Introduction
Acute myeloid leukemia (AML) accounts for
approximately 20% of acute leukemia cases in children (1). Acute promyelocytic leukemia (APL) is
morphologically identified as AML-M3 according to the
French-American-British (FAB) classification. APL represents 5–8%
of AML cases in children (2). It
is characterized by accumulation of immature granulocytes called
promyelocytes, which feature bilobulated nuclei and Auer rods
(3). Cytogenetically, APL
promyelocytes carry a balanced reciprocal translocation between
chromosomes 15 and 17, t(15;17), which results in a fusion protein
PML/RARα encoded by the promyelocytic leukemia (PML) gene
fused with the gene for retinoic acid receptor α (RARα) (4). Immunophenotypically, APL
promyelocytes express CD33, CD13 and CD117 antigens, and less
frequently CD34 and human leukocyte antigen DR (5). Five decades ago APL was considered
the most fatal type of acute leukemia and the treatment of APL was
a nightmare for physicians; event-free survival (EFS) of APL
patients including children was only 35% (6).
Due to the development of various combinations of
chemotherapy based on all-trans-retinoic acid (ATRA) and
anthracycline, the 5-year disease-free survival (DFS) or EFS rate
of pediatric APL patients has reached 75–80% based on recent
studies (7–17). Thus, APL is now curable in most
cases. Recently a study on childhood AML in Japan reported a 93.1%
overall survival (OS) rate and 94% EFS rate for patients treated
with ATRA, anthracyclines and cytarabine (at both the induction and
consolidation stages) (16).
Despite of the reported high survival rate, the outcome of APL
treatment in many developing countries including China appears to
vary dramatically, largely depending on how the therapeutic regimes
are designed and delivered (14,18).
A study based in Taiwan reported that the overall survival and EFS
of 6 APL children treated with ATRA during induction were 83 and
67%, respectively (19). Zhang
et al (20) reported that
65 Chinese APL children had better outcome with EFS, DFS, and
overall survival achieved 77.5, 85.4 and 88.9%, respectively.
However, another study found that the EFS of 16 APL children
treated with an in-house protocol and 14 with a modified PETHEMA
LPA99 protocol were 38 and 79.6%, respectively (12). Such large variations prompted us to
identify which components included in the widely used regimes play
a pivotal role in prognosis of the disease. Here, we conducted a
retrospective, multiple center-based study on 119 cases of
pediatric APL following treatment with four different chemotherapy
regimes based on ATRA. We found that the overall outcomes were more
favorable after treatment with regimes 2 and 3 than with regimes 1
and 4, and this added benefit may have been due to the presence of
a Chinese herbal medicine formula, Realgar-Indigo naturalis formula
(RIF), and the absence of high-dose cytarabine (Ara-C) in regimes 2
and 3.
Materials and methods
Eligibility of patients
Informed consents were obtained from the parents or
guardians of the children (under the age of 18) diagnosed with APL
who were enrolled at the Departments of Pediatrics, in the leukemia
wards of six collaborative hospitals in China from September 1997
to December 2008. The diagnosis was based on the FAB
classification, detection of the PML/RARα fusion gene by RT-PCT or
fluorescent in situ hybridization (FISH), and detection of
t(15;17) in bone marrow cells aspirated from the patients, as well
as the morphology of the cells. Following the eligibility
screening, 119 cases were retrospectively enrolled in this study.
The patients were divided into four groups based on the therapeutic
regimes received, with 36, 16, 35 and 32 patients in regimes 1–4,
respectively as described below.
Treatment
The therapeutic regimes consisted of multistage
treatments including induction and consolidation (for all 4
regimes), maintenance (for regimes 2, 3 and 4), and reinforcement
(for regime 3 only) (Fig. 1).
Regime 1 used a protocol developed in-house including ATRA,
daunomycin (DNR), Novantrone (NVT), and high-dose Ara-C (2
g/m2, IV). Regime 2 used a modified PETHEMA LPA99
protocol including ATRA, methotrexate (MTX), NVT, DNR, and RIF.
