Introduction
Rodent teeth are sensitive to chemicals and are good
models for the evaluation of chemically induced effects in humans.
However, their biological features, particularly those of the
incisors, are quite different from those of human teeth (1,2).
Rats have 16 teeth, comprising an incisor and three molars in each
quadrant. Rodent incisors grow, calcify and erupt continuously
throughout life. A longitudinal section shows the complete life
cycle of tooth development from inception to maturity (3,4). The
frequency of odontogenic tumors is low in rodents (5) and humans. The odontogenic epithelium
is responsible for tooth development under physiological conditions
and can give rise to tumors and cysts in the jaw (6,7).
Therefore, tumors derived from the perpetually erupting ameloblasts
of the rodent incisor may differ from those in human adults. The
chemical compounds that cause morphological changes in rodent teeth
include various antitumor or DNA-alkylating agents, such as
5-fluorouracil, doxorubicin, mitomycin C, vinblastine sulphate,
docetaxel, irinotecan hydrochloride, cisplatin and nitrosoureas.
These compounds cause a variety of dental lesions in the incisors
and/or molars (5,8–10).
Among these chemicals, N-ethyl-N-nitrosourea (ENU)
and N-methyl-N-nitrosourea (MNU) have deleterious
effects on odontogenic tissues, resulting in tooth deformation and
malocclusion and eventually odontogenic tumors in rats (9,11–17)
and hamsters (8,15,18–20).
Epithelial rests of Malassez (ERM) were first
described by Malassez in 1884. In studies of human teeth, Malassez
noted that ERM form a network around the tooth root and that the
number of ERM decreases with age (21). ERM are quiescent epithelial
remnants of Hertwig’s epithelial root sheath that remain in the
periodontal ligament throughout life (22–24);
the exact function of these structures has not been clarified. ERM
subcultured with primary dental pulp cells can differentiate into
ameloblast-like cells and generate enamel-like and/or dentin-like
tissues (25). ERM have the
potential to proliferate in response to appropriate extracellular
signals such as mitogens, and the proliferation of ERM may lead to
the formation of odontogenic tumors or cysts (26,27).
However, the changes that occur in ERM during the process of
odontogenic tumor development have not been closely examined. The
present study focuses on the changes in ERM during the development
of odontomas in the molar region and the morphological and
immunohistochemical characterization of these lesions induced by a
single intraperitoneal injection of MNU into female Lewis rats.
Materials and methods
Animals
A total of 43 3-week-old female Lewis rats
[LEW/CrlCrlj] were purchased from Charles River Japan (Atsugi,
Japan). All rats were housed 4–5 in a plastic cage with paper
bedding (Paper Clean, SLC, Hamamatsu, Japan) in a temperature-
(22±2°C) and humidity- (60±10%) controlled animal room under a 12-h
light/dark cycle.
Experimental procedures
After an acclimatization period of 1 week, rats were
divided into two groups. A total of 25 rats received an
intraperitoneal injection of 50 mg/kg MNU (Sigma, St. Louis, MO,
USA). MNU was dissolved in physiologic saline containing 0.05%
acetic acid immediately prior to injection. The control group
consisted of 18 rats that were injected with vehicle only
(physiologic saline containing 0.05% acetic acid). All rats were
fed a commercial pellet diet (CMF 30 kGy, Oriental Yeast, Chiba,
Japan) and had ad libitum access to water throughout the
experiment. During the experiments, clinical signs were observed
once a day, and body weight was measured once a week. At 12 and 18
weeks of age, 5 randomly selected rats in the control group and 6
in the MNU-treated group were sacrificed. The remaining rats in the
control group (n=8) and the MNU-treated group (n=13) were
sacrificed at 30 weeks of age. Complete necropsies were conducted
on all animals. All procedures involving animals were approved by
the Animal Experimentation Committee of Kansai Medical
University.
Tissue sampling
The rats were anesthetized with isoflurane
(Forane®; Abbot Japan, Tokyo, Japan), and the left and
right sides of the mandible and maxilla were separately dissected
from the surrounding tissues. The collected tissue was immersed in
10% neutral buffered formalin for one week and demineralized in a
10% EDTA solution (pH 7.0–7.3; Osteosoft®, Merk KGaA,
Darmstadt, Germany) at room temperature for 4–6 weeks. The samples
were then dehydrated with graded ethanol and embedded in paraffin.
Sagittal sections were cut in the mesiodistal direction and
included the first, second and third molars and the incisors, as
previously described (28).
Sections were stained with hematoxylin and eosin (H&E) or used
for immunohistochemistry. ERM of the first, second, and third
molars and the induced tumors were histopathologically evaluated.
Histopathological and immunohistochemical evaluations were reviewed
by a toxicologic pathologist certified by the Japanese Society of
Toxicologic Pathology and/or by the International Academy of
Toxicologic Pathology (K.Y. and A.T.), according to the previously
defined histopathological terminology and diagnostic criteria
(2,29,30).
Immunohistochemistry
The labeled streptavidin biotin (LSAB) technique was
performed with an LSAB staining kit (Dako, Carpinteria, CA, USA).
