Introduction
Statins inhibit 3-hydroxy-3-methylglutaryl-coenzyme
A reductase, which catalyzes the conversion of
3-hydroxy-3-methylglutaryl-coenzyme A to mevalonate, a
rate-limiting step in cholesterol synthesis (1). In addition to their efficacy for
cholesterol lowering, statins have been reported to have anabolic
effects on bone. A number of studies have demonstrated a
bone-promoting effect when simvastatin was applied locally with
different carriers in various animal models (2).
Previous studies have provided information regarding
effective dosage and certain underlying mechanisms (1,3). It
was shown that enhanced expression of bone morphogenetic protein-2
(BMP-2) mRNA is achieved by simvastatin, and that this may trigger
osteoblast differentiation (4).
However, the combined effects of simvastatin and BMP-2 on the
differentiation of osteoblasts have not been fully
investigated.
This study aimed to examine the dose-dependent
impact of simvastatin and BMP-2 on the differentiation of
osteo-precursor cells. In addition, the impact of the molecules on
cell viability was also evaluated. The alkaline phosphatase
activity (ALP) test was performed to assess differentiation, and
protein expressions related to bone formation, including that of
phospho-Smad1/5/8 (pSmad1/5/8), were measured using western blot
analysis to evaluate the underlying mechanism. To the author’s
knowledge, this investigation is the first to elucidate the
combined effect of simvastatin and BMP-2 on the expression of
pSmad1/5/8 in relation to osteoblast differentiation.
Materials and methods
Cell culture
Murine osteoprecursor (MC3T3-E1) cells were cultured
in α-minimum essential medium (αMEM) supplemented with 10% fetal
bovine serum and antibiotics (100 U/ml of penicillin and
streptomycin 100 μg/ml) (Invitrogen, Carlsbad, CA, USA). To
induce osteogenic differentiation, culture media were replaced with
osteogenic differentiation medium [αMEM supplemented with 50
μg/ml ascorbic acid and 10 mM β-glycerolphosphate (Sigma,
St. Louis, MO, USA)]. The cultures were maintained in a humidified
atmosphere with 5% CO2 and 95% air at 37°C. Simvastatin
was dissolved in dimethyl sulfoxide (DMSO; Sigma) and
filter-sterilized. The water used was distilled and deionized
(ddH2O). In order to minimize any difference in cellular
growth and differentiation between the controls and treated
cultures, an equal amount of DMSO was applied in the control and
treated cultures of each experiment.
Cellular proliferation
Cells were plated at a density of 1.0x104
cells 1 ml/well in 12-well plates, and the cultures were stimulated
with simvastatin and BMP-2 at final concentrations ranging from 0.1
to 1 μM for simvastatin and from 6 to 60 ng/ml for BMP-2,
respectively. The effects of simvastatin and BMP-2 on the cellular
proliferation of the osteoprecursor cells were assessed at day 5.
The 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide
(MTT) reagents (0.5 mg/ml) were then added, and the cells were
incubated for 1 h at 37°C. The mitochondrial activities of the
cells after various concentrations of simvastatin and BMP-2
treatments were determined by colorimetric assay, which detects the
conversion of MTT to an insoluble blue formazan product. The
solubilizing reagent, DMSO (1 ml) was added to all wells and mixed
thoroughly to dissolve the blue crystals. After ensuring that all
crystals were dissolved, the plates were read on a microplate
reader using a test wavelength of 560 nm against a reference
wavelength of 670 nm.
Alkaline phosphatase (ALP) activity
assays
The ALP assay for osteoblast differentiation was
performed on day 5 of culture. Cells that were pre-lysed in a
radioimmunoprecipitation assay buffer were sonicated for 20 sec at
4°C. The lysate was centrifuged at 14,000 rpm for 10 min at 4°C in
a microcentrifuge to remove cellular debris. An enzyme activity
assay was performed in an assay buffer containing 10 mM
p-nitrophenylphosphate as substrate.
