Introduction
An effective cancer immunotherapy approach requires
the activation of host T cells capable of recognizing a tumor
target antigen and T-cell activation requires the appropriate
antigen presentation by antigen-presenting cells. Dendritic cells
(DCs) are the most potent professional antigen-presenting cells,
with an excellent ability to interact with T cells and initiate
their responses (1,2). The antigen-presenting ability of DCs
makes them attractive vehicles for the delivery of therapeutic
cancer vaccines (3,4). Numerous studies have focused on
finding a feasible and effective DC-based vaccine, including
pulsing DCs with tumor antigen peptide or protein, transducing
genes encoding tumor antigen into DCs and fusing tumor cells with
DCs (5–8). Among these, the genetic modification
of DCs to express tumor antigens has been documented to be
efficient for inducing antitumor immunity. In our previous study
(5), we used α-fetoprotein (AFP)
gene-modified DCs (AFP-DCs) to explore the potential of a DC-based
tumor vaccine against hepatocellular carcinoma (HCC). Despite the
induction of significant cytotoxic T-lymphocyte (CTL) responses,
the antitumor effect was limited. Other investigators have also
reported that the results of DC-based therapeutic vaccines were far
from encouraging in some animal models (9,10).
Interleukin-2 (IL-2) is a potent stimulator of
lymphocyte proliferation and increases the activity of CTLs
(11). The systemic administration
of IL-2 in vivo has a broad range of immunologic effects,
including the induction of specific T-helper cells, natural killer
(NK) and lymphokine-activated killer (LAK) cells and autoantibody
production in congenitally T cell-deficient mice (12,13),
and the enhancement of the restoration of immune function in
irradiated or cyclophosphamide-treated animals (14,15).
IL-2 is capable of specifically enhancing alloimmune responses in
normal or primed mice and is able to enhance the antitumor activity
of adoptively transferred LAK cells or specifically immune T
lymphocytes (16). Of note, IL-2
alone is able to mediate the regression of selected, established
murine and human tumors by mechanisms that involve the stimulation
of in vivo lymphoid proliferation in tissues and by the
activation of host-derived T cells (17).
Based on these studies, we explored potential
therapeutic regimens based on the combined AFP and IL-2 transfected
DCs. Moreover, we aimed to determine whether IL-2 is able to
potentiate the antitumor effects of AFP-transfected DCs in
vivo. Thus, the study was designed to provide a rationale for
the development of clinical trials in humans using DC-based vaccine
strategies.
Materials and methods
Construction and production of
recombinant adenovirus (Ad) encoding AFP and IL-2
The AdAFP and AdIL-2 vectors were constructed using
the AdEasy system. Briefly, fragments of the AFP and IL-2 genes
were cloned into the pTrack plasmid containing a CMV promoter. To
generate the recombinant adenoviral plasmid, following
linearization with PmeI, the pTrack-CMV-AFP or
pTrack-CMV-IL-2 vectors were co-transformed into E. coli
BJ5183 with supercoiled pAd-Easy-1. The resultant AdAFP and AdIL-2
plasmids were characterized by restriction endonuclease digestion.
To generate the adenoviral vector particles, the
PacI-digested plasmids AdAFP and AdIL-2 were infected into
293 cells by calcium phosphate co-precipitation methods. The AdAFP
and AdIL-2 virus particles were propagated and purified as
described in the AdEasy system instructions. The control AdGFP
vector containing a green fluorescent protein (GFP) gene under the
control of a CMV promoter was plaque-purified and amplified in the
293 cells. After two cycles of purification by CsCl
ultra-centrifugation, the adenoviral vector titers were determined
as viral genome particle numbers.
Preparation of DCs
DCs were prepared as described in a previous study
(18). PBMCs from
HLA-A2+ HCC patients were isolated by Ficoll-Hypaque
(Sigma, St. Louis, MO, USA) density gradient separation. Cells were
either used immediately or cryopreserved in agents containing 50%
X-VIVO 15 medium, 40% fetal calf serum (FCS) and 10% dimethyl
sulfoxide (DMSO; Sigma). The PMBCs were then cultured in serum-free
X-VIVO 15 medium (Cambrex Bioscience, Walkersville, MD, USA) in
6-well plates at 37°C with 5% CO2. After 2 h,
non-adherent cells were removed by gently washing with
phosphate-buffered saline (PBS) solution. Adherent cells were
replenished with 30 ml X-VIVO 15 medium containing 100 ng/ml
granulocyte macrophage-colony stimulating factor (GM-CSF;
Peprotech, Inc., Rocky Hill, NJ, USA) and 10 ng/ml interleukin-4
(IL-4; Peprotech) and incubated for 7 days at 37°C with 5%
CO2.
Generation and genetic modification of
DCs
Ad vectors were purified by two rounds of CsCl
density centrifugation, dialysed and stored at −70°C in 3% sucrose.