The following antibodies were used: rabbit anti-tyrosine receptor
kinase A (TrkA) polyclonal antibody (sc-118, 1:50 dilution; Santa
Cruz Biotechnology, Santa Cruz, CA, USA) as a nerve growth factor
receptor in periodontal ligament epithelium (31,32);
mouse anti-human CK14 monoclonal antibody (clone LL002, 1:20
dilution; Leica, Microsystems, Wetzlar, Germany) as a basal cell
keratin marker (25); mouse
anti-human p63 monoclonal antibody (clone 4A4, 1:50 dilution; Dako,
Glostrup, Denmark) as an epithelial stem cell marker in oral tissue
(33–38); and rabbit anti-porcine amelogenin
polyclonal antibody (raised by T.U., 1:5000 dilution) (39,40)
as an ameloblast marker (41,42).
Each primary antiserum or antibody was incubated overnight at 4°C
without antigen retrieval. The reaction products were visualized
using 3-3′-diaminobenzidine tetrahydrochloride.
Morphometric analysis
H&E-stained sections of the jaw were scanned
with a high-resolution digital slide scanner (NanoZoomer 2.2
Digital Pathology, Hamamatsu Photonics, Hamamatsu, Japan) to
prepare digital images. The ndpi image files were opened in color
mode with NDP.view software (Hamamatsu Photonics), and the area of
ERM was measured in both sides of the maxillary and mandibular
jaws; the number of ERM was counted in the cervical and furcation
regions of the first, second, and third molars of the mandible and
maxilla. An experimental dentist (A.K.) performed morphometric
analysis using Image J Windows software (National Institutes of
Health, Bethesda, MD, USA).
Statistical analysis
All discrete values are expressed as mean ± standard
error (SE) and were analyzed with the two-tailed independent
Student’s t-test for unpaired samples after confirming the
homogeneity of variance. The statistical analysis was used to
examine the significance of differences in the number and the area
of ERM between MNU-treated rats and control rats at each time point
and between different time points. P-values <0.05 were
considered to indicate statistical significance.
Results
General remarks
None of the animals died during the study period.
Body weight gain was lower in the MNU-treated rats than in the
control rats (data not shown). By the age of 30 weeks, the
MNU-treated rats developed mammary tumors (data not shown).
Morphological analysis of proliferative
changes in molars
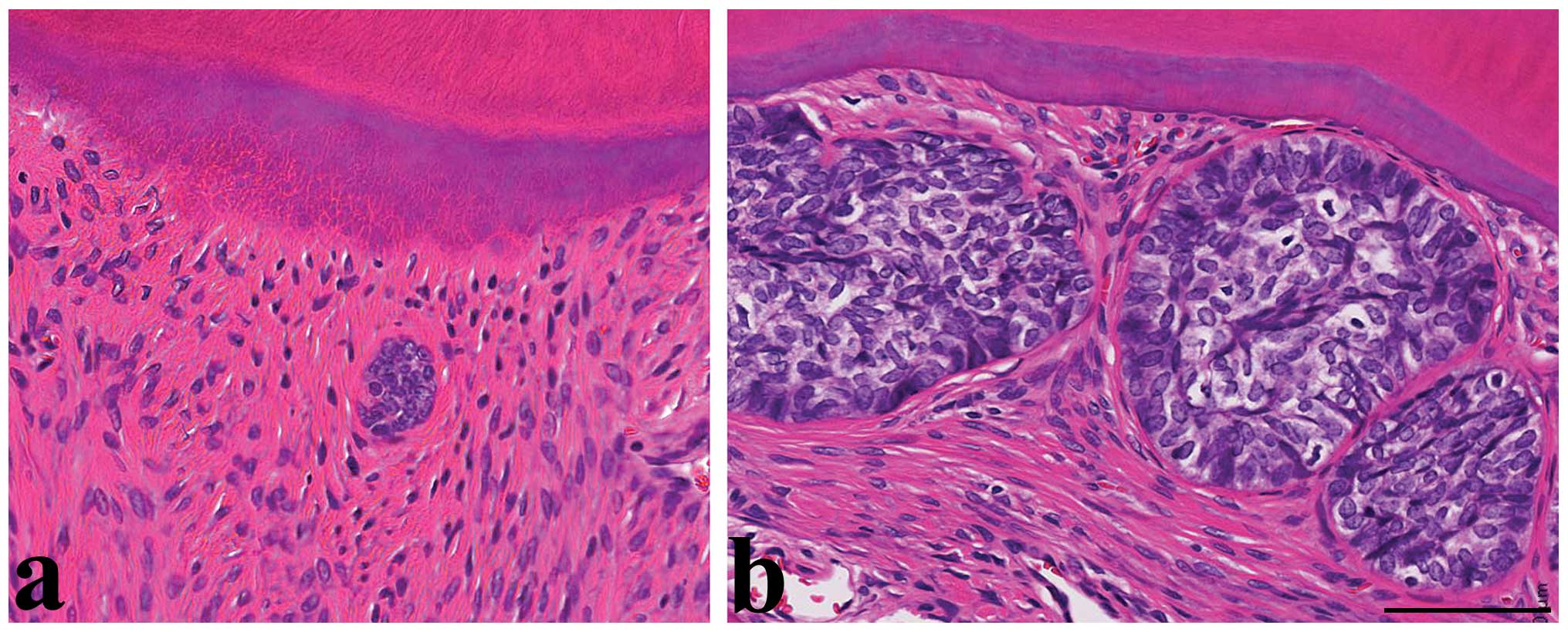
In the MNU-untreated control rats, ERM were observed
in the cervical and furcation regions of the first, second, and
third mandibular and maxillary molars and were characterized by a
high nuclear/cytoplasmic ratio and condensed nuclei (Fig. 1a). Moreover, the size of the ERM
remained small, and there were no neoplastic changes in the
periodontal ligament at any time point. The ERM in MNU-treated
animals at 12, 18, and 30 weeks of age had similar morphology to
the ERM in control rats, but the size of the ERM gradually
increased after MNU treatment (Fig.