Western blot analysis
Osteoprecusor cells were washed twice with ice-cold
phosphate-buffered saline and solubilized in lysis buffer. The
lysates were centrifuged at 14,000 rpm for 20 min at 4°C to remove
the nuclear pellet. The supernatants were boiled in a sodium
dodecyl sulfate sample buffer containing β-mercaptoethanol. Equal
quantities of cell extracts were separated using sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and transferred onto
polyvinylidene fluoride (PVDF) microporous membranes (Immobilon-P
membranes; Millipore Corporation, Billerica, MA, USA). Membranes
were then blocked for at least 1 h in 0.1% (v/v) phosphate-buffered
saline and Tween-20 (Tween-20/phosphate-buffered saline) containing
5% (w/v) powdered milk before being probed with the desired
antibodies diluted in the same buffer at the recommended
concentrations. The membrane was incubated with horseradish
peroxidase-conjugated secondary antibody. The washed blot was
developed using enhanced chemiluminescence reagent. Antibodies
against TGF-β1, pSmad1/5/8 and β-actin and secondary antibodies
linked with horseradish peroxidase were purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA), BD Pharmingen (San
Diego, CA, USA) and Santa Cruz Biotechnology (Santa Cruz, CA,
USA).
Statistical analysis
Results are expressed as the means ± SD of the
experiments and a one-way analysis of variance (ANOVA) was used to
determine differences between groups using a commercially available
program (PASW Statistics 18; SPSS Inc., Chicago, IL, USA).
Statistical significance was set at P<0.05
Results
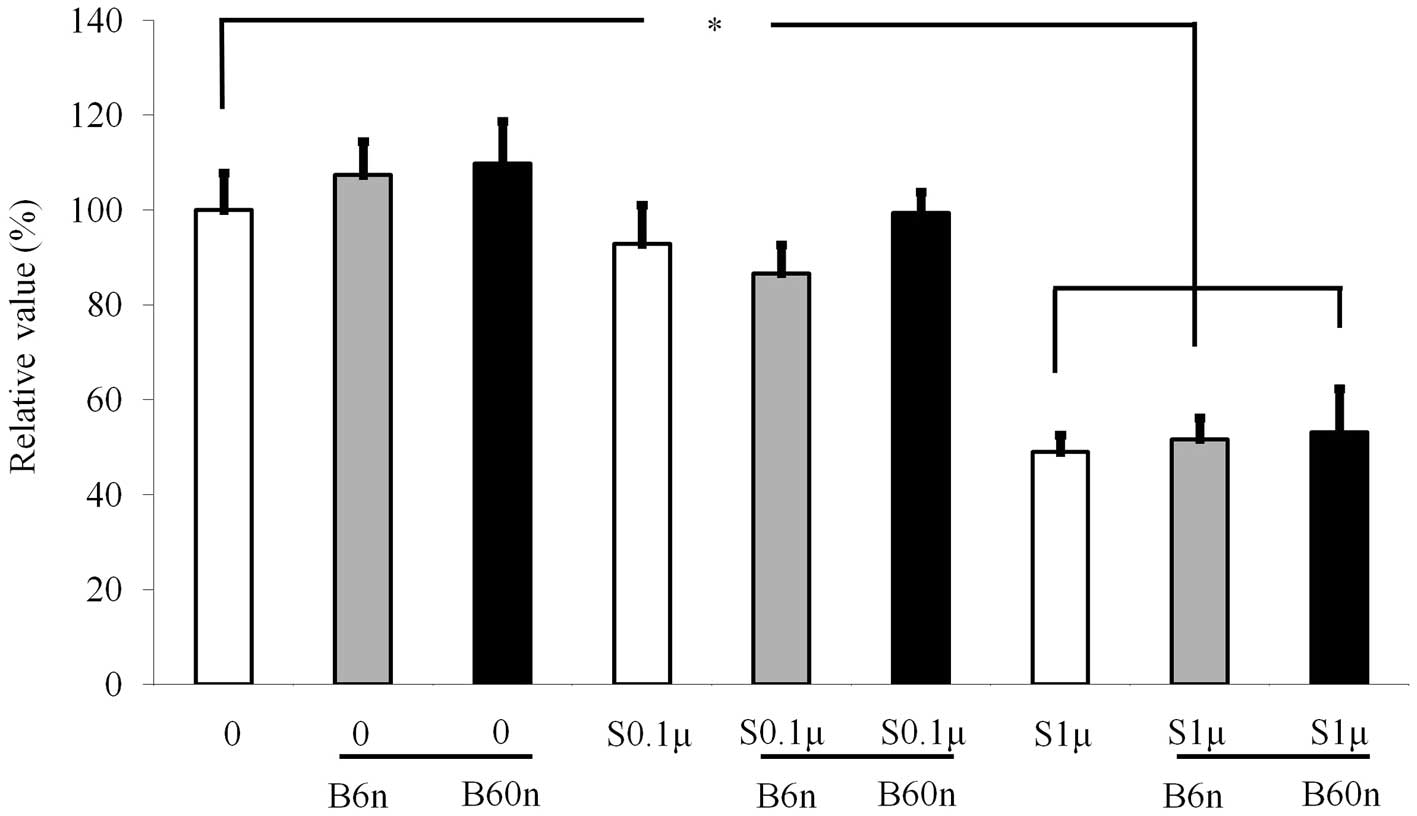
Cellular proliferation
Cultures growing in the presence of BMP-2 at 6 and
60 ng/ml did not show any changes in the MTT assays, and there were
no significant differences among the three groups (Fig. 1). However, addition of 1 μM
simvastatin significantly reduced the growth of the cells.