Vector preparations were demonstrated to be free of
replication-competent adenovirus. To assess the ability of the Ad
vectors to transfer and express genes in DCs, the cells were
infected with AdGFP for 2 h after 7 days of culture using a
multiplicity of infection (MOI) of 50, 100 or 200. Two days later,
GFP expression was quantified by flow cytometry. We observed a
dose-dependent response to the adenoviral infections, with maximal
staining (74%) at a MOI of ≥200. Therefore, a MOI of 200 was
selected for the transfection of DCs with AdAFP and AdIL-2 in this
study.
Ad vector-mediated infection and AFP and
IL-2 expression in DCs
The Ad-mediated genetic modification of DCs was
carried out by incubating the DCs with AdGFP, AdAFP, AdIL-2 or
AdAFP combined with AdIL-2 at a MOI of 200 for 2 h and then washing
the cells twice with complete X-VIVO 15 medium. The above DC
vaccines are referred to as GFP-DC, AFP-DC, IL-2-DC and
IL-2/AFP-DC, respectively. The DCs were collected 24 h after
genetic modification to evaluate the gene transfer efficacy. The
genetically-modified DCs were subjected to reverse
transcription-PCR (RT-PCR) analysis of AFP and/or IL-2 expression.
The primers used were β-actin, forward: 5′-ACAATGAGCTGCGTGTGGCT-3′
and reverse 5′-TCTCCTTAATGTCACGCACGA-3′, with an expected PCR
product of 344 bp. The specific forward primer for human IL-2 was
5′-AGCAAGCTTACCATGCAACTCCTGTC-3′ and reverse:
5′-GCGGATCCTTATGTTGAGATGATGC-3′, with a product size of 447 bp. The
specific primers for human AFP were AFP1 (outer forward):
5′-CTCTTCCAGA AACTAGGAGAA-3′, AFP2 (outer reverse): 5′-CTCTTCAGC
AAAGCAGACTT-3′, AFP3 (inner forward): 5′-GCTGACA TTATTATCGGACAC-3′,
AFP4 (inner reverse): 5′-AGCCTC AAGTTGTTCCTCTGT-3′, with an
expected product size of 282 bp.
Detection of AFP and IL-2 release by
enzyme-linked immunosorbent assay (ELISA)
The supernatants of the cells from the four groups
(GFP-DC, AFP-DC, IL-2-DC and IL-2/AFP-DC) were collected. The AFP
release in the supernatants was evaluated by ELISA using an AFP
ELISA detection kit (CanAg Diagnostics, Gothenburg, Sweden) and the
culture supernatants of the genetically-modified DCs were collected
for detection of the production of IL-2 using an ELISA kit (R&D
Systems, Minneapolis, MN, USA).
Flow cytometric analysis
DCs (2×105 cells) were washed and
resuspended in PBS solution containing 0.02% sodium azide and 1%
bovine serum albumin. The cells were incubated with various
fluorochrome-conjugated monoclonal antibodies at 4°C for 20 min in
the dark. Phytoerythrin (PE)-conjugated antibodies against CD83,
HLA-DR and CD86 and fluorescein isothiocyanate (FITC)-conjugated
antibodies against CD80 (BD Biosciences Pharmingen, San Diego, CA,
USA) were used. The cells were then washed twice and fixed in PBS
solution containing 1% formaldehyde. The phenotypes were analysed
by flow cytometry using a FACScan analytical flow cytometer.
CTL generation and interferon-γ (IFN-γ)
release ELISA
The CD8+ T cells from the HLA-A2+ HCC
patients were positively selected using an anti-CD8 isolation kit
(Dynal Biotech). The cells were suspended in complete X-VIVO 15
medium. DCs transfected with Ad or treated with TNF-α were plated
with the effector T cells at a ratio of 1:20 (1×105 DCs:
2×106 effectors) in a total volume of 2 ml in 24-well
tissue culture plates and cultured for seven days at 37°C with 5%
CO2. The effector T cells were then harvested, washed,
counted and restimulated with newly infected and mature DCs. The
effector cells were serially stimulated a total of three times.
Five days after the third stimulation, IFN-γ release in the
supernatants of the effector cells was evaluated by ELISA using an
IFN-γ ELISA detection kit. In brief, the effector T cells were
collected and incubated with the target cells (HepG2) at a 20:1
ratio in a total volume of 200 μl in 96-well plates for 24 h. The
supernatants were harvested from the cultures and IFN-γ assays were
performed according to the manufacturer’s instructions.
Cytotoxicity assay
The CD8+ T cells were incubated with
stimulators (GFP-DCs, AFP-DCs, IL-2-DCs and IL-2/AFP-DCs) at a
ratio of 20:1 (2×106 T cells vs. 1×105
stimulators) in 24-well culture plates in X-VIVO 15 medium for 5
days at 37°C with 5% CO2. The cytotoxicity analysis was
performed using a lactate dehydrogenase (LDH) release assay.