Regime 3 used a modified European-APL93 protocol including ATRA,
RIF, DNR, NVT, DA [DNR plus low-dose Ara-C (150 mg/m2,
IV)], NA [NVT plus low-dose Ara-C (150 mg/m2, IV)] and
6-mercaptopurine (6MP). Regime 4 used a protocol suggested by the
British Committee for Standards in Haematology, including ATRA,
DNR, and Ara-C [at a low-dose (200 mg/m2, IV) and high
dose (2 g/m2, IV) alternatively at various stages]. The
details of the regimes are shown in Fig. 1.
 | Figure 1.Therapeutic regimes and patient groups
included in the study. Ara-C, cytarabine; ATRA,
all-trans-retinoic acid; DNR, daunomycin; HD, high-dose; DA
(DNR and Ara-C); MTX, methotrexate; NVT, Novantrone; 6MP,
6-mercaptopurine; NA (NVT and Ara-C); RIF, Realgar-Indigo naturalis
formula; PO, per os; IV, intravenously; d, day; wk, week;
q12h, every 12 hours; qwk, every week; qn, every night; Tab./#,
tablets; IH, hypodermic injection. |
Supportive therapy
Supportive therapy was provided to almost all of the
patients, which was crucial to prevent the development of serious
or even fatal complications such as coagulopathy and retinoic acid
syndrome. Counter-coagulopathy therapy included transfusions of
platelets, plasma, and cryoprecipitate to maintain the fibrinogen
level above 1.5 g/l and the platelet count above
3x1010/l until clinical resolution of coagulopathy
(21). Retinoic acid syndrome is
another complication characterized by dyspnea, unexplained fever,
weight gain, peripheral edema, pulmonary nodular infiltrates and
pleuropericardial effusion. It develops rapidly and is potentially
fatal. Upon observation of the earliest signs of retinoic acid
syndrome, treatment with high-dose steroids was initiated
immediately by administering dexamethasone at 0.3 mg/kg/dose twice
daily intravenously for at least 4 days to the children (22). In addition, hydroxyurea was
administered to control leukocytosis when required.
Definitions
Complete remission (CR) was defined according to the
US National Cancer Institute criteria as the presence of less than
5% of blast cells in bone marrow aspirates (23). DFS refers to the duration from the
date of diagnosis until the date of the last follow-up for any
event, e.g., failure to achieve remission, relapse, secondary
malignancy, or death from any cause. The DFS was calculated based
on time from the date of hematologic CR until death or hematologic
relapse.
Statistical methods
The relationship of a patient’s clinical
characteristics to his/her treatment outcome was analyzed using the
Cox Regression Proportional Hazard Model. Multivariate analysis was
used. Clinical, demographic, biologic characteristics, treatment
outcomes, and toxicity of the patients were compared using
χ2 tests, Fisher exact tests, and one-way ANOVA. DFS of
the four regimes was further analyzed using the Kaplan-Meier
method, and the log-rank test was used for comparison between
different treatment groups. Survival rates were represented as the
mean percentage ± standard error (SE). Statistical differences were
considered significant at a P-value (2-sided) <0.05 or highly
significant at a P-value (2-sided) <0.01.
Results
Clinical characteristics and laboratory
test results of the patients
The 119 patients included 80 males and 39 females.
Data were retrieved for a median follow-up time of 43 months
(ranging from 1 to 147 months). Approximately half of the patients
were from rural areas. The median age of the patients was 8.9
years, and 3.4% of the patients were under the age of 2. Paleness,
hemorrhage, and organomegalies were present in 90.8, 74.6 and 35.5%
of the patients, respectively. Fever was found in 63.8% of the
patients, and bone pain was experienced by 22.7% of the
patients.