1b). No odontogenic tumors were detected at 12 or 18 weeks of
age. At 30 weeks of age, odontogenic tumors were found in three of
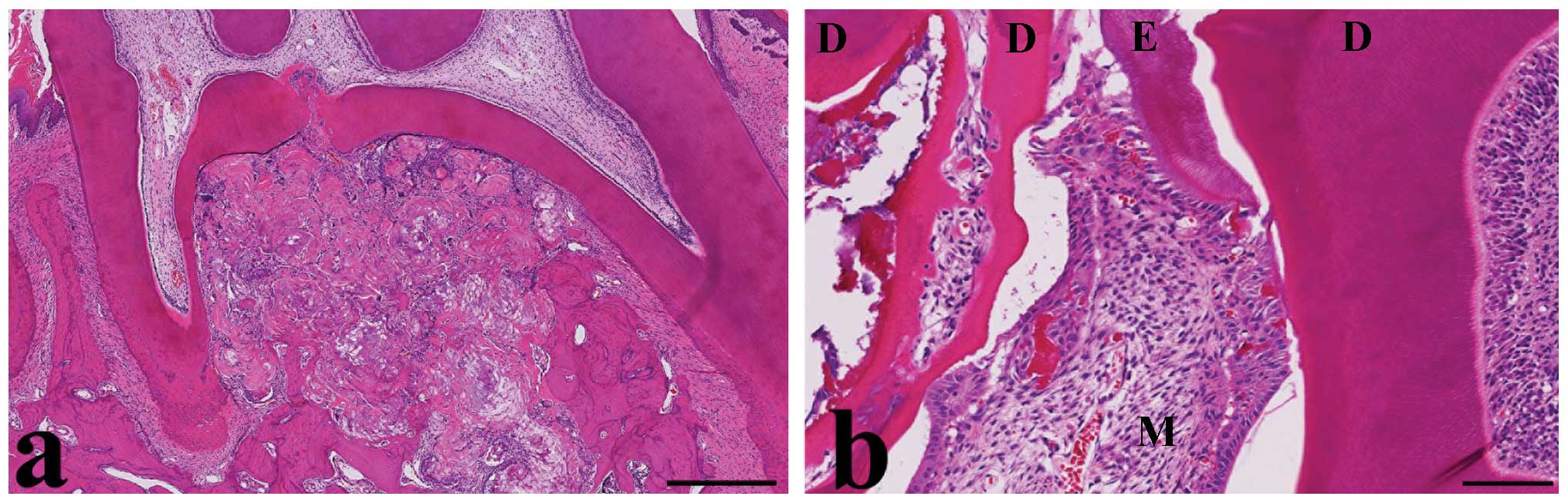
13 MNU-treated rats (23% incidence). These tumors were located near
the third mandibular molars. Two odontogenic tumors appeared in the
cervical and furcation regions, indicating molar origin (Fig. 2a). These tumors contained a mixture
of ameloblast-like cells with enamel-like tissue, odontoblast-like
cells with multinucleated giant cells and dentin-like tissue, and
dental pulp-like mesenchymal cells (Fig. 2b). The third tumor had similar
morphology. It was difficult to determine the origin of this tumor,
but it was located at the base of the incisor, indicative of
incisor origin. The morphology of the three MNU-induced tumors was
indicative of odontoma (complex type).