Briefly, the effector T cells were collected and incubated with the
target cells (HepG2, SMMC7721 and K562) at ratio of 40:1 in
96-microwell plates at 37°C with 5% CO2 for 4 h. The
plates were then centrifuged for 5 min. Supernatants from each well
(100 μl) were transferred to 96-flat bottom microwell plates and
100 μl of the LDH substrate mixture was added. After 15 min, the
absorbance was measured at 570 nm with an ELISA reader (Denley
Dragon MK2; Labsystems, Helsinki, Finland). The CTL-mediated
cytotoxicity was calculated using the equation:
Cytotoxicity=[1-(effector-target-effector)/target] x100%.
Immunizations and tumor challenge
We subcutaneously injected 1×105
IL-2/AFP-DCs or the same number of DCs, GFP-DCs, IL-2-DCs or
AFP-DCs, or PBS into the right flank region of C57BL/6 mice. The
same immunization was repeated once after 1 week. Tumor challenge
was initiated by the subcutaneous injection of 2×105
HepG2 cells into the rear leg of the immunized mice 1 week after
the last immunization to evaluate the specificity of the antitumor
immunity induced by the DC vaccines in the immunized mice. Tumor
occurrence was observed every other day. The length and width of
the tumor mass were measured with calipers every other day and the
tumor size was expressed as 0.5 × (length + width).
Results
Ad-mediated IL-2 and/or AFP genetic
modification of DCs
The DCs were cultured in vitro for 7 days and
then characterized using composite criteria of typical morphology.
Flow cytometric analysis was performed 48 h after infection with
AdGFP (at MOIs of 50–200) to determine the infection efficiency and
the impact of Ad infection on the DC phenotypes. The DCs proved
amenable to the in vitro Ad-mediated gene transfer and the
efficiency was increased in a MOI-dependent manner. A MOI of 200
achieved transgene expression in 74.61% of cells (Fig. 1A). The GFP expression was stable