Laboratorial test results of the 119 patients
indicated that the median white blood cell (WBC) count was
8.79x109/l (range, 0.9x109/l to
191x109/l), the median hemoglobin level was 71 g/dl
(range, 25–117 g/dl), and the median platelet number was
28x109/l (range, 4x109/l to
344x109/l). Elevated lactic dehydrogenase (LDH) (>450
U/l) was present in 34 of 106 tested patients, of whom 5.7% (6/106)
had LDH levels over 1,000 U/l. The immunophenotyping for CD13, CD33
and MPO was carried out on 91 of the 119 patients, and the rates of
positive specimens were 98.3, 97.2 and 87% for the three antigens,
respectively. Our data demonstrated no significant difference
between the four regimes in terms of age, gender, WBC count,
hemoglobin level, platelet count, and LDH level of the patients
tested before the treatment (Table
I).
 | Table I.Clinical characteristics and
laboratory results at diagnosis of the APL patients classified into
the four treatment groups. |
Table I.
Clinical characteristics and
laboratory results at diagnosis of the APL patients classified into
the four treatment groups.
| Characteristics | Regime 1 | Regime 2 | Regime 3 | Regime 4 | P-value |
|---|
| Age, years, median
(range) | 7.7 (1.6–12.4) | 8.2 (2.2–17.5) | 8.7 (0.5–14.6) | 10 (2.2–14.9) | 0.196 |
| Male/female (n) | 26/10 | 9/7 | 22/13 | 20/12 | 0.065 |
| WBC,
109/l, range (median) | 1.2–127.5 (3) | 1.1–143.4 (7.7) | 0.2–145.7 (11.1) | 1.9–191 (32.3) | 0.055 |
| Hb, g/l, range