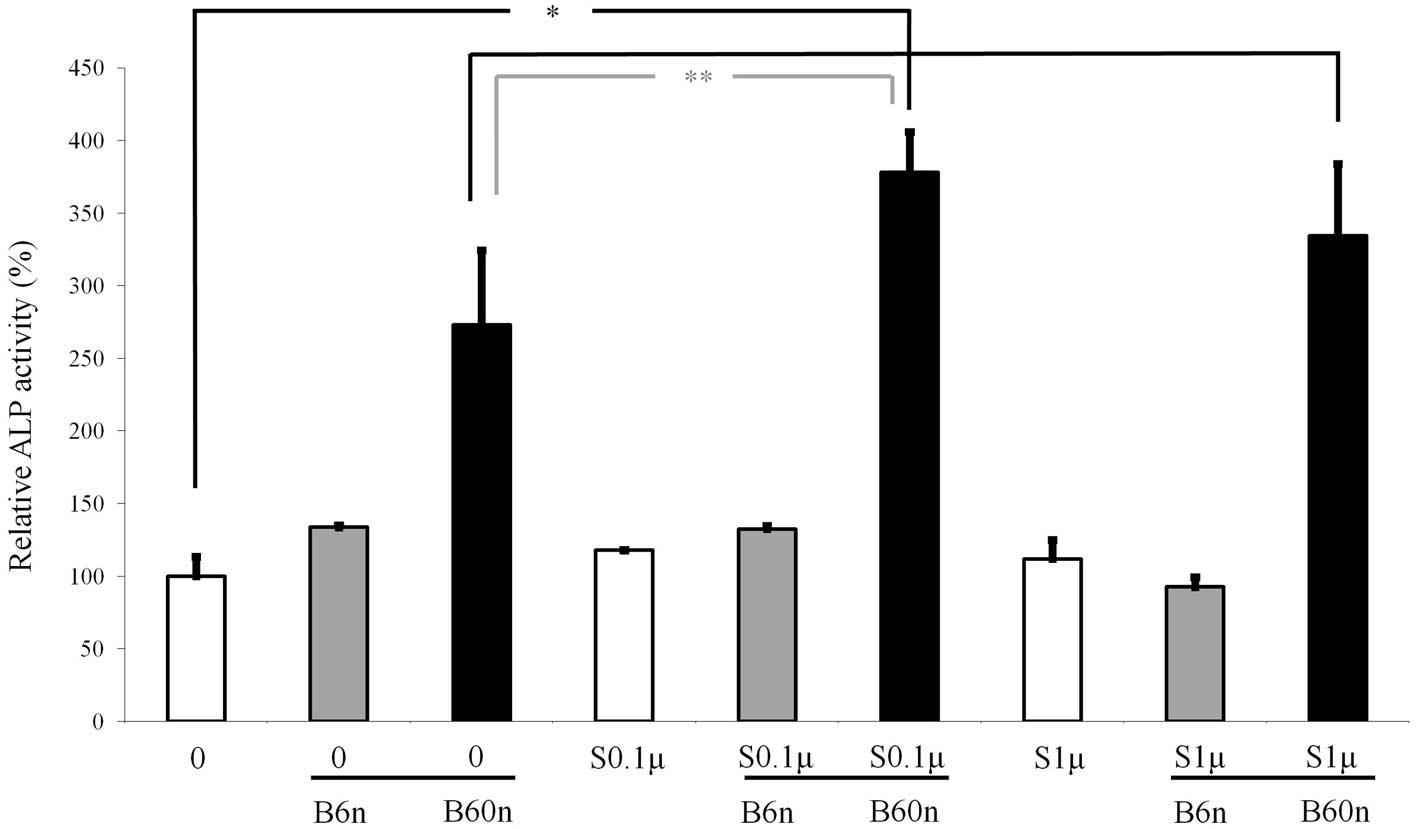
ALP activity assay
The ALP activity was increased when cells were
treated with simvastatin, with the highest value at 0.1 μM
(Fig. 2). The ALP activity
increased accordingly when 6 or 60 ng/ml BMP-2 was added to the
simvastatin-unloaded and 0.1 and 1 μM simvastatin groups,
with a significantly higher value observed at 60 ng/ml BMP-2.