and persisted while the DCs remained in culture.
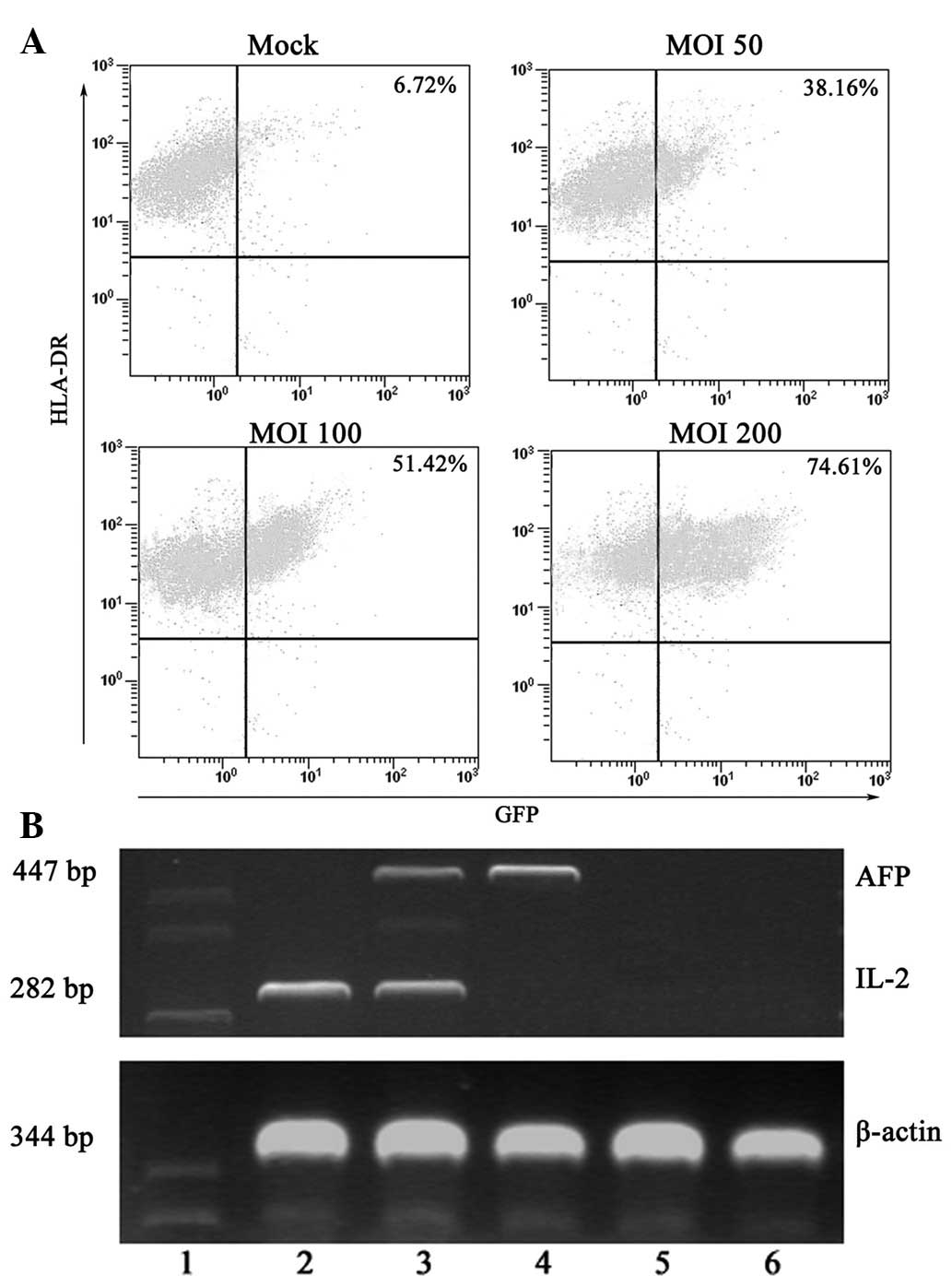 | Figure 1Transduction efficay of an Ad vector
at various MOIs and surface marker expression of
adenovirus-infected DCs. (A) Recombinant AdGFP was used to
transduce day 7 immature DCs, which were then cultured for 48 h in
the presence of GM-CSF and IL-4. On day 9, flow cytometric analysis
of GFP expression by the Ad-GFP-DCs was carried out. Typically,
>74% of cells were GFP+ at a MOI of 200. (B) IL-2
and/or AFP expression by gene-modified DCs is shown. PCR products
of β-actin, IL-2 and AFP were visualized by electrophoresis in a 2%
agarose gel containing 0.5 μg/ml ethidium bromide. Lane 1 Marker,
lane 2 IL-2-DC, lane 3 IL-2/AFP-DC, lane 4 AFP-DC, lane 5 GFP-DC,
lane 6 DC group. Ad, adenovirus; MOI, multiplicity of infection;
DCs, dendritic cells; GFP, green fluorescent protein; GM-CSF,
granulocyte macrophage-colony stimulating factor; IL-2,
inter-leukin-2; AFP, α-fetoprotein; PCR, polymerase chain
reaction. |
Subsequently, we evaluated the efficacy of the
Ad-transduced transfer of AFP and IL-2 genes into the DCs. IL-2
and/or AFP expression levels in the genetically-modified DCs were
analysed by semiquantitative RT-PCR. As shown in Fig. 1B, DCs, GFP-DCs and IL-2-DCs did not
express any detectable AFP, whereas AFP was detected in AFP-DCs and
IL-2/AFP-DCs. IL-2 expression was detected in DCs transfected with
or without various Ad, but the expression levels in DCs transfected
with AdIL-2 alone or combined with AFP were significantly higher
than in DCs, GFP-DCs and AFP-DCs. The results indicated that AFP
and/or IL-2 genes were efficiently transfected. The culture
supernatants of DCs following Ad transfection were collected and
analysed for IL-2 production by ELISA.
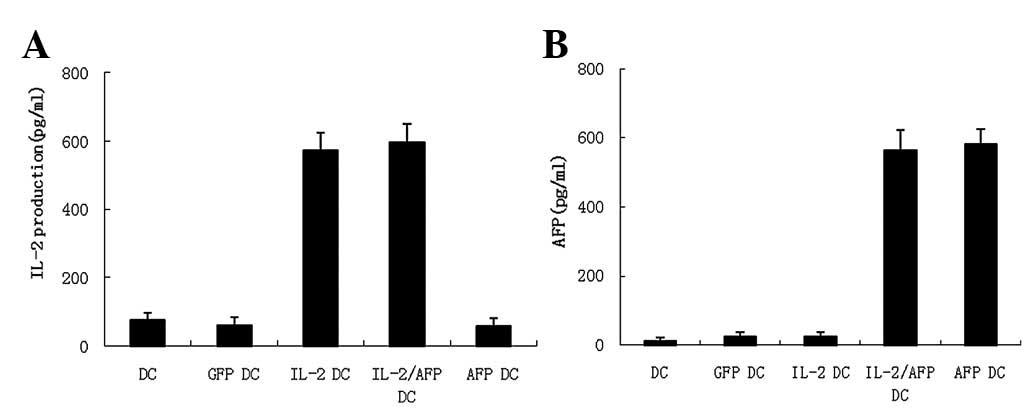
As shown in Fig.
