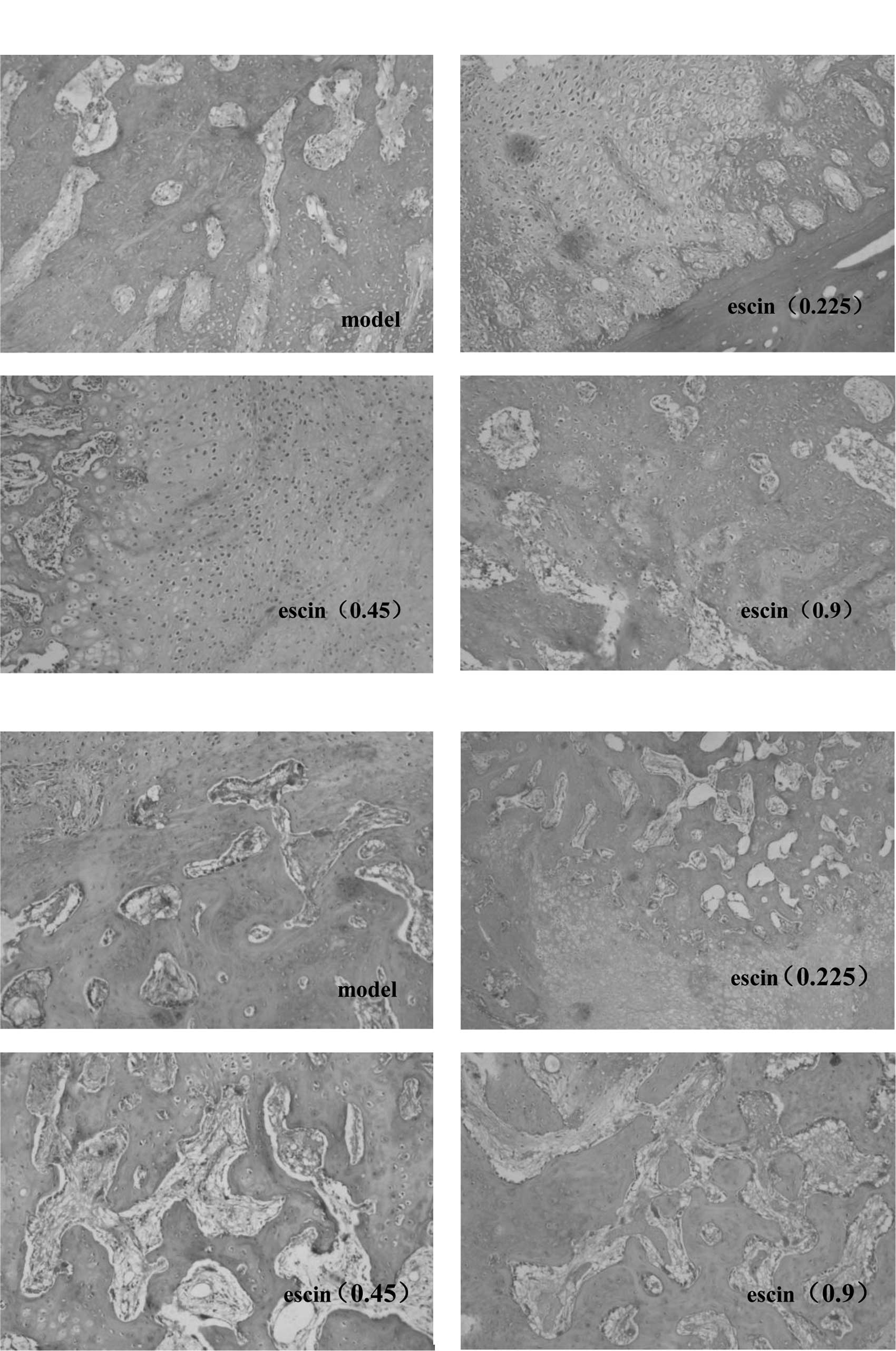
Effects of escin on the histology of bone
fracture
Histological examination at week 4 after the
operation demonstrated a large number of fibroblasts and
chondrocytes both in mature and immature stages, while no
significant difference was observed among all groups (Fig. 3A). Histology at week 8
post-operation showed also no significant difference among groups
with respect to cartilage cells, mature bone cells, bone lamella
and haversian system (Fig.
3B).