Similarly, cultures grown in the presence of 60 ng/ml of BMP-2
displayed an increased ALP activity when 0.1 or 1 μM
simvastatin was added. The highest ALP activity was achieved when
0.1 μM simvastatin and 60 ng/ml BMP-2 were loaded
simultaneously.
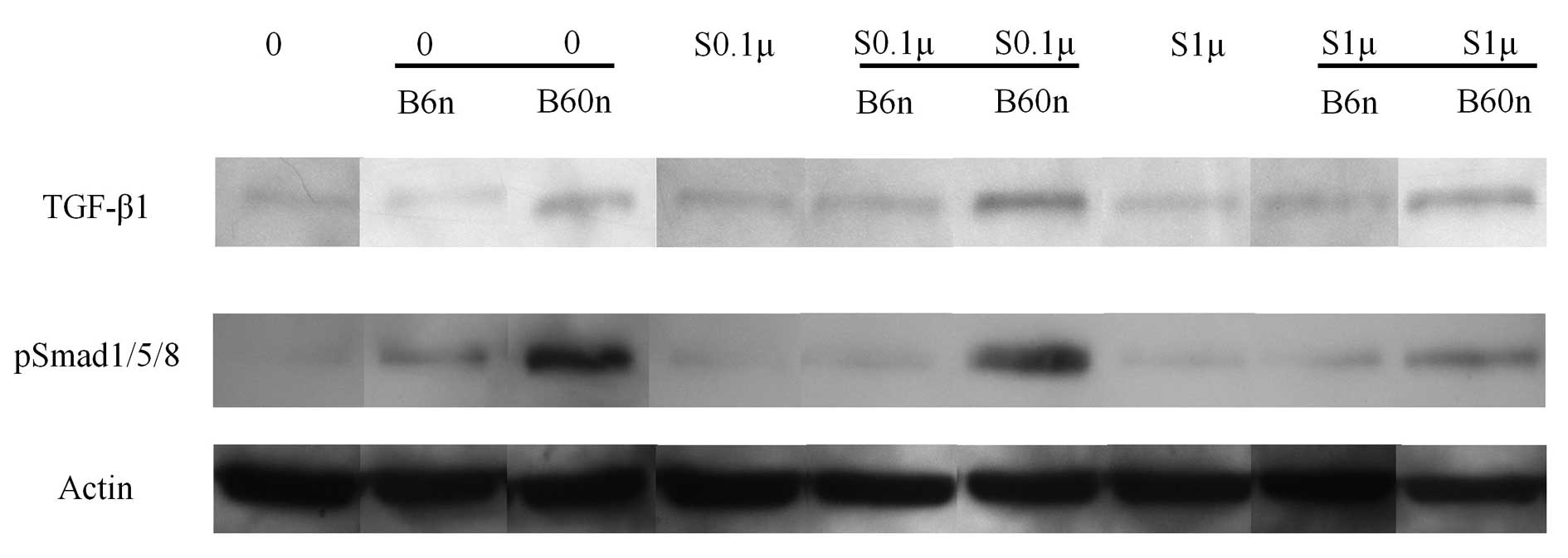
Western blot analysis
Western blot analysis was performed to detect the
protein expression following treatment with simvastatin and BMP-2.
