Introduction
Dendritic cells (DCs) have been found to be the most
potent professional antigen-presenting cells (APCs) to date and are
initiators of the immune response. They play central roles in the
induction, regulation and maintenance of anti-tumor immunity
(1). Human peripheral blood
mononuclear cells (PBMCs) can be induced into DCs in the presence
of granulocyte-macrophage colony-stimulating factor (GM-CSF) and
interleukin-4 (IL-4) and using this method a large amount of DCs
with high quality can be obtained for clinical application
(2). Biological therapy of tumors
based on DCs has been a focus of research, and extensive progress
has been made. Autogenous antigen-bearing DCs following in
vitro culture have been re-transfused to induce specific
immunity, which has been a routine method (3). Following culture for 5–7 days, PBMCs
can be induced into immature DCs which may be further induced into
mature DCs in the presence of inflammatory factors. The maturation
of DCs is critical for the function of DCs. Only mature DCs can
function to activate an immune response. Currently, the DC vaccine
used in clinical practice consists of mature DCs, and the
therapeutic efficacy is closely related to the degree of DC
maturation. Thus, induction of DC maturation is a key step in the
preparation of DC vaccine. Various cytokines including TNF-α, IL-1β
and PGE2, CD40L, LPS and CpG ODN have been applied to induce DC
maturation. In the present study, human PBMCs were induced into
immature DCs which were then induced to mature DCs using different
stimulants. Our study aimed to compare the effectiveness of these
various stimulants in inducing DC maturation and to identify an
optimal method for clinical preparation of DC vaccine.
Materials and methods
Collection of human PBMCs
Peripheral blood (50 ml) was collected from
volunteers aged 20–40 years and isolation of PBMCs was carried out
within 2 h.
Main reagents
RPMI-1640 powder containing L-glutamine (Gibco),
fetal bovine serum (FBS; Hangzhou Sijiqing Biotech Co., Ltd.),
penicillin-streptomycin (P-S; Gibco), Ficoll lymphocyte separation
solution (relative density, 1.077±1 g/l; Lymphoprep™), recombinant
human GM-CSF (rhGM-CSF), recombinant human IL-4 (IL-4), tumor
necrosis factor-α (TNF-α), recombinant human IL-6, recombinant
human IL-1β, CD40L (Pepro Tech), prostaglandin E2 (PGE2;
Cayman), LPS (Sigma), FITC-dextran (40,000 MW, Sigma),
phycoerythrin (PE)-conjugated mouse anti-human CD83 and HLA-DR,
fluorescein isothiocyanate (FITC)-conjugated mouse anti-human CD86
and CD80 fluorescence monoclonal antibody (BD Pharmingen) and human
IL-12(p40) ELISA kit (Dia Clone) were used in the present
study.
Main instruments
FACS Calibur flow cytometer, CellQuest analysis
system (B–D) and Wellscan Mk3 microplate reader (Labsystems Dragon)
were used in the present study.
Isolation of PBMCs
PBMCs were isolated using Ficoll density gradient
centrifugation. In brief, heparin-treated fresh blood was diluted
in normal saline of equal volume. Then, 9 ml of lymphocyte
separation solution was added to a 50-ml centrifuge tube followed
by addition of 15 ml of diluted blood. Centrifugation was carried
out at 20°C for 20 min at 250 x g. The mononuclear cells at the
interface were collected and washed in PBS twice. These cells were
used for DC induction.
Culture and maturation induction of
DCs
The PBMC density was adjusted to 5x109
cells/l with complete RPMI-1640 medium containing 10% inactivated
FBS, 2 mmol/l L-glutamine, 0.05 mmol/l 2-mercaptoethanol and 100
U/ml P-S solution. The cell suspension was added to a
25-cm2 flask followed by incubation at 37°C in an
atmosphere with 5% CO2 for 2 h. The suspended cells were
removed, and the adherent cells were maintained in complete
RPMI-1640 medium containing 1000 U/ml rhGM-CSF and 500 U/ml IL-4.