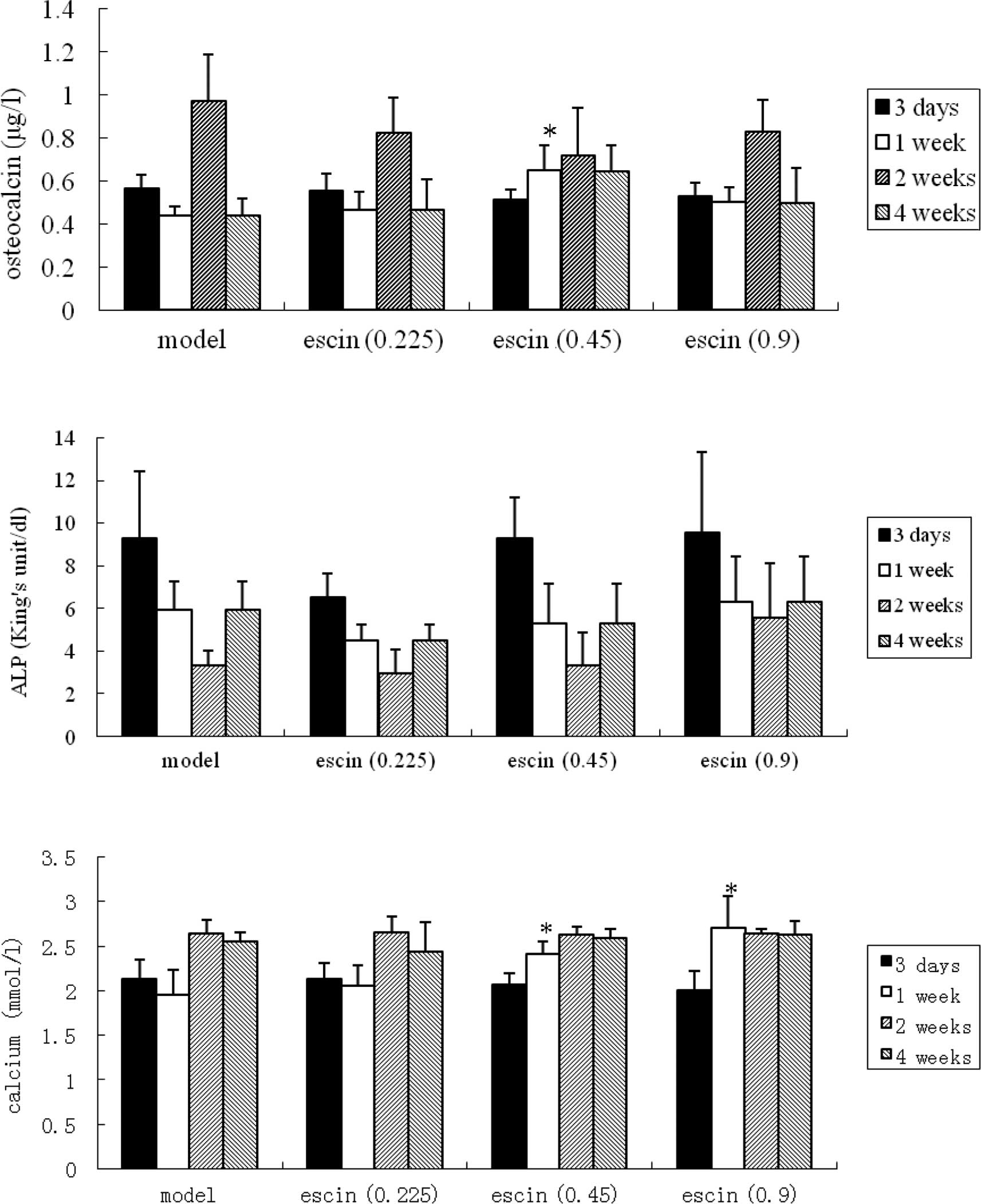
Effects of escin on the serum osteocalcin
and phosphatase alkaline after fracture
One week after the operation, the serum osteocalcin
levels in the escin-treated group (0.45 mg/ kg) was higher than
that in the model group (p<0.05), while no significant
difference was found between the treated groups and the model group
at other time intervals (i.e., day 3 and weeks 2 and 4
post-operation) (Fig. 4A).
At different time intervals (i.e., day 3 and weeks
1, 2 and 4 post-operation), the phosphatase alkaline levels of the
treated groups were comparable to that of the model group, and the
differences were not statistically significant (Fig. 4B).