Morphometrical analysis of epithelial
rests of Malassez (ERM)
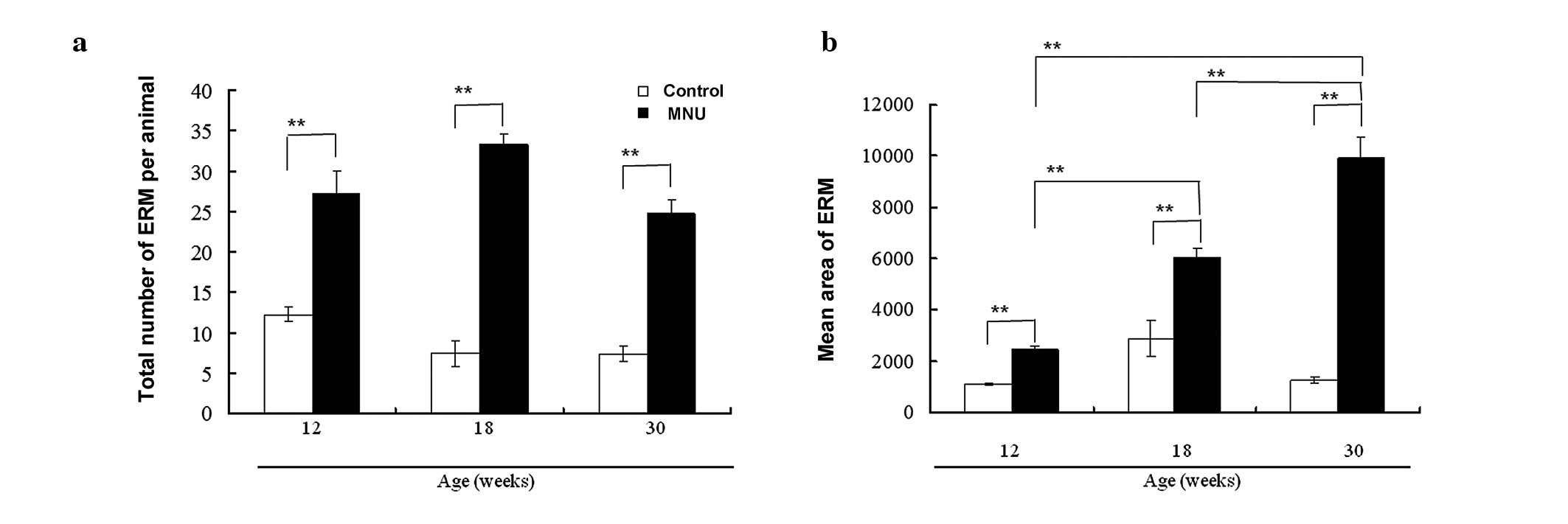
The number of ERM per rat remained low in control
rats (12.2, 7.4, and 7.3 at 12, 18, and 30 weeks, respectively),
but was significantly higher in the MNU-treated rats at each time
point (27.2, 33.2, and 24.7 at 12, 18, and 30 weeks, respectively)
(Fig. 3a). Although there was no
time-dependent increase in the number of ERM in the control or
MNU-treated groups, MNU-treated rats had significantly larger ERM
as compared with controls, and there was a time-dependent increase
in the mean area of ERM (Fig. 3b)
(1108, 2878, and 1268 μm2 in control groups vs. 2428,
6061, and 9930 μm2 in MNU-treated groups at 12, 18, and
30 weeks, respectively). This discrepancy between the number and
area of ERM may be due to the fusion of enlarged ERM.
Immunohistochemical analysis of
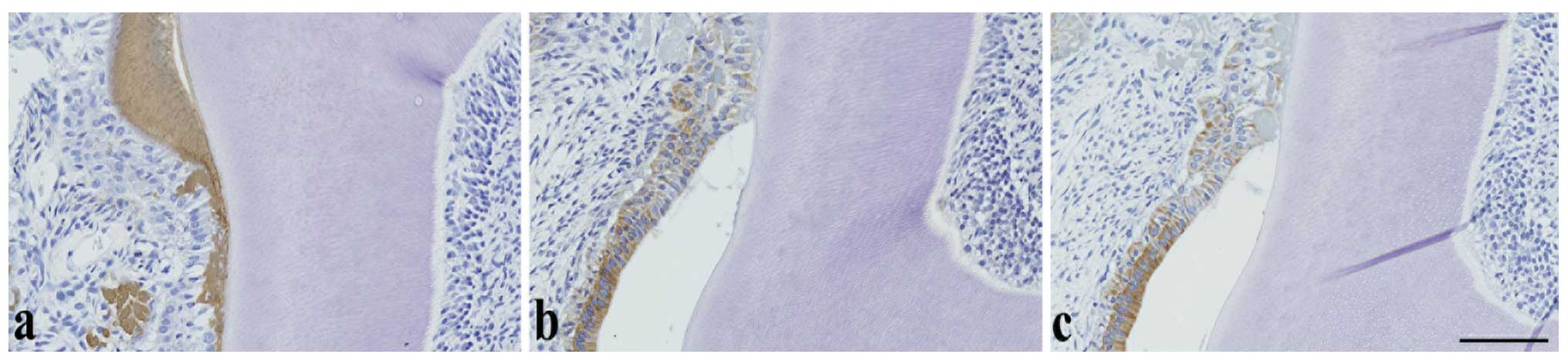
proliferative changes in molars
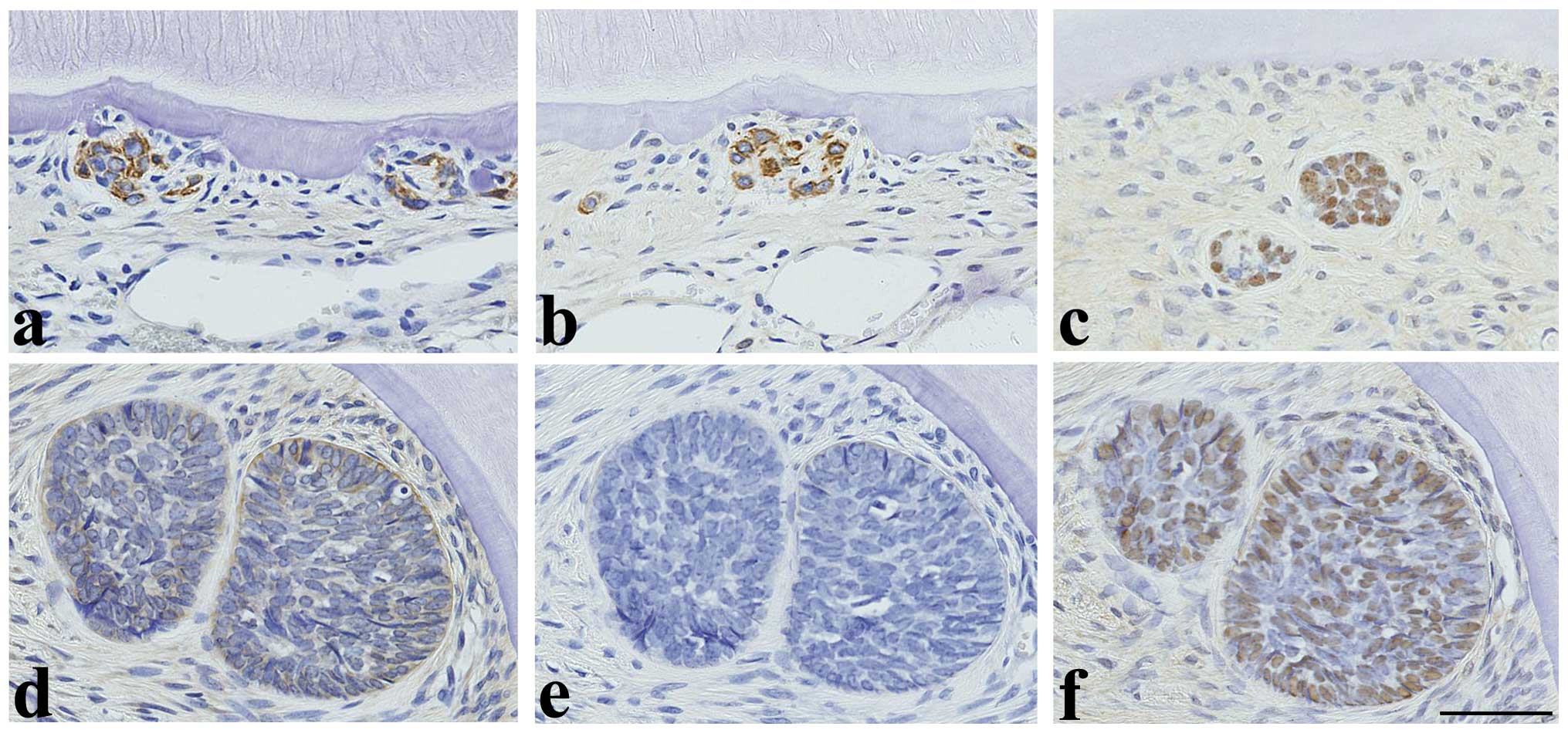
Regardless of MNU treatment, TrkA (Fig. 4a) and CK14 (Fig. 4b) immunoreactivity was observed in
the cell cytoplasm and p63 immunoreactivity was observed in the
cell nuclei (Fig. 4c) of the
normal or relatively small ERM in the cervical and furcation
regions of the molars, whereas amelogenin was negative (figure not
shown). However, in the larger ERM observed following MNU
treatment, TrkA and CK14 expression decreased or disappeared
(Fig. 4d and 4e), whereas p63 expression (Fig. 4f) remained constantly high and
amelogenin remained negative (figure not shown). In tumors, the
expression of amelogenin (Fig.
5a), TrkA (Fig. 5b), and CK14
(Fig. 5c) was detected in the
cytoplasm of ameloblast-like cells and cells with tall columnar or
cuboidal-shaped cells (secretory ameloblasts). Amelogenin was also
expressed in enamel-like tissue, whereas p63 was negative. None of
the antigens examined were expressed in odontoblast-like cells,
dentin-like tissue or dental pulp-like mesenchymal cells.
Discussion
The present study examines changes in ERM as a
precursor to odontogenic tumor development in the molar region
following a single intraperitoneal injection of MNU into female