On Day 3, fresh medium containing rhGM-CSF and IL-4 was added. On
Day 6, the immature DCs were collected and seeded into a 24-well
plate at a density of 2x105/ml (1 ml/well). These cells
were further induced with rhGM-CSF and IL-4 and then divided into 5
groups: Group A, control group (cells were treated with rhGM-CSF
and IL-4 alone); Group B, CD40L group (cells were treated with
rhGM-CSF and IL-4 as well as 500 ng/ml CD40L); Group C, LPS group
(cells were treated with rhGMCSF and IL-4 as well as 1 μg/ml
LPS); Group D, TNF-α group (cells were treated with rhGM-CSF and
IL-4 as well as 1000 U/ml TNF-α); Group E, cocktail of cytokine
group (cells were treated with rhGM-CSF and IL-4 as well as 1000
U/ml TNF-α, 10 ng/ml IL-6, 10 ng/ml IL-1β and 1 μg/ml
PGE2). DCs were harvested 24 h later and subjected to
detection of pheno-types by flow cytometry. At the same time, the
supernatant was also collected and stored at −70°C for the
detection of IL-12p40.
Detection of maturation markers of DCs by
flow cytometry
The maturation markers (CD80, CD83, CD86 and HLA-DR)
of DCs were determined with direct immunofluorescence method. The
DC density was adjusted to 2–5x105 cells/ml. Then, 50
μl of cell suspension was mixed in 5 μl of antibody
working solution followed by incubation in the dark at 4°C for 30
min. After washing in PBS twice, cells were fixed in 450 μl
of 1% paraformaldehyde. Cells were re-suspended and then subjected
to flow cytometery (FACS Calibur flow cytometer). CellQuest
software was employed to calculate the positive expression rate of
these markers in 1x104 cells. Fluorescence-conjugated
isotype IgG was used in the negative control group.
Detection of endocytic activity of
DCs
The extent of FITC-dextran uptake reflects the
antigen uptake ability of DCs. Detection of endocytic activity of
DCs was performed according to the method developed by John et
al (4) with modification. DCs
were maintained for 7 days and then DCs were collected and cell
density was adjusted to 4x108 cells/l with RPMI-1640
medium containing 10% FCS. Then, 2x105 cells were mixed
in 0.5 mg/ml FITC-dextran solution followed by incubation at 4°C
and 37°C for 2 h. After washing in cold PBS twice, cells were
re-suspended in 1% paraformaldehyde and subjected to flow
cytometry. The mean fluorescence density represents the uptake
ability. In each group, cells were consistently incubated at 4°C as
a control and the fluorescence density was subtracted to avoid the
non-active uptake.
Detection of IL-12 secretion by DCs with
ELISA
A double-antibody sandwich enzyme-linked
immunosorbent assay (ELISA) was employed to measure the IL-12p40
content according to the manufacturer’s instructions. Following
visualization, absorbance (A) was measured at 450 nm in a
microplate reader. The IL-12 content was calculated according to
the standard curve. The lower limit in the detection of IL-12p40
was 20 pg/ml.
Mixed lymphocyte reaction
Allogeneic lymphocytes were used as effector cells.
These cells were seeded into a 96-well plate at a density of
2x105/well with the ratio of DCs to effector cells of
1:10. The control group included lymphocytes alone. Three wells
were included in each group. Incubation was performed at 37°C in an
atmosphere with 5% CO2 for 3 days. Then, 10 μl of
5 mg/ml MTT was added to each well followed by further incubation
for 4 h. The supernatant was removed and 100 μl of DMSO was
added to each well followed by gentle shaking. When the blue-violet
formazan crystals dissolved, absorbance was measured at 595 nm
(A595) 10 min later. The proliferation index (PI) was calculated as
follows: PI = Aexperiment/Acontrol.
Statistical analysis
Data are expressed as the mean ± standard deviation
(SD) and statistical analysis was carried out with SPSS version
10.0. Analysis of variance and correlation analysis were
performed.
Results
DC morphology
After 2-h culture of PBMCs, the adherent cells were
small and round mononuclear cells. On Day 1, after culture, the DCs
had a large volume and were adherent and pleomorphic. The suspended
cells increased over time. On Day 3, colonies were present. On Day
6, cells were still pleomorphic and the majority of cells were
suspended DCs. Following maturation induction, the mature DCs were
largely suspended and had obvious processes and large volume. These
cells were irregular and presented evidence of dendritic
processes.