Effects of escin on serum calcium and
phosphate levels after fracture
One week after the operation, the serum calcium
levels in the escin-treated groups (0.45 and 0.9 mg/kg) were higher
compared to the model group (p<0.05). At other time intervals
(i.e., day 3 and weeks 2 and 4 after the operation) however, no
significant difference was observed between the treated groups and
the model group (Fig. 4C). On the
other hand, at week 2 after the operation, the serum phosphate
levels of the escin-treated group (0.9 mg/kg) were lower than those
of the model group (p<0.05), while no significant difference was
noted between the treated groups and the model group at other time
intervals (Fig. 4D).
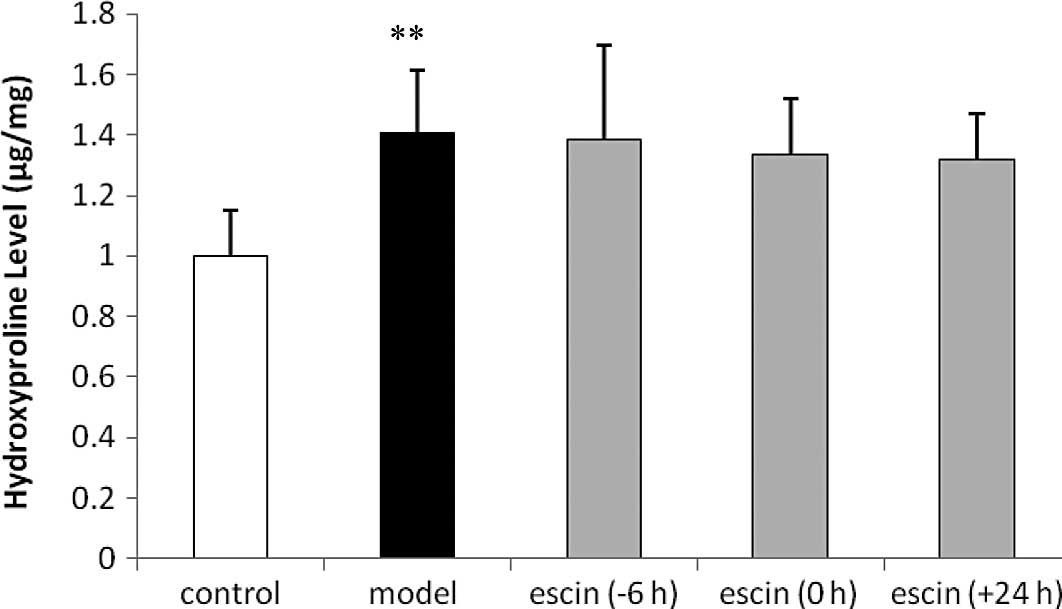
Effects of escin on the hydroxyproline
levels after the operation
The hydroxyproline content of the abdominal wall
samples in the model group was higher compared to that in the
control group (p<0.01). However, there were no significant
differences between the escin-administered and the model groups
(Fig. 5).
Discussion
Glucocorticoids exhibit excellent anti-inflammatory
and anti-edematous effects, and have been widely used clinically to
prevent inflammatory edema after trauma. However, glucocorticoids
exhibit multiple effects to inhibit the immune system, and are also
associated with a risk for osteoporosis and an increased
susceptibility to reduced wound healing (12). Accumulating experimental evidence
in previous studies suggests that escin exerts potent
anti-inflammatory and anti-edematous effects, such as inflammatory
edema after fracture. However, it is unclear whether escin affects
fracture healing and whether escin has an inhibitory effect on
wound healing. In the present study, we investigated the direct
effects of escin on tibia fracture healing and abdominal wound
healing in rabbit and rat models, respectively, and the dosage of
escin was determined according to the therapeutic dose for
inflammatory edema treatment in the clinic.
In order to study the fracture healing process, many
methods including the radiological and histological findings, DEX
absorptiometry (13–16) and serum determination of
osteocalcin, phosphatase alkaline, calcium and phosphate (17,18)
have been used.
Radiographic follow-up provides a visual evaluation
and monitoring of regenerative tissue formation. The callus size
and fracture gap reflect the extent of bone healing (13,15,19).
In our study, no significant difference was observed between the
escin-treated groups and the model group. This indicates that escin
does not affect the progress of bone healing.
BMD values reflect the mineralization of bone
healing, which is important for the strength and function of the
bone (13–15). In this study, no significant
difference was observed between the escin-treated and the model
rabbits. The results indicate that the administration of escin does
not affect the mineralization during the fracture healing
process.
The physiological process of fracture healing can be
summarized as follows (20,21).
