Introduction
Oxidative stress is defined as an imbalance in the
pro-oxidant-antioxidant equilibrium in favor of pro-oxidants
(1). It is known that there are
relationships between oxidative stress and the development of
various diseases such as cancer, arteriosclerosis, diabetes
mellitus, hypertension and metabolic syndrome (1–6). The
concentration of lipid peroxidation in peripheral blood is
generally higher in pregnant women than in non-pregnant women
because the rapidly growing fetoplacental unit in utero
requires a large amount of oxygen (7–9). In
addition, pregnancy-induced hypertension is associated with
maternal oxidative stress (7,10–12).
Previous studies suggest that an oxidative DNA
damage biomarker, 8-hydroxydeoxyguanosine (8-OHdG), is elevated in
bladder carcinoma, prostate cancer (13), childhood cancers (14,15),
diabetes mellitus (16,17), coronary heart disease (18) and myoma uteri (19), as well as in smokers (20–22).
Moreover, the levels of 8-OHdG are higher in males than females
(23,24). Maternal mental and physical stress
during pregnancy increases not only pregnancy complications, such
as miscarriage, premature birth and low birth weight, but also the
risk of diseases in later life as well as hematopoietic dysfunction
in the fetus/neonate (25,26). Furthermore, physiologically,
pregnancy leads to an increase in free radicals due to the high
energy demands of maternal physical functions and this causes
maternal oxidization damage.
The placental/umbilical cord blood (CB) is the
peripheral blood of the fetus; in addition, various hormones and
molecules of oxidative stress derived from the maternal body are
generally transported into the CB (27). Dziaman et al (28) reported that the levels of 8-OHdG in
the urine of newborn children are ∼2.5 times higher than those of
adult subjects, indicating that 8-OHdG may be a good marker of
oxidative stress in newborns. However, little is known about the
involvement of 8-OHdG during the perinatal period, and there have
been few studies investigating the precise correlations between the
levels of 8-OHdG in CB and the physical conditions of the mother
and neonate. Knowing the level of 8-OHdG in CB may facilitate
long-term health strategies after birth. In the present study, to
clarify the involvement of 8-OHdG during the perinatal period, the
relationships between the levels of CB 8-OHdG and maternal/neonatal
characteristics in vaginal deliveries were determined.
Materials and methods
Subjects and cord blood sample
collection
Between November 2010 and April 2011, CB units were
collected at a single Hospital (Hirosaki National Hospital,
Hirosaki, Japan) after obtaining informed consent from all mothers
and approval from the Committee of Medical Ethics of Hirosaki
National Hospital (Hirosaki, Japan) and the Committee of Medical
Ethics of Hirosaki University Graduate School of Medicine
(Hirosaki, Japan). The inclusion criteria were singleton gestation
vaginal deliveries and birth without resuscitation or immediate
rescue procedures. A segment of the umbilical cord was double
clamped immediately after neonatal delivery, and the blood was
obtained from the umbilical vein before placental delivery (i.e.,
in utero collection). The CB was collected into a sterile
collection bag containing 28 ml citrate phosphate dextrose
anticoagulant (CBC-20; Nipro, Co., Osaka, Japan) until the flow
ceased. A total of 28 CB units were collected and serum was
separated within 24 h of CB collection. Eppendorf test tubes filled
with separated serum were stored at −80°C until analysis for
biochemical parameters. Relevant perinatal data (i.e., maternal
age, smoking status, gestational age, duration of labor, birth
weight, Apgar score, and umbilical artery acid/base status and gas
values) were obtained from hospital records.
Quantitative analysis of 8-OHdG
The concentration of 8-OHdG in CB was analyzed using
highly sensitive 8-OHdG ELISA monitoring kits (Jaica, Fukuroi,
Japan). Each assay was performed immediately after thawing of the
serum sample. To remove high-molecular-weight proteins, which
interfered with the analysis, each CB serum sample was filtered
through an ultrafiltration membrane (molecular weight cut-off,
10,000; Amicon). The obtained filtrate was concentrated by a
SpeedVac® centrifugal evaporator (Thermo Scientific
Savant SPD1010; Thermo Fisher Scientific, Suwanee, GA, USA).