2A, AFP release was detected in the supernatants of the DCs
following transfection with AdIL-2/AFP and AdAFP. Although the
levels of AFP were higher than in the supernatants from DCs
transfected with AdIL-2 and AdGFP and untransfected DCs, they were
nevertheless low levels and did not have any negative effect on the
functions of DCs. In addition, the culture supernatants from DCs
and genetically-modified DCs showed that IL-2 expression reached
the highest levels of approximately 562.45 and 585.21 pg/ml at 24 h
in the supernatants from the IL-2-DCs and IL-2/AFP-DCs,
respectively, while <65 pg/ml IL-2 was detected in the
supernatants from the untransfected DCs, GFP-DCs and AFP-DCs
(Fig. 2B). The results indicate
that low levels of IL-2 are secreted by DCs and that
IL-2-genetically modified DCs are able to secrete IL-2 at
relatively high levels.
Gene transduction with the adenovirus
vector did not impair DC function
DCs were assessed for cell surface phenotypes by
flow cytometry prior to and following transfection. A greater
degree of maturation was observed following transfection. This
finding was characterized by an increased expression of the cell
surface molecules CD83, CD80, CD86 and HLA-DR (Fig. 3). Genetically-modified DCs
exhibited a phenotypic and functional change toward antigen
presentation. Following transfection with Ad for 24 h, the levels
of the cell surface molecules of the DCs were increased and the
transfected DCs expressed higher levels of CD80, CD86, CD83 and
HLA-DR compared with the immature DCs. Moreover, no significant
differences in the expression levels of CD80, CD83, CD86 and HLA-DR
were observed between the mature DCs and the infected DCs,
indicating that gene transduction with the Ad vector did not alter
the surface phenotypes of the DCs.
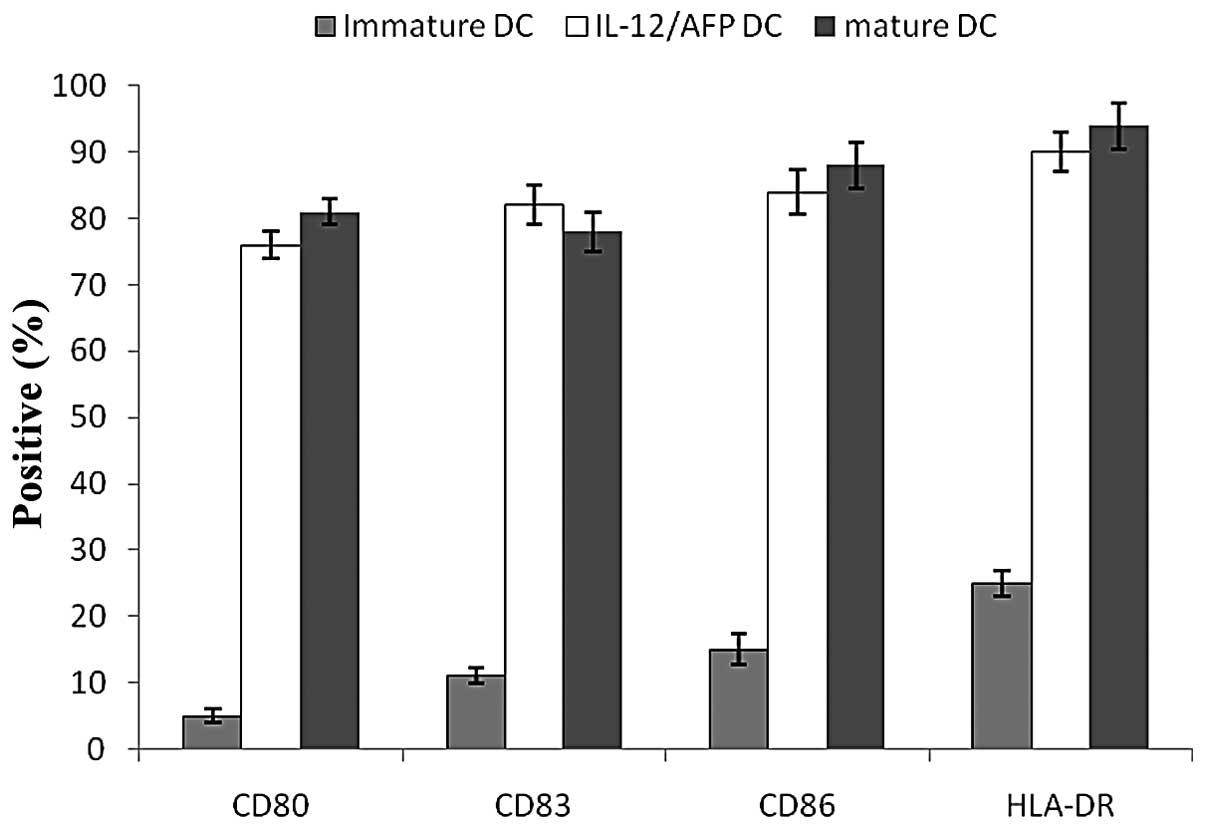 | Figure 3Cell surface markers of infected DCs
measured by flow cytometry. Before DCs were infected with Ad, the
expression levels of CD80, CD83, CD86 and HLA-DR were low, but
following infection with Ad, the DCs expressed higher levels of
CD80, CD83, CD86 and HLA-DR. Following infection with AdIL-2/AFP,
the expression levels of CD80, CD83, CD86 and HLA-DR were 76.5,
82.1, 84.7 and 90.3%, respectively. The results indicate that the
infected DCs exhibited a mature phenotypic change towards antigen
presentation. DC, dendritic cells; IL-2, interleukin-2; AFP,
α-fetoprotein. |
More effective specific CTL response by
DCs co-transfected with IL-2 and AFP
The cytotoxic activity of the DCs was assayed
against HCC cells. Target cells comprised HepG2, SMMC7721 and K562
cells. The results indicated that IL-2/AFP-DCs specifically induced
the highest CTL activity against AFP-expressing HepG2 cells.