A few hours after fracture, the extravascular blood cells form a
blood clot, known as a hematoma. Then, fibroblasts replicate and
form granulation tissue. Days after fracture, the periosteal cells
replicate and develop into chondroblasts and osteoblasts, which
form hyaline cartilage and woven bone. The fibroblasts within the
granulation tissue develop into chondroblasts which also form
hyaline cartilage. Subsequently, the hyaline cartilage and woven
bone are replaced with lamellar bone and the haversian system takes
shape. Meanwhile, the osteoblasts form new lamellar bone upon the
recently exposed surface of the mineralized matrix. This new
lamellar bone is in the form of trabecular bone. Eventually, the
trabecular bone is replaced by compact bone. Therefore, the amount
of fibroblasts, chondroblasts and osteoblasts, the area of hyaline
cartilage, woven bone, lamellar bone and the thickness of bone
trabeculae show the extent of fracture healing (14,16,19).
In the present study, 4 weeks after the operation, a large number
of fibroblasts and chondrocytes in mature and immature stages were
found. Eight weeks post-surgery, bone cells reached maturity, while
the haversian system and a small number of cartilage cells were
observed. However, there was no significant difference among
groups, which again confirms that escin does not affect the
fracture healing process.
In humans, ALP is produced mostly in the liver and
bone. Elevated ALP indicates that there may be active bone
formation occurring as ALP is a by-product of osteoblast activity
(22). In our study, no
significant difference was found between the treated groups and the
model group, with respect to the serum levels of ALP, which
demonstrated that escin does not affect osteoblast activity.
Osteocalcin is synthesized by osteoblasts, and a small fraction of
the newly synthesized protein is released into the circulation
where it can be measured (23). As
osteocalcin is bone-specific, it has been widely used as a marker
of bone formation (24). Calcium
and phosphate are important components of bone matrix, and their
serum contents directly reflect the metabolism of bone (18). In our study, 1 week after the
operation, the serum osteocalcin levels in the escin-treated group
(0.45 mg/kg) were significantly higher than that in the model group
(p<0.05), while the calcium levels in the escintreated groups
(0.45 and 0.9 mg/kg) were higher than that in the model group
(p<0.05). The data showed that the treatment with escin
increased the calcium and osteocalcin contents in the serum, while
no significant difference was found between the escin-treated
groups and the model group at other time intervals; the mechanism
remains unclear and further study is required.
Wound healing is the process of repair that follows
injury to the skin and other soft tissues. A number of factors
regulate wound repair. Hydroxyproline is an amino acid and a
subproduct of collagen synthesis. The tissue hydroxyproline assay
presents a parallel increase with the tissue collagen level;
therefore, hydroxyproline measurement is an important test for
wound healing (11,25). In the present study, the
hydroxyproline levels showed a significant increase in the model
rat group. However, there were no significant differences between
the escin-administered rat groups and the model rat group. The
results revealed that escin did not affect the wound healing of the
abdominal wall in rats.
In conclusion, according to the present study escin
does not affect the process of fracture healing and wound healing,
which provides rational evidence for using escin after fracture in
the clinic.
Acknowledgements
This study was supported by Taishan
Scholar Project, the 11th Five Years Key Programs for Science and
Technology Development of China (grant no. 2008ZX09202-008), and
the National Natural Science Foundation of China (grant no.
30772760). The authors would like to thank Professor Tongshen Liu
and all the staff of the Health Examination Center of the Yantai
Mountain Hospital for their technical assistance.
References
|
1.
|
Fu F, Hou Y, Jiang W, Wang R and Liu K:
Escin: inhibiting inflammation and promoting gastrointestinal
transit to attenuate formation of postoperative adhesions. World J
Surg. 29:1614–1620. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Zhang L, Fu F, Zhang X, Zhu M, Wang T and
Fan H: Escin attenuates cognitive deficits and hippocampal injury
after transient global cerebral ischemia in mice via regulating
certain inflammatory genes. Neurochem Int. 57:119–127. 2010.
View Article : Google Scholar
|
|
3.
|
Xin W, Zhang L, Fan H, Jiang N, Wang T and
Fu F: Escin attenuates acute lung injury induced by endotoxin in
mice. Eur J Pharmaceut Sci. 42:73–80. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Matsuda H, Li Y, Murakami T, Ninomiya K,
Yamahara J and Yoshikawa M: Effects of escins Ia, Ib, IIa, and IIb
from horse chestnut, the seeds of Aesculus hippocastanum L.,
on acute inflammation in animals. Biol Pharmaceut Bull.