Statistical analysis
Statistical analysis was performed using SPSS
software version 16.0 (SPSS Japan, Inc., Tokyo, Japan) and Origin
(OriginLab, Northampton, MA, USA) for Windows. Descriptive
statistics are presented as arithmetic median (range). Data were
also analyzed by univariate analysis using the Mann-Whitney U test,
Kruskal-Wallis test, and Spearman’s rank correlation coefficient
depending on the distribution pattern of the data. The level of
significance was set at P<0.05.
Results
Perinatal data of the study
population
The perinatal data of the study population are
summarized in Table I. The median
maternal age was 30 years; 57.1% of the mothers were non-smokers
and 42.9% were smokers. The median gestational duration was 38.5
weeks; 53.6% of the newborn infants were males and 46.4% were
females. The median birth weight was 3047 g. The median Apgar
scores at 1 and 5 min were both 9. The median umbilical arterial pH
was 7.33, and base excess was −2.0 mM/l. The 8-OHdG levels in CB
ranged from 0.1 to 1.39 ng/ml (median, 0.37 ng/ml).
 | Table IPerinatal characteristics of the study
population. |
Table I
Perinatal characteristics of the study
population.
| Maternal factors | Median (range) |
|
| Maternal age
(years) | 30.0 (16–42) |
| Gestational age
(weeks) | 38.5 (32–41) |
| Maternal smoking
status | |
| Non-smokers, n
(%) | 16 (57.1) |
| Smokers, n (%) | 12 (42.9) |
| Parity | |
| Primipara, n
(%) | 20 (71.4) |
| Multipara, n
(%) | 8 (28.6) |
| Total duration of
labor (min) | 360.5 (50–2664) |
| First stage of labor