Changes in the phenotype of DCs following
maturation induction
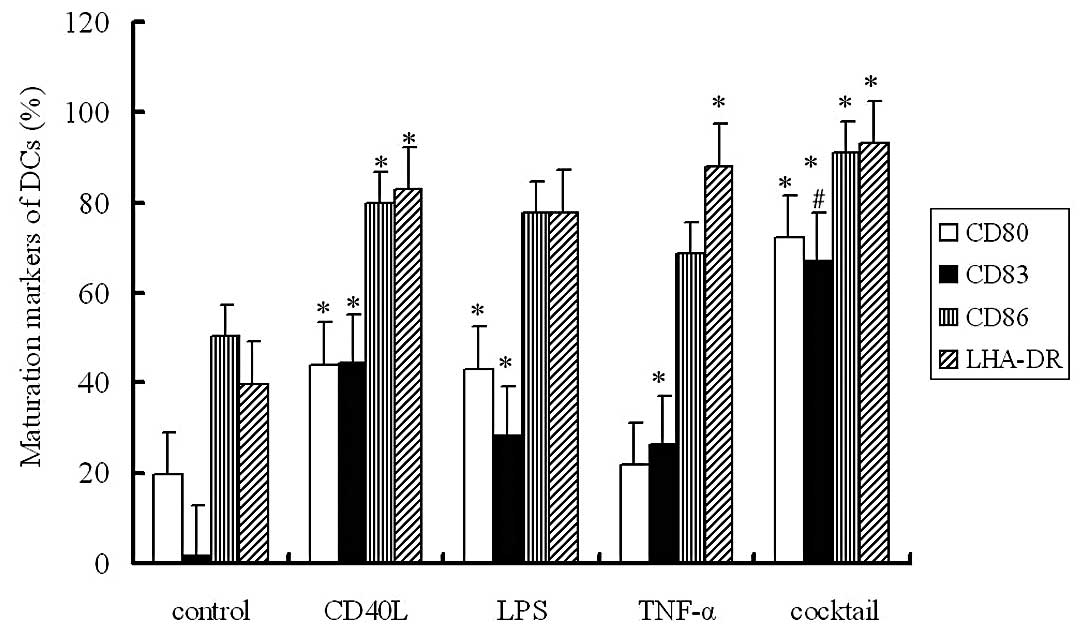
The expression levels of CD80, CD86, CD83 and HLA-DR
were determined by flow cytometry. Results showed the expression
levels of these markers in Groups B, C, D and E were markedly
increased when compared with levels in Group A. The expression of
CD83, a known marker of DC maturation, demonstrated the most
evident increase, and immature DCs almost had no CD83 expression.
In Group E, the maturation induction was the most efficient and the
expression of all markers was dramatically elevated. The mean
positive expression rate of CD83 was 66.91% in Group E. These
findings suggest that induction with a cocktail of cytokines is an
optimal method with which to induce DC maturation (Fig. 1)
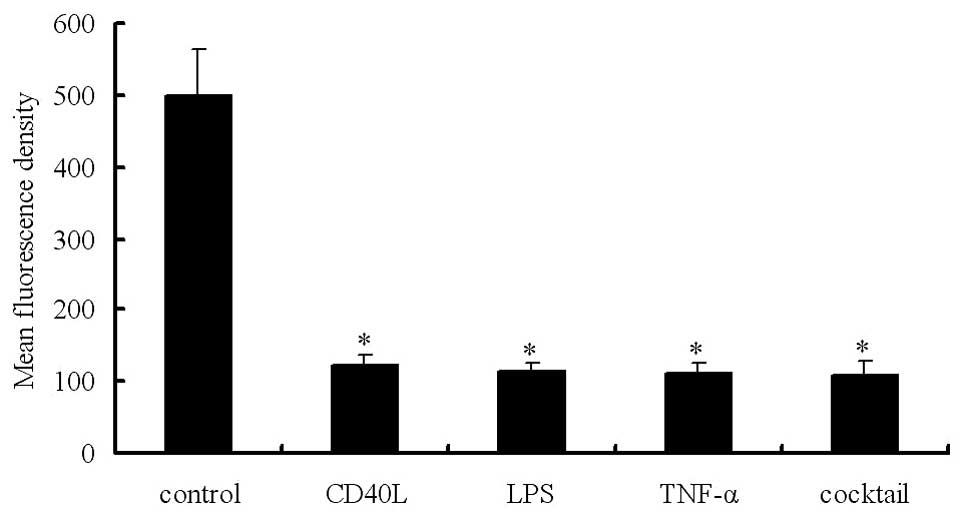
Mature DCs exhibit an obvious reduction
in endocytic activity
DCs have a mannose receptor on their surface which
mediates dextran phagocytosis. The extent of active uptake of
FITC-dextran reflects the antigen uptake ability of DCs. Flow
cytometry and measurement of FITC-dextran uptake demonstrated the
endocytic activity. In Groups A, B, C, D and E, the mean
fluorescence density was 499.04±63.40, 123.86±14.21, 113.17±13.68,
112.11±14.66 and 108.73±18.41, respectively. Our results showed
that the immature DCs exhibited potent endocytic activity, which,
however, was markedly reduced in mature DCs (Fig. 2). The endocytic activity was
negatively related to the expression levels of maturation markers
(P<0.05).