(median) | 25–116 (70) | 38–98 (76) | 32–117 (72) | 34–128 (71) | 0.500 |
| Platelet,
109/l, range (median) | 4–344 (29) | 7–190 (30) | 8–164 (26) | 9–146 (40) | 0.984 |
| LDH, U/l, range
(median) | 153–1,314 (245) | 120–1,561 (357) | 28–1,470 (283) | 15–1,995 (314) | 0.607 |
Treatment outcome
After the remission induction, the CR rate for the
four regimes was 88.9 (32/36), 87.5 (14/16), 97.1 (34/35) and 87.5%
(28/32), respectively, without statistical difference between the
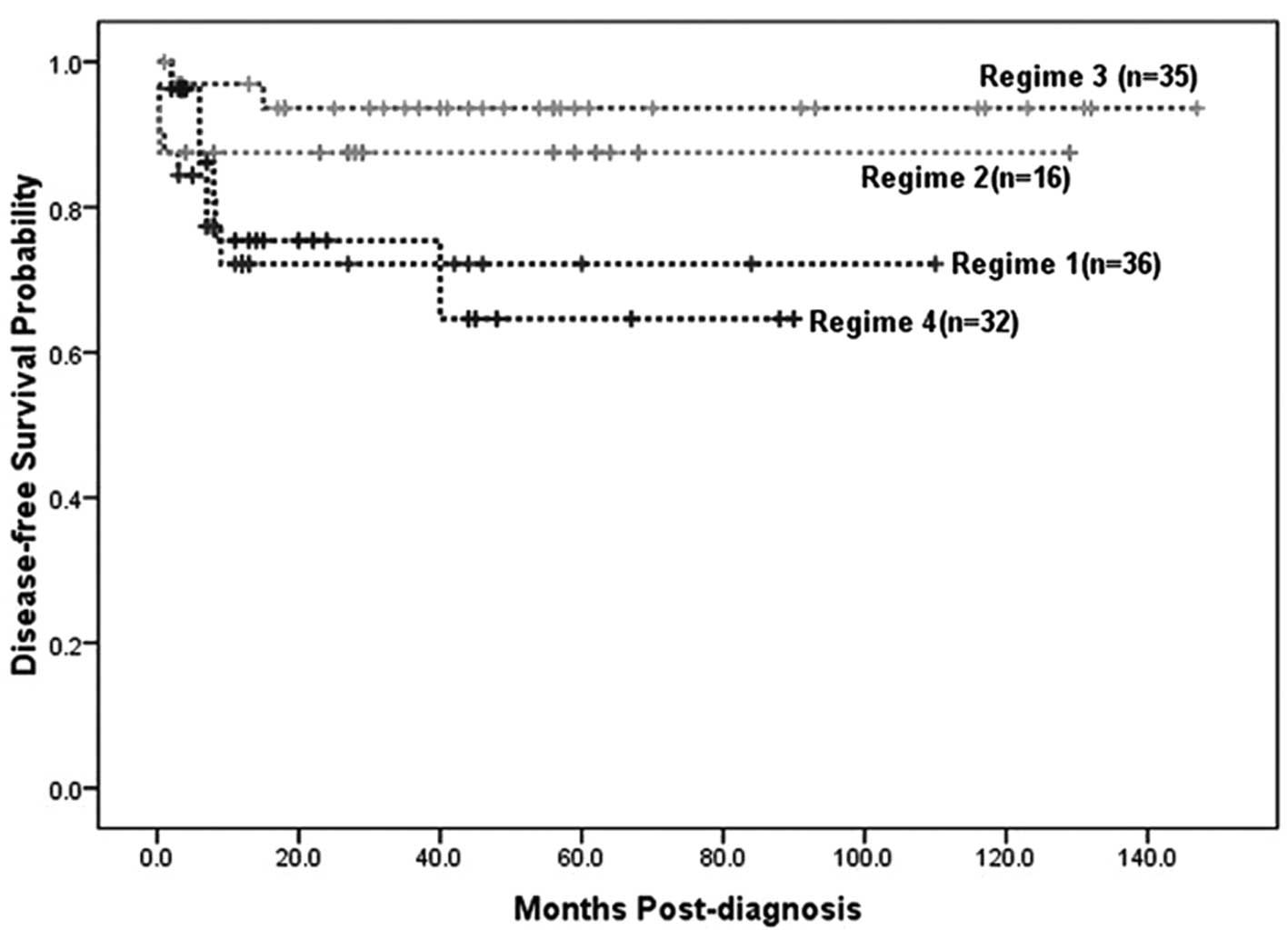
regimes. The 3.5-year DFS for the four treatment regimes was
72.2±8.6, 87.5±8.3, 93.6±4.4 and 64.6±13%, respectively, with the
DFS for regime 2 statistically higher than that for regime 1
(P<0.05), and DFS for regime 3 statistically higher than those
for regimes 1 (P<0.01) and 4 (P<0.01) (Table II and Fig. 2). The relapse rate for the 4
regimes was 25, 6.3, 5.7 and 28.1%, respectively, with a
statistically significant difference detected between regimes 1 and
2 (P<0.05), and between regimes 3 and 4 (P<0.05) (Table II and Fig. 2). Although all four regimes
resulted in high CR rates, regimes 1 and 4 were associated with
lower DFS and higher relapse rates than regimes 2 and 3. Thus, it
appears that the overall outcome for regimes 2 and 3 was more
favorable than that for regimes 1 and 4.
 | Table II.Outcome of the patients following
treatment with the four different regimes. |
Table II.
Outcome of the patients following
treatment with the four different regimes.