(min) | 279.5 (38–2497) |
| Second stage of labor
(min) | 24.0 (1–181) |
| Oxygen
administration, n (%) | 4 (14.3) |
|
| Neonatal factors | Median (range) |
|
| Birth weight (g) | 3047.0
(2038–3764) |
| Placental weight
(g) | 545.0 (400–800) |
| Neonatal gender | |
| Males, n (%) | 15 (53.6) |
| Females, n (%) | 13 (46.4) |
| Apgar score | |
| 1 min | 9 (8–9) |
| 5 min | 9 (8–10) |
|
| Placental/umbilical
cord blood | Median (range) |
|
| pHa | 7.33 (7.21–7.39) |
| pCO2
(mmHg)a | 46.7 (33.3–63.9) |
| pO2
(mmHg)a | 17.0 (8.0–41.0) |
|
HCO3–
(mmol/l)a | 24.3 (18.7–28.1) |
| Base excess
(mmol/l)a | −2 (−9 to 1) |
| 8-OHdG
(ng/ml)b | 0.37 (0.10–1.39) |
Relationship between 8-OHdG levels and
perinatal characteristics
The relationships between 8-OHdG levels and
perinatal characteristics were analyzed. The 8-OHdG level detected
in the non-smoking group was significantly lower than that in the
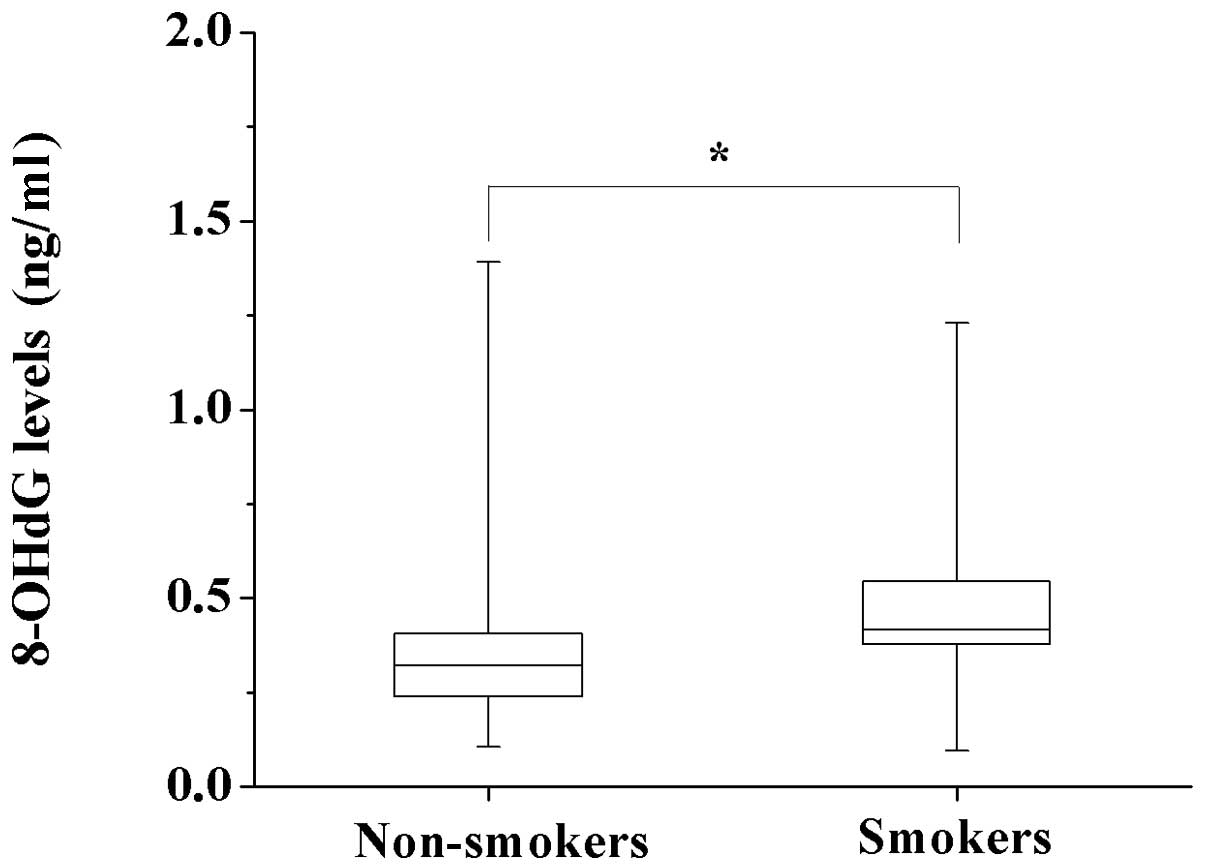
smoking group (0.33 vs. 0.42 ng/ml, P<0.05) (Fig. 1). However, no significant
correlation was observed between 8-OHdG levels and other
maternal/neonatal factors, including umbilical artery acid/base and
gas values. Four cases received oxygen during labor; the 8-OHdG
levels in CB ranged from 0.13 to 0.41 ng/ ml. Both above-mentioned
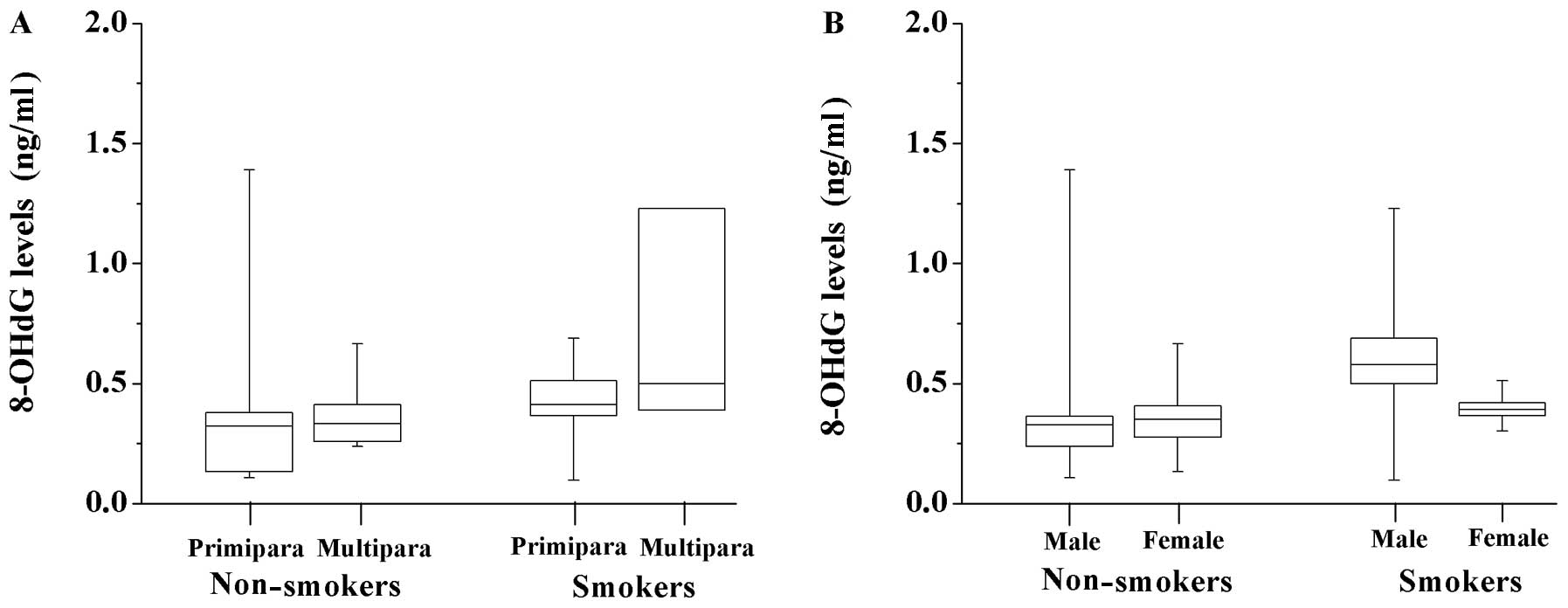
groups were further classified as primipara or multipara and male
or female. No significant differences were observed between any
subgroups (Fig. 2).
The relationships between CB 8-OHdG levels and