Moreover, the AFP-DCs also exhibited a more potent tumor-specific
CTL response. CTLs were also induced by the IL-2-DC vaccine, but no
significant CTL induction was observed by the GFP-DCs, DCs and T
cells alone. Moreover, the CTLs specifically lysed the AFP-positive
carcinoma cells, while the AFP-negative carcinoma cells were not
lysed, indicating that the CTL response was antigen-specific
(Fig. 5).
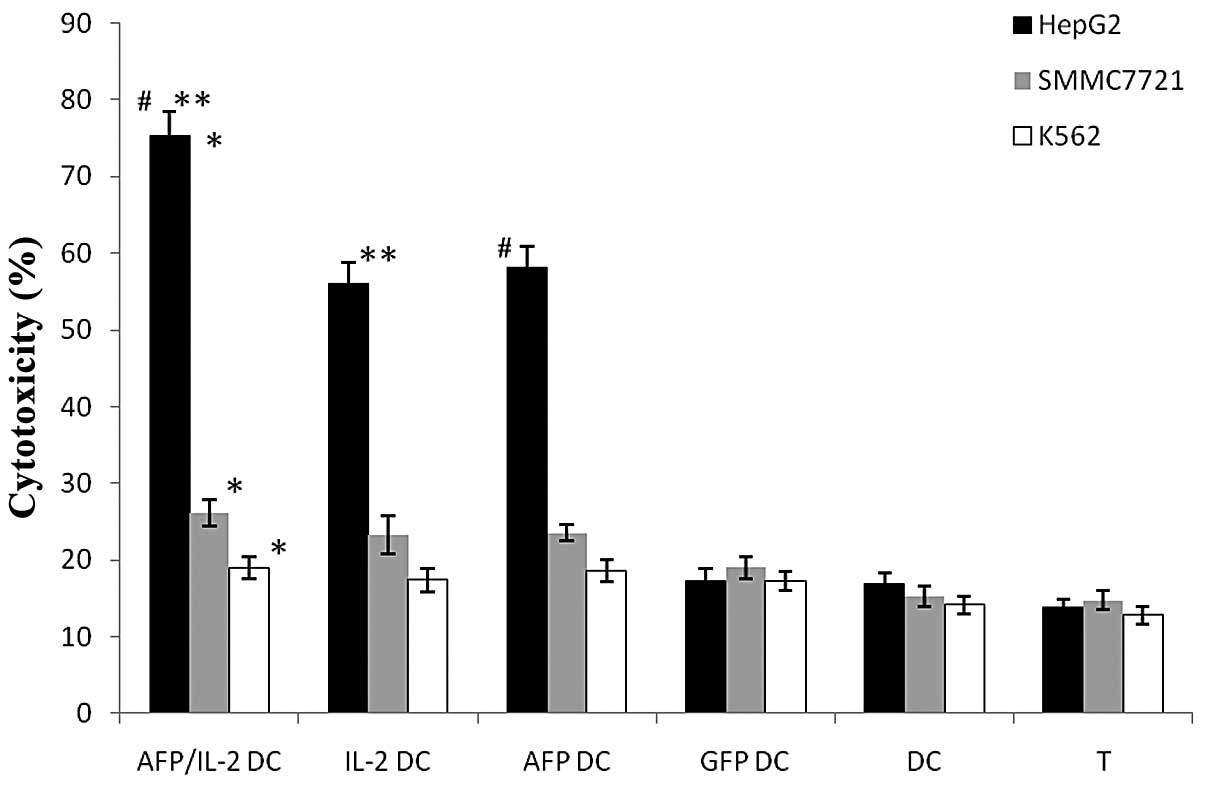 | Figure 5Cytotoxicity measured by LDH assay.