| Treatment
outcome | Regimes
| P-value
|
|---|
| 1 | 2 | 3 | 4 | <0.05 | <0.01 |
|---|
| Number of
cases | 36 | 16 | 35 | 32 | | |
| Complete response,
n (%) | 32 (88.9) | 14 (87.5) | 34 (97.1) | 28 (87.5) | | |
| Relapse, n (%) | 9 (25.0) | 1 (6.3) | 2 (5.7) | 9 (28.1) | 1/3, 3/4 | |
| 3.5-year DFS,
% | 72.2±8.6 | 87.5±8.3 | 93.6±4.4 | 64.6±13 | 1/2 | 1/3, 3/4 |
| Toxicity, n
(%) | | | | | | |
|
Hepatotoxicity | 22 (61.1) | 3 (18.8) | 7 (20.0) | 15 (46.9) | 2/4, 3/4 | 1/2, 1/3 |
| Headache | 4 (11.1) | 2 (12.5) | 4 (11.4) | 5 (15.6) | | |
| Skin
reaction | 7 (19.4) | 3 (18.8) | 8 (22.9) | 7 (21.9) | | |
| Dryness of
lips | 13 (36.1) | 4 (25.0) | 13 (37.1) | 11 (34.4) | | |
| Fluid
retention | 3 (8.3) | 2 (12.5) | 10 (28.6) | 3 (9.4) | 1/3, 3/4 | |
| Cardiac
arrhythmia | 2 (5.6) | 0 | 0 | 6 (18.8) | 1/4, 2/4 | 3/4 |
| Differentiation
syndrome | 0 | 0 | 0 | 4 (12.5) | 1/2, 2/4 | 3/4 |
| Sepsis | 25 (69.4) | 3 (18.8) | 6 (17.1) | 20 (62.5) | | 1/2, 1/3, 2/4,
3/4 |
Toxicity
The occurrence of hepatotoxicity, headache, skin
reaction, dryness of lips, fluid retention, cardiac arrhythmia,
differentiation syndrome, and sepsis was recorded for all the
patients during all the therapeutic stages (Table II). Of note, hepatotoxicity and
sepsis occurred statistically more frequently in regimes 1 and 4
than in regimes 2 and 3. Fluid retention was observed more with
regime 3 than with regimes 1 and 4. Cardiac arrhythmia occurred
more with regimes 1 and 4 than regimes 2 and 3. Differentiation
syndrome occurred only in regime 4. Based on the above findings,
the incidence of general toxicity and sepsis was lower for
treatment regimes 2 and 3 than with regimes 1 and 4.
Discussion
In the present study, data was collected and
analyzed regarding the clinical characteristics, laboratory test
results and treatment outcome of 119 cases of pediatric APL treated
with four different chemotherapy regimes. Similar to previous
reports (7–9), the WBC counts of 47% of the patients
were higher than 10x109/l. High WBC counts combined with or without
low platelet counts have been considered as indices of relapse risk
(9,17,24).
However, we found that the WBC and platelet counts and hemoglobin
and LDH levels, tested before treatment, had no significant
influence on prognosis of the pediatric APL patients (data not
shown). Instead, the various therapeutic regimes imposed
significantly different impacts on the outcomes of the
patients.
Regimes 1–4 resulted in a CR rate of 88.9, 87.5,
97.1 and 87.5%, respectively, a 3.5-year DFS rate of 72.2, 87.5,
93.6 and 64.6%, respectively, and a relapse rate of 25, 6.3, 5.7
and 28.1%, respectively. Although all four regimes led to high CR
rates, regimes 1 and 4 resulted in lower DFS and higher relapse
than regimes 2 and 3. Therefore, the overall outcome for regimes 2
and 3 was more favorable than regimes 1 and 4.
It does not appear that the comparably short
treatment without maintenance for regime-1 patients was accountable
for their poor survival, as the regime-4 patients had maintenance
treatment, yet both their CR and 3.5-year DFS rates were the
lowest. Therefore, these different outcomes might have mainly
resulted from the different components in the regimes. Although all
four regimes included ATRA and DNR, plus other various
chemotherapeutic agents, regimes 2 and 3 had two obvious features.
They included the Chinese herbal medicine formula RIF, and no Ara-C
(regime 2) or only low-dose (150 mg/m2) Ara-C (regime
3). In contrast, Ara-C was used at a high dose (2 g/m2)
during all the consolidation treatments in regime 1, and at low
doses (30 or 45 mg/m2) in two of the consolidation
treatments and intermediate (300 mg/m2) and high (2
g/m2) doses, respectively, in the other two
consolidation treatments in regime 4. It appears that the use of
the Chinese herbal medicine formula RIF and avoidance of the
high-dose Ara-C may have reduced the treatment-related toxicity,
relapse rate, and the frequency of sepsis, hence contributing to
the optimal outcome of the patients treated with regimes 2 and
3.
Traditional Chinese medicine (TCM) is a unique
medical system used for thousands of years by the Chinese and other
ethnic populations in China as well as many other countries. TCM
doctors usually prescribe a combination of plant species or
minerals to treat diseases (25,26).