biochemical markers and blood cells detected in the pre-delivery
maternal peripheral blood were also analyzed. A significant
positive correlation was found between 8-OHdG level and maternal
white blood cells (data not shown). However, no significant
correlation was observed between 8-OHdG level and maternal
C-reactive protein (CRP), which is an inflammatory marker.
Discussion
In the present study, the relationships between CB
8-OHdG levels and perinatal maternal/neonatal characteristics were
determined. 8-OHdG is formed when DNA is oxidatively modified by
reactive oxygen species (ROS). Thus, 8-OHdG is one of the most
sensitive biomarkers of oxidative stress and is, therefore, widely
used as a biomarker of oxidative DNA damage (29). Forlenza and Miller (30) report that serum 8-OHdG levels are
much lower than urine 8-OHdG levels; serum 8-OHdG levels are ∼0.20
to 1.26 ng/ml in healthy adults. In addition, Schulpis et al
(31) compared the 8-OHdG levels
in CB between vaginal and Cesarean section deliveries; the mean
8-OHdG level was 0.25–0.27 ng/ml, indicating that there was no
significant difference between delivery modes. In the present
study, the 8-OHdG levels in CB obtained from singleton gestation
vaginal deliveries ranged from 0.10 to 1.39 ng/ml (Table I), which is the same as that
detected in healthy adult serum. In addition, the 8-OHdG levels in
smokers were higher than those in non-smokers (Fig. 1). Tobacco causes the creation of
ROS, which lead to DNA damage; this subsequently elevates 8-OHdG
levels in urine and serum (20–22).