The percentage of HepG2 cell-mediated lysis by IL-2/AFP-DCs
(74.57±4.24%) was much higher than that of SMMC7721 and K562 cells,
*P= 0.0017; the percentage of lysed SMMC7721 cells was
higher than that of lysed K562 cells. However, no significant
differences among the lyses of the three target cells mediated by
GFP-DCs, DCs or T cells were detected, P>0.05. For HepG2 cells,
the percentage of lyses mediated by IL-2/AFP-DC were higher than
those mediated by AFP-DCs (#P= 0.0421), IL-2-DCs (**P=0.0315),
GFP-DCs, DCs and T cells alone, P<0.05. For SMMC7721 cells, the
results were similar: the percentage of cell lyses mediated by
IL-2/AFP-DC were higher than those mediated by AFP-DCs, IL-2-DCs,
GFP-DCs, DCs and T cells alone, P<0.05. Lyses of K562 cells by
CTLs, GFP-DCs, DCs or T cells alone were not significantly
different, P>0.05. LDH, lactate dehydrogenase; IL-2,
interleukin-2; AFP, α-fetoprotein; DC, dendritic cell; T, T cell;
GFP, green fluorescent protein. |
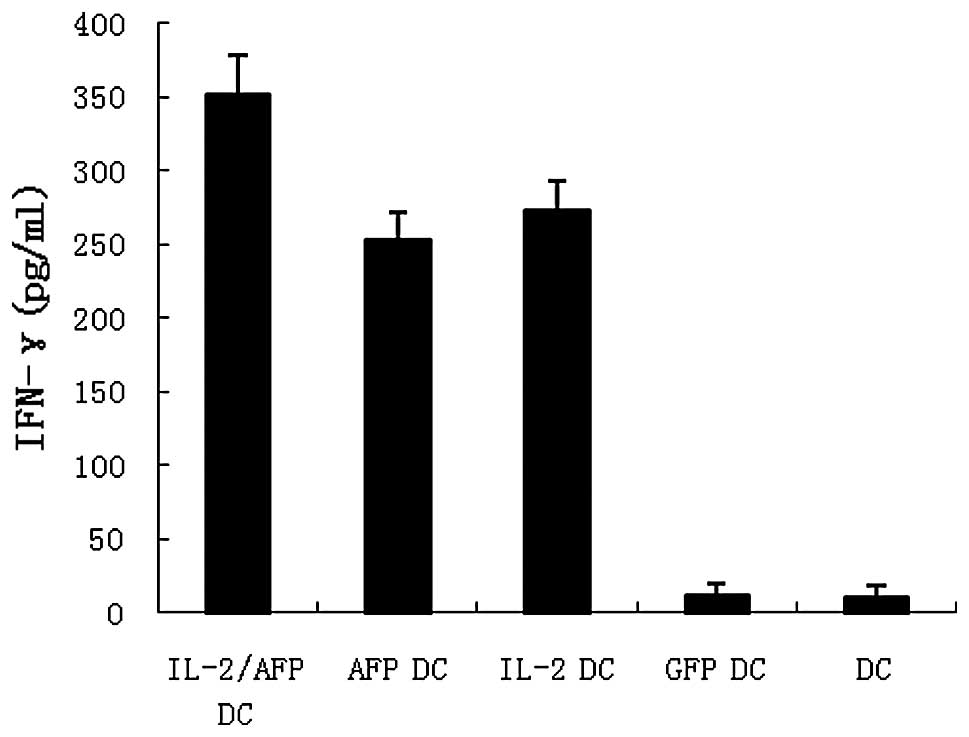
AFP-specific CTLs were generated as described in
Materials and methods. IFN-γ release in the supernatants of the
effector cells was evaluated by ELISA. The results revealed that
IL-2/AFP-DCs co-cultured with HepG2 exhibited higher levels of
IFN-γ than the other groups (Fig.
4).
More effective elicitation of protective
antitumor immunity by immunization with DC co-transfected with IL-2
and AFP
C57BL/6 mice were immunized subcutaneously twice
with DCs, GFP-DCs, IL-2-DCs, AFP-DCs or AFP/IL-2-DCs. Seven days
after the second immunization the mice were challenged with HepG2
cells. The results in Fig. 6
demonstrate that immunization with AFP-DCs or IL-2-DCs markedly
inhibited the tumor growth compared with immunization with GFP-DCs
or DCs or PBS injection (P<0.01). Additionally, inhibition of
tumor growth was observed significantly in mice following
vaccination with AFP/IL-2-DC compared with mice vaccinated with
other vaccines (P<0.01). This result suggests that AFP/IL-2-DC
is a potent vaccine that is able to induce specific antitumor
immunity efficiently.