Increasing evidence has demonstrated that TCM can be used as
alternative medicine to treat diseases that have no cure using
conventional medicine, or as supportive medicine to enhance
therapeutic efficacy and reduce adverse effects of conventional
medicine such as chemotherapy. RIF contains realgar, indigo
naturalis, tetra-arsenic tetrasulfide, indirubin and tanshinone IIA
as its major active ingredients.
Wang et al (27) reported that RIF, when used in a
murine APL model, promoted ubiquitination and degradation of the
PML/RARα oncoprotein by inducing expression and transportation of
aquaglyceroporin-9 which degraded PML/RARα. It also enhanced G1/G0
arrest of APL cells by regulating multiple targets of the cell
cycle. Notably, recent multi-center clinical trials showed that a
CR rate of 98% and a 5-year overall survival rate of 87% were
achieved in adult APL patients receiving RIF, with only moderate
adverse effects such as gastrointestinal discomfort and rash
(25,26,28).
Furthermore, Luo et al (12) reported that a modified PETHEMA
LPA99 protocol by including RIF had an improved overall outcome for
13 Chinese children with APL. These lines of evidence are
consistent with the added beneficial effect of including RIF in
regimes 2 and 3 in the present study (Table I and Fig. 2). In addition, compared to arsenic
trioxide, a widely used anti-leukemia drug analogous to
tetra-arsenic tetrasulfide, RIF is relatively inexpensive, can be
taken orally and shortens the hospital stay of patients (29).
Ara-C is an anti-metabolite chemotherapeutic drug,
which acts by impeding cancer cells from making and repairing DNA
required for cell proliferation. Ara-C has been used to treat acute
leukemia, several types of head and neck cancers, and non-Hodgkin’s
lymphoma. In induction or consolidation treatment for AML, high
doses of either DNR or Ara-C often result in improved remission and
survival rates (30–33). However, among these studies, only
Weick et al (33) compared
two doses of Ara-C, 1,400 and 24,000 mg/m2, for
induction chemotherapy, and found no difference in overall survival
rate of the patients.
The dose of Ara-C used during consolidation has also
been extensively explored in single-arm trials. Mayer et al
(34) reported a large, randomized
study of 596 patients with AML in first remission, which suggested
a dose-response relationship with Ara-C. Patients who received the
dose of 3,000 mg/m2 had an improved disease-free and
overall survival, especially for those who were under 60 years of
age. However, an important finding of this study is that high-dose
Ara-C was effective only in patients who had ‘favorable’,
‘intermediate’ or normal karyotypes upon treatment (34). As our patients all had abnormal
karyotypes by the nature of the disease, high (2 g/m2)
doses of Ara-C used in the regimes may not have helped, but instead
counteracted, the outcome of the young patients. One of the serious
side effects of Ara-C, like many other chemotherapeutics, is
increased risk of fatal infection due to reduced WBC count, which
might have contributed to the short 3.5-year EFS and CR rate of the
patients on regimes 1 and 4.
Overall, our present study, the largest scale study
to date concerning pediatric APL in China to our knowledge, has
revealed important information for the treatment of pediatric APL.
We found that the modified PETHEMA LPA99 protocol (for the regime
2) and the modified European ALP93 protocol (regime 3), both
including RIF and the absence of high-dose Ara-C, achieved a more
favorable overall outcome, less chemotherapy treatment-related
toxicity, and lower frequency of sepsis and relapse for APL
children, than the in-house protocol (regime 1) and the protocol
suggested by the British Committee for Standards in Haematology
(regime 4). The inclusion of RIF and exclusion of high-dose Ara-C
may have contributed to the beneficial effects of regimes 2 and 3
on the prognosis of pediatric APL cases.
Acknowledgements
We thank the Huazhong University of
Science and Technology and the Sun Yat-sen University for funding
this study.
References
|
1
|
Smith MA, Ries LA, Gurney JG, et al:
Leukemia. Cancer incidence and survival among children and
adolescents United States SEER Program 1975–1995. National Cancer
Institute, SEER Program; Bethesda, MD: pp. 17–34. 1999
|
|
2
|
Cantu-Rajnoldi A, Biondi A, Jankovic M, et
al: Diagnosis and incidence of acute promyelocytic leukemia (FAB M3
and M3 variant) in childhood. Blood. 81:2209–2210. 1993.PubMed/NCBI
|
|
3
|
Catovsky D, Matutes E, Buccheri V, et al:
A classification of acute leukaemia for the 1990s. Ann Hematol.