Previous studies demonstrate that maternal smoking increases the
risk of childhood disorders including preterm delivery (32), intrauterine growth retardation
(IUGR), low birth weight (32,33),
cleft lip/cleft palate (34),
congenital heart disease (35) and
attention-deficit hyperactivity disorder (36). Furthermore, some studies report
that 8-OHdG levels are elevated in childhood cancer (14), diabetes mellitus (16,17)
and coronary heart disease (18).
The results of the present study do not indicate any relationships
between 8-OHdG levels and various indicators of fetal developmental
outcomes. However, additional approaches regarding the
characteristics of oxidative stress during the perinatal period are
required to improve the health and developmental outcomes of
fetuses and infants.
Maternal leukocyte count is well known to increase
during pregnancy. Although the mechanism underlying the increase in
leukocytes is unclear, it is reported that the count is affected by
cortisol, which increases during pregnancy and as a result of
various placenta-derived cytokines (37). In the present study, a significant
positive correlation was observed between CB 8-OHdG level and
pre-delivery maternal white blood cell count (data not shown);
meanwhile, CB 8-OHdG level was not related to CRP, an inflammatory
response marker (data not shown). Because maternal leukocyte count
was measured at different times depending on individuals, its
precise changes were unclear. However, there may be a relationship
between the number of leukocytes and 8-OHdG level as a result of
maternal oxidative stress.
In conclusion, the results of the present study
indicate that CB 8-OHdG levels in smokers are significantly higher
than those in non-smokers. However, no relationship was found
between 8-OHdG level and quantity or duration of smoking. Oxidative
stress possibly influences the long-term health outcomes of
infants. Although 8-OHdG is a relatively stable chemical substance,
it can lead to G→T mutations during DNA replication if generated
near chromosomal DNA, increasing the risk of cancer (2). Future investigations focusing on
leukocytes, 8-OHdG level, and CB inflammatory cytokine levels are
required. Healthcare personnel involved in perinatal medicine
should try to reduce oxidative stress during pregnancy to improve
the health of the fetus after birth.
Acknowledgements
This study was supported by a grant
from Hirosaki University Institutional Research (2011). The study
was performed by the research group of Ikuo Kashiwakura, who
designed the study, performed a series of pilot experiments and had
overall responsibility. The authors thank Ayako Tarakida who
assisted with the collection of the CB units from normal vaginal
deliveries at the Obstetrics and Gynecology Department of Hirosaki
National Hospital (Hirosaki, Japan) and recorded all perinatal data
of the study population. All authors contributed equally to the
preparation of the manuscript.
References
|
1.
|
Sies H: Oxidative stress: from basic
research to clinical application. Am J Med. 91:S31–S38. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Shibutani S, Takeshita M and Grollman AP:
Insertion of specific bases during DNA synthesis past the
oxidation-damaged base 8-oxodG. Nature. 349:431–434. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Ceriello A, Bortolotti N, Motz E, et al:
Meal-induced oxidative stress and low-density lipoprotein oxidation
in diabetes: the possible role of hyperglycemia. Metabolism.
48:1503–1508. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Wu LL, Chiou CC, Chang PY and Wu JT:
Urinary 8-OHdG: a marker of oxidative stress to DNA and a risk
factor for cancer, atherosclerosis and diabetics. Clin Chim Acta.
339:1–9. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Olinski R, Siomek A, Rozalski R, et al:
Oxidative damage to DNA and antioxidant status in aging and
age-related diseases. Acta Biochim Pol. 54:11–26. 2007.PubMed/NCBI
|
|
6.
|
Cangemi R, Angelico F, Loffredo L, et al:
Oxidative stress-mediated arterial dysfunction in patients with
metabolic syndrome: Effect of ascorbic acid. Free Radic Biol Med.