Lewis rats. The number of ERM in MNU-treated rats was significantly
higher at all time points as compared to control rats. The area of
ERM in the MNU-treated rats was significantly larger than in the
control rats, and it increased in a time-dependent manner. Finally,
odontomas were observed in the molar and incisor regions in
MNU-treated rats at 30 weeks of age.
Herrold was the first to induce odontogenic tumors
in animals by exposure to MNU (18). Morphologically, MNU-induced tumors
in Syrian hamsters resembled human ameloblastomas. Rats were later
found to be susceptible to MNU-induced odontogenic tumors (15). MNU-induced odontogenic tumors in
rodents are derived from the ameloblasts of the continuously
erupting incisors, which is located adjacent to the root of the
incisors (12,14,17,26).
A single injection of 150 mg/kg MNU causes death in approximately
one third of rats in three weeks. Younger rats (6 weeks old or
younger) were more susceptible to MNU than older rats (8 weeks
old), and the susceptibility to the development of odontogenic
lesions appears to end at 8 weeks of age (17). Local and multiple injections of
ENU, a chemical closely related to MNU, coupled with mechanical
injuries (incisor wounding) were found to accelerate the production
of odontogenic tumors in the incisor region (9), which indicates that promoter stimuli
may be required for a high yield of odontogenic tumors. Odontogenic
tumors in the molar region occur in the cervical and furcation
regions (but not in the apical region) where ERM always remain as a
result of local administration of MNU mixed with alginate
impression material to preserve MNU at the treatment site (26). Due to the similarity in locations
of ERM and tumors, enlarged ERM may have acquired neoplastic
characteristics. In contrast to tumors derived from ameloblasts of
incisors, tumors derived from ERM of multi-rooted rat molars are
similar to human tumors. However, to the best of our knowledge, it
has not been previously reported that a single systemic
administration of MNU causes odontogenic tumors in the molar
region. In the present study, odontogenic tumors developed in the
molar regions of female Lewis rats treated with MNU.