The results showed that addition of BMP-2 amplified the expression
of pSmad1/5/8 (Fig. 3). Similarly,
the addition of simvastatin increased the expression of pSmad1/5/8
with the highest value observed in the 0.1 μM simvastatin
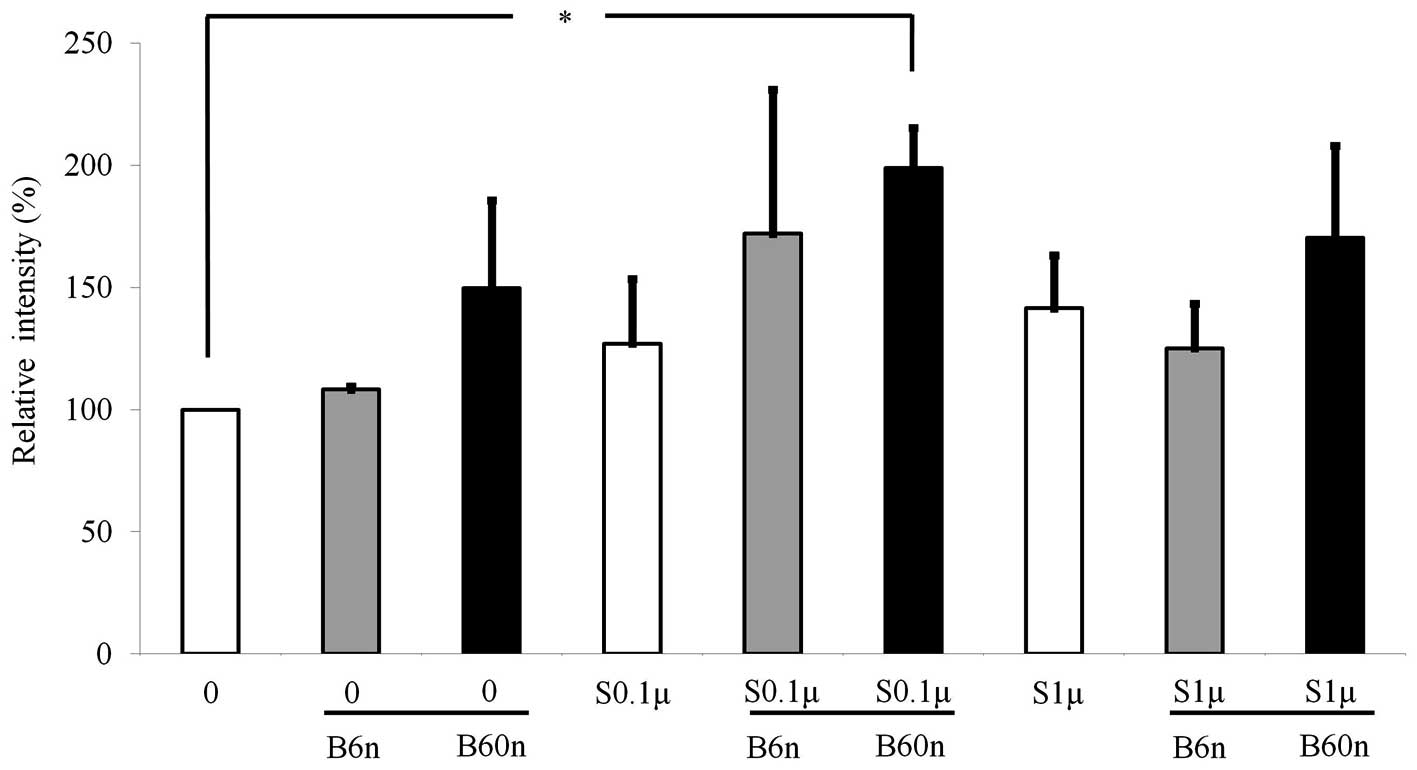
group. Normalization of the protein expression levels revealed that
the group treated with 60 ng/ml BMP-2 yielded 149.6±36.1%
pSmad1/5/8 expression. The expression of pSmad1/5/8 increased when
BMP-2 was co-delivered with simvastatin. The relative values of 0.1
μM simvastatin + 60 ng/ml BMP-2 and 1 μM simvastatin
+ 60 ng/ml BMP-2 were 198.8±16.5 and 170.4±37.6%, respectively
(Fig. 4).
Discussion
In this report, the combined effects of simvastatin
and BMP-2 on cell viability, differentiation and protein expression
of osteoblast progenitor cells were examined under predetermined
concentrations of simvastatin (0.1 and 1 μM) and BMP-2 (6
and 60 ng/ml). In addition, evaluations were carried out to
identify whether the combination of simvastatin and BMP-2 produces
effects additive, synergistical or competitive effects.
The MTT assay was used to evaluate cell viability,
since it is considered a sensitive assay with which to assess
osteoblast proliferation through the determination of mitochondrial
dehydrogenase activity (3,5). Equal amounts of DMSO were loaded to
each culture to offset the effect of the vehicle (3). The results indicated that there was
significant reduction in obtained MTT values, suggesting that 1
μM simvastatin may effect the number of cells in this
cultured condition. It can be suggested that high concentrations of
simvastatin may exert a toxic effect on the cells (6); however, there may be variations in
doses related to the deleterious effect, depending on the type of
cells, the stage of differentiation and the culturing period
(3,6,7).