20:1092–1095. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Arnould T, Janssens D, Michiels C and
Remacle J: Effect of aescine on hypoxia-induced activation of human
endothelial cells. Eur J Pharmacol. 315:227–233. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Xiao GM and Wei J: Effects of β-aescin on
the expression of nuclear factor-kappa B and tumor necrosis
factor-alpha after traumatic brain injury in rats. J Zhejiang Univ
Sci B. 6:28–32. 2005.
|
|
7.
|
Xin W, Zhang L, Sun F, et al: Escin exerts
synergistic anti-inflammatory effects with low doses of
glucocorticoids in vivo and in vitro. Phytomedicine. 18:272–277.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Ma CF and Li L: Clinical observation of
the curative effects of a combination of sodium aescinate and
glycerol fructose on joint swelling after calcaneal fracture. China
Pharmacist. 11:445–446. 2008.
|
|
9.
|
Tang L: Clinical observation of the
curative effects of sodium aescinate on swelling after ankle
fracture. Mod Med J. 36:105–106. 2008.
|
|
10.
|
Yang LD and Liu F: Clinical observation of
the curative effects of sodium aescinate on limb swelling resulting
from fracture of tibia and fibula. Acta Academiae Medicinae
Nantong. 29:34–35. 2009.
|
|
11.
|
Bergman I and Loxley R: New
spectrophotometric method for the determination of proline in
tissue hydrolyzates. Anal Chem. 42:702–706. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Christian LM, Graham JE, Padgett DA,
Glaser R and Kiecolt- Glaser JK: Stress and wound healing.
Neuroimmunomodulation. 13:337–346. 2006. View Article : Google Scholar
|
|
13.
|
Wang CJ, Yang KD, Wang FS, Hsu CC and Chen
HH: Shock wave treatment shows dose-dependent enhancement of bone
mass and bone strength after fracture of the femur. Bone.
34:225–230. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Morgan EF, Mason ZD, Bishop G, et al:
Combined effects of recombinant human BMP-7 (rhBMP-7) and
parathyroid hormone (1–34) in metaphyseal bone healing. Bone.
43:1031–1038. 2008.PubMed/NCBI
|
|
15.
|
Aleksyniene R, Thomsen JS, Eckardt H,
Bundgaard KG, Lind M and Hvid I: Parathyroid hormone PTH (1–34)
increases the volume, mineral content, and mechanical properties of
regenerated mineralizing tissue after distraction osteogenesis in
rabbits. Acta Orthopaedica. 80:716–723. 2009.
|
|
16.
|
Saghieh S, Khoury NJ, Tawil A, et al: The
impact of zoledronic acid on regenerate and native bone after
consolidation and removal of the external fixator: an animal model
study. Bone. 46:363–368. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Taniguchi T, Matsumoto T and Shindo H:
Changes of serum levels of osteocalcin, alkaline phosphatase, IGF-I
and IGF-binding protein-3 during fracture healing. Injury.
34:477–479. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Andreen O and Larsson SE: Effects of 1,
25-dihydroxycholecalciferol on fracture healing. Calcium,
phosphate, and zinc in callus and serum. Arch Orthop Trauma Surg.
103:257–262. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
O'Connor JP, Capo JT, Tan V, Cottrell JA,
Manigrasso MB, Bontempo N and Parsons JR: A comparison of the
effects of ibuprofen and rofecoxib on rabbit fibula osteotomy
healing. Acta Orthopaedica. 80:597–605. 2009.PubMed/NCBI
|
|
20.
|
Brighton CT and Hunt RM: Early
histological and ultrastructural changes in medullary fracture
callus. J Bone Joint Surg. 73:832–847. 1991.PubMed/NCBI
|
|
21.
|
Brighton CT and Hunt RM: Early histologic
and ultrastructural changes in microvessels of periosteal callus. J
Orthop Trauma. 11:244–253. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Coleman JE: Structure and mechanism of
alkaline phosphatase. Ann Rev Biophys Biomol Struct. 21:441–483.
1992. View Article : Google Scholar
|
|
23.
|
Akesson K, Ljunghall S, Gärdsell P, Sernbo
I and Obrant KJ: Serum osteocalcin and fracture susceptibility in
elderly women. Calcif Tissue Int. 53:86–90. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Herrmann M, Klitscher D, Georg T, Frank J,
Marzi I and Herrmann W: Different kinetics of bone markers in
normal and delayed fracture healing of long bones. Clin Chem.
48:2263–2266. 2002.PubMed/NCBI
|
|
25.
|
Brown GL, Curtsinger LJ, White M, et al:
Acceleration of tensile strength of incisions treated with EGF and
TGF-beta. Ann Surg. 208:788–794. 1988. View Article : Google Scholar : PubMed/NCBI
|