In general, odontogenic tumors are rarely
encountered as spontaneous lesions in rodents (1,5,43).
In Tg.AC mice, a mouse line created in the FVB/N background by
pronuclear injection of v-Ha-ras oncogene, the incidences of
odontogenic tumors (odontoma) were 16% in a 26-week study and 35%
in a 1-year study (5,44). In other transgenic mice, such as
Hedgehog transcriptional effector Gli2-overexpressed mice,
keratocystic-type odontogenic tumors arise from proliferative ERM
(45). In Smad4-gene
knockout mice (Smad4Co/Co OC-Cre), a
similar type of odontogenic tumor arises from proliferative ERM
(46). In the present study, the
incidence of odontogenic tumors was 23% at the age of 30 weeks,
which is compatible with the incidence in Tg.AC mice.
Several genetic and molecular alterations appear to
promote the development and progression of odontogenic tumors via
multiple steps associated with tooth development, bone metabolism,
and the malignant potential of tumors (6). In an in vitro study, ERM in
combination with dental pulp cells generate enamel-like or
dentin-like tissues in a similar manner to cervical loop epithelial
cells (25). ERM have
characteristics of stem/progenitor cells (36). Morphological continuity exists
between ERM and the induced tumors in rats treated with local
administration of MNU (26). TrkA
(47), CK14 (25), and p63 (48) are markers for ERM in the
periodontal ligament. p63 is a candidate epithelial stem cell
marker in oral tissues (33–38).
TrkA, CK14 and p63 were constantly expressed in small ERM of
control rats, although TrkA and CK14 expression tended to decrease
and disappear, respectively, in the larger ERM of MNU-treated rats.
p63 was consistently detected in ERM of various sizes. However, ERM
of various sizes were negative for amelogenin (25,49).
In the present study, the expression of TrkA and CK14 in large ERM
was different from that in normal-sized ERM. The decreased levels
of expression may corroborate with the change at the early
transition or dedifferentiation stage of odontogenic
carcinogenesis.
MNU-induced tumors were diagnosed as odontomas
(complex type), since they contained dentin-like, enamel-like, and
dental pulp-like mesenchymal cells (2,30).
Amelogenin is not detected in ERM (49), but it is detected in the cytoplasm
of the tall columnar odontogenic epithelium, stellate
reticulum-like cells, and their associated extracellular components
together with enamel-like tissue in epithelial odontogenic tumors
(50). In the present study,
amelogenin was detected in the enamel-like matrix, in secretory
ameloblasts adjacent to enamel-like matrix, and in cells assumed to
be ameloblasts, but not in dentin-like or dental pulp-like
mesenchymal cells. Amelogenin-CK14 interactions in ameloblasts
occur during enamel formation (51) and cultured ERM express amelogenin
and CK14 (25). Epithelial
odontogenic tumors express various degrees of amelogenin and CK14
(50,52). Co-expression of amelogenin and CK14
may be a characteristic of epithelial odontogenic tumors,
indicating that these tumors have ameloblastic differentiation or
odontogenic epithelial properties. In addition, TrkA was expressed
in tumor cells with ameloblastic differentiation, although the
reasons for this are unclear. p63 is essential for tooth
development (53) and is expressed
in dental germ cells (35),
ameloblasts (37), and various
odontogenic tumors including ameloblastomas (48,54).
Although p63 was consistently detected in ERM of various sizes, the
compound odontomas in the present study were p63-negative. p63
expression correlates to malignancy in odontogenic tumors (48). In conclusion, a single
intraperitoneal injection of MNU resulted in the development of
odontogenic tumors with mixed enamel and dentin differentiation
(complex odontomas) in the molar region, and these tumors may have
been derived from ERM. Detailed mechanistic investigations of the
molecular basis of odontogenic carcinogenesis are required to
further elucidate the relationship between ERM and odontogenic
tumors in rodents and humans.
Acknowledgements
The authors thank Ms. T. Akamatsu for
her technical assistance and Ms. A. Shudo for manuscript
preparation. This work was supported in part by a grant-in-aid for
Scientific Research (C) (22591954).
References
|
1
|
Brown HR and Hardisty JF: Oral cavity,
esophagus, and stomach. Pathology of the Fischer Rat: Reference and
Atlas. Boorman GA, Montgomery CA Jr and MacKenzie WF: Academic
Press; San Diego: pp. 9–30. 1990
|
|
2
|
Long PH and Leininger JR: Teeth. Pathology
of the Mouse: Reference and Atlas. Maronpot RR, Boorman GA and Gaul
BW: Cache River Press; Vienna: pp. 13–28. 1999
|
|
3
|
Abe T and Miyajima H: Effect of drugs on
developing enamel in rat incisors. J Toxicol Pathol. 3:245–256.
1990.(Abstract in English, text in Japanese).
|
|
4
|
Kuijpers MH, van de Kooij AJ and Slootweg
PJ: The rat incisor in toxicologic pathology. Toxicol Pathol.
24:346–360. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Weber K: Induced and spontaneous lesions
in teeth of laboratory animals. J Toxicol Pathol. 20:203–213. 2007.