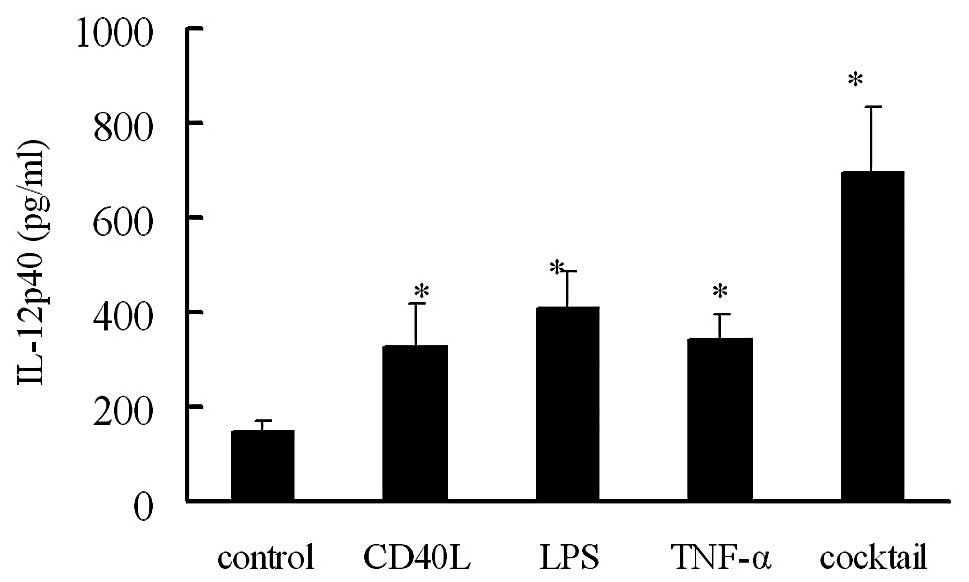
IL-12 secretion by DCs following
maturation induction using different methods
ELISA was employed to detect the IL-12p40 content in
the supernatant of DCs following maturation induction for 24 h. The
IL-12p40 content was calculated in 2x105/ml cell
suspension. In Groups A, B, C, D and E, the IL-12p40 content was
146.93±26.39, 330.76±87.23, 410.33±75.99, 342.45±52.60 and
693.65±138.52 pg/ml, respectively (Fig. 3). The IL-12p40 content in mature
DCs in Groups B, C, D and E were markedly higher than that in Group
A (P<0.05). The highest IL-12p40 content was found in Group E,
while there was no marked difference among Groups B, C, D and E
(P>0.05).