43:853–859. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Little RE and Gladen BC: Levels of lipid
peroxides in uncomplicated pregnancy: a review of the literature.
Reprod Toxicol. l13:347–352. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Argüelles S, Machado MJ, Ayala A, Machado
A and Hervias B: Correlation between circulating biomarkers of
oxidative stress of maternal and umbilical cord blood at birth.
Free Radic Res. 40:565–570. 2006.PubMed/NCBI
|
|
9.
|
Burton GJ and Jauniaux E: Oxidative
stress. Best Pract Res Clin Obstet Gynaecol. 25:287–299. 2011.
View Article : Google Scholar
|
|
10.
|
Wang Y and Walsh SW: Placental
mitochondria as a source of oxidative stress in pre-eclampsia.
Placenta. 19:581–586. 1998.PubMed/NCBI
|
|
11.
|
Morris JM, Gopaul NK, Endresen MJ, et al:
Circulating markers of oxidative stress are raised in normal
pregnancy and pre-eclampsia. Br J Obstet Gynaecol. 105:1195–1199.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Ishihara O, Hayashi M, Osawa H, et al:
Isoprostanes, prostaglandins and tocopherols in pre-eclampsia,
normal pregnancy and non-pregnancy. Free Radic Res. 38:913–918.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Chiou CC, Chang PY, Chan EC, Wu TL, Tsao
KC and Wu JT: Urinary 8-hydroxydeoxyguanosine and its analogs as
DNA marker of oxidative stress: development of an ELISA and
measurement in both bladder and prostate cancers. Clin Chim Acta.
334:87–94. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Matsubasa T, Uchino T, Karashima S, et al:
Oxidative stress in very low birth weight infants as measured by
urinary 8-OHdG. Free Radic Res. 36:189–193. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Yang Y, Tian Y, Yan C, Jin X, Tang J and
Shen X: Determinants of urinary 8-hydroxy-2′-deoxyguanosine in
Chinese children with acute leukemia. Environ Toxicol. 24:446–452.
2009.
|
|
16.
|
Hinokio Y, Suzuki S, Hirai M, Suzuki C,
Suzuki M and Toyota T: Urinary excretion of 8-oxo-7,
8-dihydro-2′-deoxyguanosine as a predictor of the development of
diabetic nephropathy. Diabetologia. 45:877–882. 2002.
|
|
17.
|
Nishikawa T, Sasahara T, Kiritoshi S, et
al: Evaluation of urinary 8-hydroxydeoxyguanosine as a novel
biomarker of macrovascular complications in type 2 diabetes.
Diabetes Care. 26:1507–1512. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Collins AR, Gedik CM, Olmedilla B, Southon
S and Bellizzi M: Oxidative DNA damage measured in human
lymphocytes: large differences between sexes and between countries,
and correlations with heart disease mortality rates. FASEB J.
12:1397–1400. 1998.
|
|
19.
|
Foksinski M, Kotzbach R, Szymanski W and
Olinski R: The level of typical biomarker of oxidative stress
8-hydroxy-2′-deoxyguanosine is higher in uterine myomas than
control tissues and correlates with the size of the tumor. Free Rad
Biol Med. 29:597–601. 2000.
|
|
20.
|
Kasai H, Iwamoto-Tanaka N, Miyamoto T, et
al: Life style and urinary 8-hydroxydeoxyguanosine, a marker of
oxidative DNA damage: effects of exercise, working conditions, meat
intake, body mass index, and smoking. Jpn J Cancer Res. 92:9–15.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Loft S, Svoboda P, Kasai H, et al:
Prospective study of 8-oxo-7,8-dihydro-2′-deoxyguanosine excretion
and the risk of lung cancer. Carcinogenesis. 27:1245–1250.