View Article : Google Scholar
|
|
6
|
Kumamoto H: Molecular pathology of
odontogenic tumors. J Oral Pathol Med. 35:65–74. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Takeda Y: Histogenesis and clinical
pathology of odontogenic tumors. Dent J Iwate Med Univ. 31:143–152.
2006.(In Japanese).
|
|
8
|
Edwards MB: Some effects of
N-methylnitrosourea on the oral and odontogenic tissues of the
Syrian golden hamster. Arch Oral Biol. 23:515–524. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Maeda H, Kameyama Y, Fujita K, Sato E and
Mizutani M: Experimental odontogenic tumors produced by
ethylnitrosourea injections and mechanical injuries. J Oral Pathol
Med. 20:296–299. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Satoh H, Usegi Y, Kawabata T, Mori K,
Fujii F and Kashimoto Y: Morphological classification of dental
lesions induced by various antitumor drugs in mice. Toxicol Pathol.
29:292–299. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Azuma T and Komori A: An experimental
study of odontogenic tumors in rats. Induction of tumors by gastric
intubation of MNU. Jpn J Oral Biol. 27:631–639. 1985. View Article : Google Scholar
|
|
12
|
Berman JJ and Rice JM: Odontogenic tumours
produced in Fischer rats by a single intraportal injection of
methylnitrosourea. Arch Oral Biol. 25:213–220. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Eblin H, Barbachan JJD, Do Valle JGC and
De Oliveira LY: N-Methyl-N-nitrosourea-induced odontogenic
neoplasms in rats. J Dent Res. 52:1771973. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Eisenberg E, Murthy AS, Vawter GF and
Krutchkoff DJ: Odontogenic neoplasms in Wistar rats treated with
N-methylnitrosourea. Oral Surg Oral Med Oral Pathol. 55:481–486.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gardner DG: Experimentally induced
ameloblastomas: a critical review. J Oral Pathol Med. 21:337–339.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Leaver DD, Swann PF and Magee PN: The
induction of tumors in the rat by a single oral dose of
N-nitrosomethylurea. Br J Cancer. 223:177–187. 1969. View Article : Google Scholar
|
|
17
|
Smulow J B, Konstantinidis A and
Sonnenschein C: Age-dependent odontogenic lesions in rats after a
single i.p. injection of N-nitroso-N-methylurea. Carcinogenesis.
4:1085–1088. 1983.PubMed/NCBI
|
|
18
|
Herrold KM: Odontogenic tumors and
epidermoid carcinomas of the oral cavity. An experimental study in
Syrian hamsters. Oral Surg Oral Med Oral Pathol. 25:262–272. 1968.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kohgo T: An experimental study of
odontogenic tumors in hamsters by N-nitrosomethylurea. J Stomatol
Soc Jpn. 39:191–212. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kohgo T, Iizuka T and Shindoh M:
Pathological evaluation of the effects of intentional disocclusion
and overloading occlusion in odontogenesis disorders in
N-methylnitrosourea-treated hamsters. Toxicol Pathol. 27:226–232.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wentz FM, Weinmann JP and Schour I: The
prevalence, distribution, and morphologic changes of the epithelial
remnants in the molar region of the rat. J Dent Res. 29:637–646.
1950. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bykov VL: Epithelial cell rests of
Malassez: tissue, cell, and molecular biology. Morfologiia.
124:95–103. 2003.(In Russian).
|
|
23
|
Kat PS, Sampson WJ, Wilson DF and Wiebkin
OW: Distribution of the epithelial rests of Malassez and their
relationship to blood vessels of the periodontal ligament during
rat tooth development. Aust Orthod J. 19:77–86. 2003.PubMed/NCBI
|
|
24
|
Huang X, Bringas P, Slavkin HC and Chai Y:
Fate of HERS during tooth root development. Dev Biol. 334:22–30.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shinmura Y, Tsuchiya S, Hata K and Honda
MJ: Quiescent epithelial cell rests of Malassez can differentiate
into ameloblast-like cells. J Cell Physiol. 217:728–738. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hamamoto Y, Hamamoto N, Nakajima T and
Ozawa H: Morphological changes of epithelial rests of Malassez in
rat molars induced by local administration of N-methylnitrosourea.
Arch Oral Biol. 43:899–906. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rincon JC, Young WG and Bartold PM: The
epithelial cell rests of Malassez - a role in periodontal
regeneration? J Periodontal Res. 41:245–252. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yagoto M, Shimojyo Y, Uotani Y, et al:
Methodology of histological preparation of rat teeth tissue
including incisor and molar. Jpn J Histotech. 9:29–32. 2000.(In
Japanese).
|
|
29
|
Long PH, Leininger JR and Lieuallen WG:
Non-proliferative lesions of bone, cartilage, tooth, and synovium
in rats. Guides for Toxicologic Pathology STP/ARP/AFIP Washington,
D.C.: 1996
|
|
30
|
Long PH, Leininger JR, Nold JB and
Lieuallen WG: Proliferative lesions of bone, cartilage, tooth, and
synovium in rats. Guides for Toxicologic Pathology STP/ARP/AFIP
Washington, D.C.: 1996
|
|
31
|
Kurihara H, Shinohara H, Yoshino H, Takeda
K and Shiba H: Neurotrophins in cultured cells from periodontal
tissues. J Periodontol. 74:76–84. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Woodnutt DA and Byers MR: Morphological
variation in the tyrosine receptor kinase A- immunoreactive
periodontal ligament. Arch Oral Biol. 46:163–171. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Brikic A, Mutlu S, Kocak-Berberoglu H and
Olgac V: Pathological changes and immunoexpression of p63 gene in
dental follicles of asymptomatic impacted lower third molars: an
immunohistochemical study. J Craniofac Surg. 21:854–857. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Casasco M, Cornaglia AI, Riva F, Calligaro
A and Casasco A: Expression of p63 transcription factor in
ectoderm-derived oral tissues. Int J Anat Embryol. 111:125–131.