ALP activity, which is an early marker of
osteoblastic cell differentiation, was used to evaluate the
osteoblast differentiation (8). In
previous experiments, significant effects of simvastatin on
osteoblast differentiation in MC3T3-E1 cells were observed at
concentrations of 0.01 and 0.1 μM (4). Similarly, a significant increase in
ALP activity was noted in the 0.1 μM simvastatin group. The
highest value was achieved when 60 ng/ml BMP-2 was loaded with 0.1
μM simvastatin. When 60 ng/ml BMP-2 or 0.1 μM
simvastatin was loaded alone, the relative increase in ALP activity
was 177.2 and 17.9%, respectively. However, when 60 ng/ml BMP-2 was
combined with 0.1 μM simvastatin, the relative increase
reached 278.2%, indicating that these two molecules synergistically
enhance osteoblast differentiation.
Western blot analysis was performed to detect
protein expression of pSmad1/5/8, and to provide information
regarding the possible mechanism. It has been reported that
simvastatin may enhance osteoblast differentiation through BMP
signaling (9). When BMP-2 and
simvastatin were delivered simultaneously, the highest protein
expression value was achieved when 60 ng/ml BMP-2 was loaded with
0.1 μM simvastatin. The relative expression levels of 60
ng/ml BMP-2 or 0.1 μM simvastatin were 149.6 and 127.0%,
respectively. However, when 60 ng/ml BMP-2 was combined with 0.1
μM simvastatin, the relative expression level reached
198.8%. Taken together, these results indicate that simvastatin and
BMP-2 provide osteo-inductive effects through the BMP pathway by
enhancing pSmad1/5/8 expression.
In conclusion, the combination of simvastatin and
BMP-2 produces positive effects on the differentiation of
osteoprecursor cells. The results also suggest that the combination
of simvastatin and BMP-2 has synergistic effects that are achieved
via the BMP pathway by enhancing the expression of pSmad1/5/8.
Acknowledgements
This work was supported by the
Bioethics Expert Development Fund by the Committee for Life in the
Archdiocese of Seoul.
References
|
1.
|
Chen PY, Sun JS, Tsuang YH, Chen MH, Weng
PW and Lin FH: Simvastatin promotes osteoblast viability and
differentiation via Ras/Smad/Erk/BMP-2 signaling pathway. Nutr Res.
30:191–199. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Park JB: The use of simvastatin in bone
regeneration. Med Oral Patol Oral Cir Bucal. 14:e485–488.
2009.PubMed/NCBI
|
|
3.
|
Park JB, Zhang H, Lin CY, et al:
Simvastatin maintains osteoblastic viability while promoting
differentiation by partially regulating the expressions of estrogen
receptors alpha. J Surg Res. 174:278–283. 2012. View Article : Google Scholar
|
|
4.
|
Maeda T, Matsunuma A, Kawane T and
Horiuchi N: Simvastatin promotes osteoblast differentiation and
mineralization in MC3T3-E1 cells. Biochem Biophys Res Commun.
280:874–877. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Meleti Z, Shapiro IM and Adams CS:
Inorganic phosphate induces apoptosis of osteoblast-like cells in
culture. Bone. 27:359–366. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Baek KH, Lee WY, Oh KW, et al: The effect
of simvastatin on the proliferation and differentiation of human
bone marrow stromal cells. J Korean Med Sci. 20:438–444. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Moursi AM, Winnard PL, Winnard AV,
Rubenstrunk JM and Mooney MP: Fibroblast growth factor 2 induces
increased calvarial osteoblast proliferation and cranial suture
fusion. Cleft Palate Craniofac J. 39:487–496. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Park JB: Effects of fibroblast growth
factor 2 on osteoblastic proliferation and differentiation by
regulating bone morphogenetic protein receptor expression. J
Craniofac Surg. 22:1880–1882. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Lee MH, Kim YJ, Kim HJ, et al:
BMP-2-induced Runx2 expression is mediated by Dlx5, and TGF-beta 1
opposes the BMP-2-induced osteoblast differentiation by suppression
of Dlx5 expression. J Biol Chem. 278:34387–34394. 2003. View Article : Google Scholar : PubMed/NCBI
|