2006.
|
|
22.
|
Yano T, Shoji F, Baba H, Koga T, Shiraishi
T and Orita H: Significance of the urinary 8-OHdG level as an
oxidative stress marker in lung cancer patients. Lung Cancer.
63:111–114. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Loft S, Vistisen K, Ewertz M, Tjønneland
A, Overvad K and Poulsen HE: Oxidative DNA damage estimated by
8-hydroxydeoxyguanosine excretion in humans: influence of smoking,
gender and body mass index. Carcinogenesis. 13:2241–2247. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Suzuki K, Ito Y, Ochiai J, et al: The
relationship between smoking habits and serum levels of 8-OHdG,
oxidized LDL antibodies, Mn-SOD and carotenoids in rural Japanese
residents. J Epidemiol. 13:29–37. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Lim FT, van Winsen L, Willemze R, Kanhai
HH and Falkenburg JH: Influence of delivery on numbers of
leukocytes, leukocyte subpopulations, and hematopoietic progenitor
cells in human umbilical cord blood. Blood Cells. 20:547–558.
1994.PubMed/NCBI
|
|
26.
|
De Jongh RF, Puylaert M, Bosmans E,
Ombelet W, Maes M and Heylen R: The fetomaternal dependency of cord
blood interleukin-6. Am J Perinatol. 16:121–128. 1999.PubMed/NCBI
|
|
27.
|
Knackstedt MK, Hamelmann E and Arck PC:
Mothers in stress: consequences for the offspring. Am J Reprod
Immunol. 54:63–69. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Dziaman T, Gackowski D, Rozalski R, Siomek
A, Szulczynski J and Zabielski R: Urinary excretion rates of
8-oxoGua and 8-oxodG and antioxidant vitamins level as a measure of
oxidative status in healthy, full-term newborns. Free Radic Res.
41:997–1004. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Aruoma OI, Halliwell B, Gajewski E and
Dizdaroglu M: Damage to the bases in DNA induced by hydrogen
peroxide and ferric ion chelates. J Biol Chem. 264:20509–20512.
1989.PubMed/NCBI
|
|
30.
|
Forlenza MJ and Miller GE: Increased serum
levels of 8-hydroxy-2′-deoxyguanosine in clinical depression.
Psychosom Med. 68:1–7. 2006.
|
|
31.
|
Schulpis KH, Lazaropoulou C, Vlachos GD,
et al: Maternal-neonatal 8-hydroxy-deoxyguanosine serum
concentrations as an index of DNA oxidation in association with the
mode of labour and delivery. Acta Obstet Gynecol Scand. 86:320–326.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Rogers JM: Tobacco and pregnancy. Reprod
Toxicol. l28:152–160. 2009. View Article : Google Scholar
|
|
33.
|
Ward C, Lewis S and Coleman T: Prevalence
of maternal smoking and environmental tobacco smoke exposure during
pregnancy and impact on birth weight: retrospective study using
Millennium Cohort. BMC Public Health. 7:812007. View Article : Google Scholar
|
|
34.
|
Little J, Card A and Munger RG: Tobacco
smoking and oral clefts: a meta-analysis. Bull World Health Organ.
82:213–218. 2004.
|
|
35.
|
Shi M, Wehby GL and Murray JC: Review on
genetic variants and maternal smoking in the etiology of oral
clefts and other birth defects. Birth Defects Res C Embryo Today.
84:16–29. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Milberger S, Biederman J, Faraone SV, Chen
L and Jones J: Is maternal smoking during pregnancy a risk factor
for attention deficit hyperactivity disorder in children? Am J
Psychiatry. 153:1138–1142. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Cunningham FG, Leveno KJ, Bloom SL, Hauth
JC, Rouse DJ and Spong GY: Maternal and fetal anatomy and
physiology. Williams Obstetrics. 23rd edition. McGraw Hill; New
York, NY: pp. 114–116. 2010
|