2006.PubMed/NCBI
|
|
35
|
Kock M, Nolting D, Kjaer KW, Hansen BF and
Kjaer I: Immunohistochemical expression of p63 in human prenatal
tooth primordia. Acta Odontol Scand. 63:253–257. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nam H, Kim J, Park J, et al: Expression
profile of the stem cell markers in human Hertwig’s epithelial root
sheath/epithelial rests of Malassez cells. Mol Cells. 31:355–360.
2011.PubMed/NCBI
|
|
37
|
Rufini A, Weil M, McKeon F, Barlattani A,
Melino G and Candi E: p63 protein is essential for the embryonic
development of vibrissae and teeth. Biochem Biophys Res Commun.
340:737–741. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xiong J, Gronthos S, Krzysztof M and
Bartold PM: Characterisation and stem-cell properties of epithelial
cell rests of Malassez. In: Abstract in IADR/AADR/CADR 89th General
Session and Exhibition; San Diego, USA. 2011
|
|
39
|
Uchida T, Tanabe T and Fukae M:
Immunocytochemical localization of amelogenins in the deciduous
tooth germs of the human fetus. Arch Histol Cytol. 52:543–552.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Uchida T, Tanabe T, Fukae M, et al:
Immunochemical and immunohistochemical studies, using antisera
against porcine 25 kDa amelogenin, 89 kDa enamelin and the 13–17
kDa nonamelogenins, on immature enamel of the pig and rat.
Histochemistry. 96:129–138. 1991.PubMed/NCBI
|
|
41
|
Hasegawa N, Kawaguchi H, Ogawa T, Uchida T
and Kurihara H: Immunohistochemical characteristics of epithelial
cell rests of Malassez during cementum repair. J Periodont Res.
38:51–56. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nishio C, Wazen R, Kuroda S, Moffatt P and
Nanci A: Disruption of periodontal integrity induces expression of
apin by epithelial cell rests of Malassez. J Periodont Res.
45:709–713. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lewis DJ, Cherry CP and Gibson WA:
Ameloblastoma (adamantinoma) of the mandible in the rat. J Comp
Pathol. 90:379–384. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wright JT, Hansen L, Mahler J, Szczesniak
C and Spalding JW: Odontogenic tumors in the v-Ha-ras (TGAC)
transgenic mouse. Archs Oral Biol. 40:631–638. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Grachtchouk M, Liu J, Wang A, et al:
Odontogenic keratocysts arise from quiescent epithelial rests and
are associated with deregulated hedgehog signaling in mice and
humans. Am J Pathol. 169:806–814. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gao Y, Yang G, Weng T, et al: Disruption
of Smad4 in odontoblasts causes multiple keratocytic odontogenic
tumors and tooth malformation in mice. Mol Cell Biol. 29:5941–5951.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yamashiro T, Fujiyama K, Fukunaga T, Wang
Y and Takano-Yamamoto T: Epithelial rests of Malassez express
immunoreactivity of TrkA and its distribution is regulated by
sensory nerve innervation. J Histochem Cytochem. 48:979–984. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lo Muzio L, Santarelli A, Caltabiano R, et
al: p63 expression correlates with pathological features and
biological behaviour of odontogenic tumours. Histopathology.
49:211–214. 2006.PubMed/NCBI
|
|
49
|
Hamamoto Y, Nakajima T, Ozawa H and Uchida
T: Production of amelogenin by enamel epithelium of Hertwig’s root
sheath. Oral Surg Oral Med Oral Pathol Oral Radiol Endod.
81:703–709. 1996.PubMed/NCBI
|
|
50
|
Kumamoto H, Yoshida M and Ooya K:
Immunohistochemical detection of amelogenin and cytokeratin 19 in
epithelial odontogenic tumors. Oral Dis. 7:171–176. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ravindranath RM, Tam WY, Bringas P Jr,
Santos V and Fincham AG: Amelogenin-cytokeratin 14 interaction in
ameloblasts during enamel formation. J Biol Chem. 276:36586–36597.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Crivelini MM, de Araújo VC, de Sousa SO
and de Araújo NS: Cytokeratins in epithelia of odontogenic
neoplasms. Oral Dis. 9:1–6. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Rufini A, Barlattani A, Docimo R, et al:
p63 in tooth development. Biochem Pharmacol. 82:1256–1261. 2011.
View Article : Google Scholar
|
|
54
|
Kumamoto H, Ohki K and Ooya K: Expression
of p63 and p73 in ameloblastomas. J Oral Pathol Med. 34:220–226.
2005. View Article : Google Scholar : PubMed/NCBI
|