62:16–21. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
de Thé H and Chen Z: Acute promyelocytic
leukaemia: novel insights into the mechanisms of cure. Nat Rev
Cancer. 10:775–783. 2010.PubMed/NCBI
|
|
5
|
Lo-Coco F and Ammatuna E: The biology of
acute promyelocytic leukemia and its impact on diagnosis and
treatment. Hematology Am Soc Hematol Educ Program. 514:156–161.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Avvisati G, Petti MC, Lo-Coco F, et al:
Induction therapy with idarubicin alone significantly influences
event-free survival duration in patients with newly diagnosed
hypergranular acute promyelocytic leukemia: final results of the
GIMEMA randomized study LAP 0389 with 7 years of minimal follow-up.
Blood. 100:3141–3146. 2002.
|
|
7
|
Mann G, Reinhardt D, Ritter J, et al:
Treatment with all-trans retinoic acid in acute promyelocytic
leukemia reduces early deaths in children. Ann Hematol. 80:417–422.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
de Botton S, Coiteux V, Chevret S, et al:
Outcome of childhood acute promyelocytic leukemia with
all-trans-retinoic acid and chemotherapy. J Clin Oncol.
22:1404–1412. 2004.PubMed/NCBI
|
|
9
|
Testi AM, Biondi A, Lo Coco F, et al:
GIMEMA-AIEOPAIDA protocol for the treatment of newly diagnosed
acute promyelocytic leukemia (APL) in children. Blood. 106:447–453.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ortega JJ, Madero L, Martin G, et al:
Treatment with all-trans retinoic acid and anthracycline
monochemotherapy for children with acute promyelocytic leukemia: a
multicenter study by the PETHEMA Group. J Clin Oncol. 23:7632–7640.
2005. View Article : Google Scholar
|
|
11
|
Powell B, Moser B, Stock W, et al:
Preliminary results from the North American acute promyelocytic
leukemia (APL) study C9710 (abstract). Blood. 108:5662006.
|
|
12
|
Luo XQ, Ke ZY, Huang LB, et al: Improved
outcome for Chinese children with acute promyelocytic leukemia: a
comparison of two protocols. Pediatr Blood Cancer. 53:325–328.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gregory J, Kim H, Alonzo T, et al:
Treatment of children with acute promyelocytic leukemia: results of
the first North American Intergroup trial INT0129. Pediatr Blood
Cancer. 53:1005–1010. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jeddi R, Ghedira H, Ben Abdennebi Y, et
al: ATRA and anthracycline-based chemotherapy in the treatment of
childhood acute promyelocytic leukemia (APL): a 10-year experience
in Tunisia. Med Oncol. 28:1618–1623. 2010.PubMed/NCBI
|
|
15
|
Creutzig U, Zimmermann M, Dworzak M, et
al: Favourable outcome of patients with childhood acute
promyelocytic leukaemia after treatment with reduced cumulative
anthracycline doses. Br J Haematol. 149:399–409. 2010. View Article : Google Scholar
|
|
16
|
Imaizumi M, Tawa A, Hanada R, et al:
Prospective study of a therapeutic regimen with all-trans
retinoic acid and anthracyclines in combination of cytarabine in
children with acute promyelocytic leukaemia: the Japanese childhood
acute myeloid leukaemia cooperative study. Br J Haematol.
152:89–98. 2011.PubMed/NCBI
|
|
17
|
Kim MHCC, Lee JW, Jang PS, et al: Outcome
of childhood acute promyelocytic leukemia treated using a modified
AIDA protocol. Korean J Hematol. 45:236–241. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ribeiro RC and Rego E: Management of APL
in developing countries: epidemiology, challenges and opportunities
for international collaboration. Hematology Am Soc Hematol Educ
Program. 2006:162–168. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yeh TC, Liu HC, Wang LY, et al: The
development of a novel protocol for the treatment of de novo
childhood acute myeloid leukemia in a single institution in Taiwan.