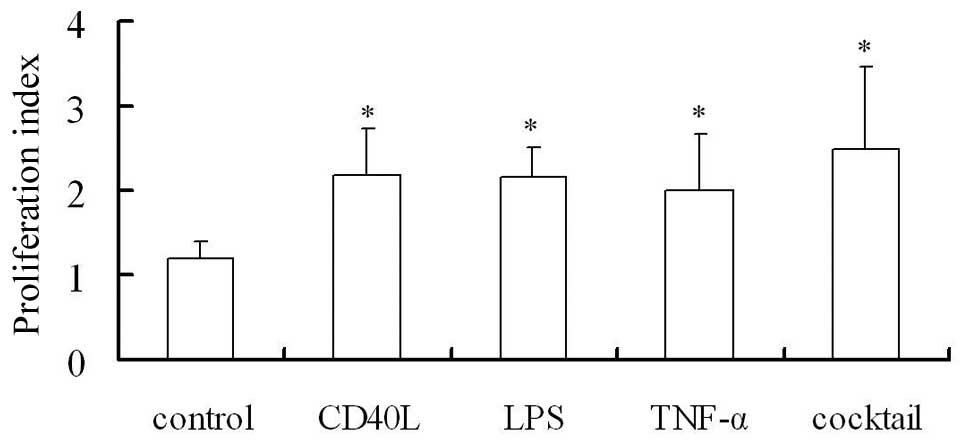
Mature DCs activate and promote
lymphocyte proliferation
Mature DCs demonstrate high expression of
costimulatory molecules and are potent to activate and stimulate
the proliferation of T lymphocytes. In the present study, MTT assay
was employed to measure proliferation (proliferation index of T
lymphocytes which were co-cultured with DCs). The proliferation
index in Groups B, C, D and E was markedly different from that in
Group A (P<0.05, Fig. 4). The
DCs in Group E (after treatment with the cocktail of cytokines)
exhibited the most potent ability to stimulate the proliferation of
DCs, which may be attributed to the favorable maturation of DCs and
high expression levels of costimulatory molecules on DCs.
Discussion
In 1973, Steinman and Cohn identified DCs in mouse
spleen which induced immune responses (5). In early 1990s, Chapuis et al
(2) first found that PBMCs could
be induced into DCs in the presence of IL-4 and GM-CSF, in which
PBMCs did not undergo proliferation and differentiation. The
differentiation and development of DCs have two distinct stages:
immature DCs and mature DCs. The immature DCs have weak ability to
stimulate the proliferation of naïve T cells and may induce the
disability of T cells. However, immature DCs can effectively
capture and process antigens. After antigen uptake or following
stimulation, immature DCs may become mature. Their antigen uptake
ability is compromised but the antigen-presenting ability is
enhanced. Mature DCs have high expression of MHC-I/II molecules,
costimul atory molecules (CD80 and CD86), adhesion molecules (such
as CD54) and other maturation markers (such as CD83) and can secret
multiple cytokines related to immunoregulation. Thus, these cells
acquire antigen-presenting ability and can stimulate the immune
response. Mature DCs have distinct characteristics when compared to
immature DCs. Only mature DCs can stimulate the proliferation of
lymphocytes and activate immune responses (6). Thus, induction of DC maturation has
been a key step in the preparation of DC vaccine.
Numerous methods have been developed to induce DC
maturation. Studies have reported that LPS, TNF-α, CD40L,
monocyte-conditioned medium (MCM) and a cocktail of cytokines
(TNF-α, IL-6, IL-1β and PGE2) can be applied to induce DC
maturation. LPS is a component of the cell wall of gram-negative
bacteria. LPS can induce DC maturation via the Toll-like receptor.
However, this is a bacterial reagent and infeasible for clinical
application. CD40L is a type II membrane glycoprotein and belongs
to the TNF superfamily. The CD40L can interact with CD40 on DCs to
induce DC maturation, reduce the antigen uptake ability of DCs,
promote the secretion of IL-12 by DCs and enhance the ability of
DCs to activate the proliferation of lymphocytes (7). MCM is a type of medium containing
several cytokines secreted by mononuclear cells including TNF-α,
IL-6 and IL-1β, and has reliable effectiveness in inducing DC
maturation (8). However, MCM
should be tailored to the individual patient in clinical use. In
addition, the preparation of MCM requires complex procedures and
needs the isolation of autogenous mononuclear cells. These
significantly limit the wide application of MCM in DC maturation
induction. TNF-α is a pro-inflammatory cytokine and is usually
applied to induce DC maturation. In the cocktail method, the
combination of cytokines aims to mimic the MCM in which TNF-α,
IL-6, IL-1β and PGE2 are used. This method was first reported by
Jonuleit et al in 1997 (9).
The cocktail method can stably and efficiently induce DC
maturation. To date, CD40L, TNF-α and a cocktail of cytokines have
been applied in clinical practice in several trials (10,11).
In the present study, the effectiveness of CD40L, TNF-α, LPS and
the cocktail of cytokines in inducing DC maturation was compared.