J Pediatr Hematol Oncol. 29:826–831. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang L, Zhao H, Zhu X, et al:
Retrospective analysis of 65 Chinese children with acute
promyelocytic leukemia: a single center experience. Pediatr Blood
Cancer. 51:210–215. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Avvisati G, Lo Coco F, Diverio D, et al:
AIDA (all-trans retinoic acid + idarubicin) in newly diagnosed
acute promyelocytic leukemia: a Gruppo Italiano Malattie
Ematologiche Maligne dell’Adulto (GIMEMA) pilot study. Blood.
88:1390–1398. 1996.
|
|
22
|
De Botton S, Chevret S, Coiteux V, et al:
Early onset of chemotherapy can reduce the incidence of ATRA
syndrome in newly diagnosed acute promyelocytic leukemia (APL) with
low white blood cell counts: results from APL 93 trial. Leukemia.
17:339–342. 2003.PubMed/NCBI
|
|
23
|
Cheson BD, Cassileth PA, Head DR, et al:
Report of the National Cancer Institute-sponsored workshop on
definitions of diagnosis and response in acute myeloid leukemia. J
Clin Oncol. 8:813–819. 1990.PubMed/NCBI
|
|
24
|
Sanz MA, Lo Coco F, Martin G, et al:
Definition of relapse risk and role of nonanthracycline drugs for
consolidation in patients with acute promyelocytic leukemia: a
joint study of the PETHEMA and GIMEMA cooperative groups. Blood.
96:1247–1253. 2000.PubMed/NCBI
|
|
25
|
Huang S, Guo A, Xiang Y, et al: Clinical
study on the treatment of acute promyelocytic leukemia with
composite Indigo Naturalis tablets. Chin J Hematol. 16:26–28.
1995.
|
|
26
|
The cooperation group phase II: Clinical
trial of compound Huangdai tablet in newly diagnosed acute
promyelocytic leukemia. Chin J Hematol. 12:801–804. 2006.
|
|
27
|
Wang L, Zhou GB, Liu P, et al: Dissection
of mechanisms of Chinese medicinal formula Realgar-Indigo naturalis
as an effective treatment for promyelocytic leukemia. Proc Natl
Acad Sci USA. 105:4826–4831. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xiang Y: The influence on long-term survey
of the patients with acute promyelocytic leukemia treated with
compound Huangdai tablets and chemotherapy. Chin J Clin Hematol.
16:204–206. 2007.
|
|
29
|
Rizzari C and Biondi A: Tailoring
treatment strategy for acute promyelocytic leukemia in low-income
countries. Pediatr Blood Cancer. 53:303–305. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Preisler H, Davis RB, Kirshner J, et al:
Comparison of three remission induction regimens and two
postinduction strategies for the treatment of acute nonlymphocytic
leukemia: a cancer and leukemia group B study. Blood. 69:1441–1449.
1987.
|
|
31
|
Dillman RO, Davis RB, Green MR, et al: A
comparative study of two different doses of cytarabine for acute
myeloid leukemia: a phase III trial of Cancer and Leukemia Group B.
Blood. 78:2520–2626. 1991.PubMed/NCBI
|
|
32
|
Schiller G, Gajewski J, Nimer S, et al: A
randomized study of intermediate versus conventional-dose
cytarabine as intensive induction for acute myelogenous leukaemia.
Br J Haematol. 81:170–177. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Weick JK, Kopecky KJ, Appelbaum FR, et al:
A randomized investigation of high-dose versus standard-dose
cytosine arabinoside with daunorubicin in patients with previously
untreated acute myeloid leukemia: a Southwest Oncology Group study.
Blood. 88:2841–2851. 1996.
|
|
34
|
Mayer RJ, Davis RB, Schiffer CA, et al:
Intensive postremission chemotherapy in adults with acute myeloid
leukemia. Cancer and Leukemia Group B. N Engl J Med. 331:896–903.
1994. View Article : Google Scholar : PubMed/NCBI
|