Our results showed these methods induced DC maturation in which the
cocktail of cytokines had the most potent ability to induce DC
maturation. The positive expression rate of CD83 was >60% in DCs
following treatment with the cocktail of cytokines, and the
expression levels of CD80, CD83 and HLA-DR were also higher than
those in the remaining groups. Our findings also demonstrated that
the DCs following induction with the cocktail of cytokines had the
highest IL-12 content and these cells had the most potent ability
to stimulate the proliferation of lymphocytes.
Taken together, in the present study, PBMCs were
employed to induce immature DCs in the presence of GM-CSF and IL-4,
and these immature DCs underwent maturation induction using
different methods. Our findings revealed that a cocktail of
cytokines stably and efficiently induced DC maturation, induced the
secretion of IL-12 by DCs and potently induced the DCs to stimulate
lymphocyte proliferation. The cytokines in the cocktail method
meets the criteria of the Good Manufacturing Practice (2012,
Ministry of Health, China) (12)
and the cocktail of cytokines is feasible for application in
clinical practice.
References
|
1
|
Reichardt VL, Brossart P and Kanz L:
Dendritic cells in vaccination therapies of human malignant
disease. Blood Rev. 18:235–243. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chapuis F, Rosenzwajg M, Yagello M, Ekman
M, Biberfeld P and Gluckman JC: Differentiation of human dendritic
cells from monocytes in vitro. Eur J Immunol. 27:431–441. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Banchereau J and Palucka AK: Dendritic
cells as therapeutic vaccines against cancer. Nat Rev Immunol.
5:296–306. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
John J, Hutchinson J, Dalgleish A and
Pandha HL: Cryopreservation of immature monocyte-derived dendritic
cells results in enhanced cell maturation but reduced endocytic
activity and efficiency of adenoviral transduction. J Immunol
Methods. 272:35–48. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Steinman RM and Cohn ZA: Identification of
a novel cell type in peripheral lymphoid organs of mice. I
Morphology, quantitation, tissue distribution. J Exp Med.
137:1142–1162. 1973. View Article : Google Scholar
|
|
6
|
Sheng KC, Pietersz GA, Wright MD and
Apostolopoulos V: Dendritic cells: activation and maturation -
applications for cancer immunotherapy. Curr Med Chem. 12:1783–1800.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brossart P, Grünebach F, Stuhler G,
Reichardt VL, Möhle R, Kanz L, et al: Generation of functional
human dendritic cells from adherent peripheral blood monocytes by
CD40 ligation in the absence of granulocyte-macrophage
colony-stimulating factor. Blood. 92:4238–4247. 1998.
|
|
8
|
Reddy A, Sapp M, Feldman M, Subklewe M and
Bhardwaj N: A monocyte conditioned medium is more effective than
defined cytokines in mediating the terminal maturation of human
dendritic cells. Blood. 90:3640–3646. 1997.PubMed/NCBI
|
|
9
|
Jonuleit H, Kühn U, Müller G, Steinbrink
K, Paragnik L, Schmitt E, et al: Pro-inflammatory cytokines and
prostaglandins induce maturation of potent immunostimulatory
dendritic cells under fetal calf serum-free conditions. Eur J
Immunol. 27:3135–3142. 1997. View Article : Google Scholar
|
|
10
|
Höltl L, Rieser C, Papesh C, Ramoner R,
Herold M, Klocker H, et al: Cellular and humoral immune responses
in patients with metastatic renal cell carcinoma after vaccination
with antigen pulsed dendritic cells. J Urol. 161:777–782. 1999.
|
|
11
|
Lee AW, Truong T, Bickham K, Fonteneau JF,
Larsson M, Da Silva I, et al: A clinical grade cocktail of
cytokines and PGE2 results in uniform maturation of
human monocyte-derived dendritic cells: implications for
immunotherapy. Vaccine. 20(Suppl 4): A8–A22. 2002.PubMed/NCBI
|
|
12
|
Berger TG, Feuerstein B, Strasser E,
Hirsch U, Schreiner D, Schuler G, et al: Large-scale generation of
mature monocyte-derived dendritic cells for clinical application in
cell factories. J Immunol Methods. 268:131–140. 2002. View Article : Google Scholar : PubMed/NCBI
|