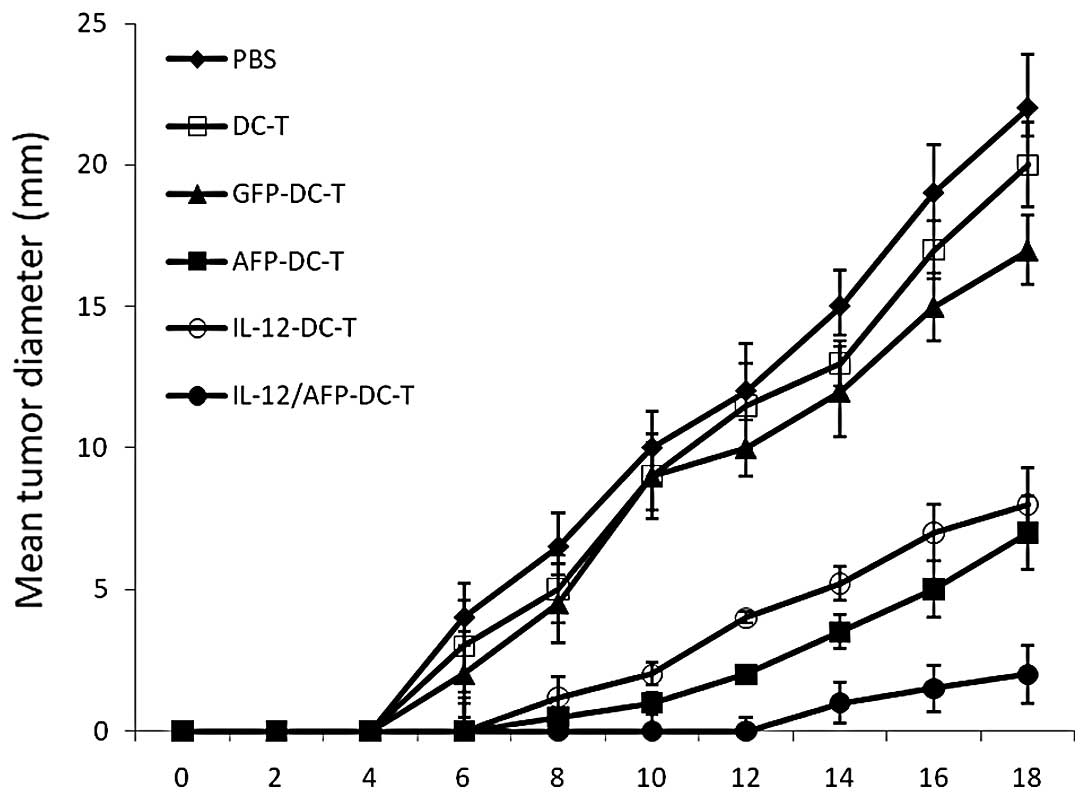 | Figure 6Induction of protective antitumor
immunity by immunization with the IL-2/AFP DC vaccine. C57BL/6 mice
were immunized subcutaneously with IL-2/AFP-DCs, AFP-DCs, IL-2-DCs,
GFP-DCs or DCs 14 and 7 days prior to challenge with HepG2 cells.
Tumor size was monitored with calipers every other day and
calculated as the product of maximal perpendicular diameters. The
results revealed that immunization with AFP-DCs or IL-2-DCs
markedly inhibits tumor growth compared with immunization with
GFP-DCs, DCs or PBS injection (P<0.01). Moreover, inhibition of
melanoma growth was observed in mice following vaccination with
IL-2/AFP-DC compared with mice vaccinated with other vaccines
(P<0.01). IL-2, interleukin-2; AFP, α-fetoprotein; DC, dendritic
cell; T, T cell; PBS, phosphate-buffered saline. |
Discussion
HCC is an aggressive disease and is the third
highest cause of cancer death due to a lack of treatment options.
Current efforts are now directed towards novel strategies for the
treatment of HCC. One of the promising approaches is to design
vaccines using DCs as the vehicle to deliver cancer antigens for an
effective induction of T-cell antitumor immunity (19). To enhance the loading of DCs with
tumor-associated antigen (TAA) in vitro and to further
increase the efficacy of the DC vaccines, various techniques for
the delivery of the priming antigen have been tested, including
pulsing with peptide, protein or tumor cell lysates and
transfection with viral vector-mediated TAA genes (20–25).
Among these, gene transfer may be one of the most promising
approaches as it may result in antigen processing naturally in the
MHC class I and II pathways by DCs and stimulation of
tumor-specific CTL and Th1 cells (26,27).
Moreover, using an Ad vector to genetically modify DCs has been
confirmed to be a good method due to its high efficacy and the
minimum risk associated with insertional mutagenesis. In our
experiment, the results revealed that the expression of Ad-GFP by
infected DCs reached the highest level of 74.61%, which indicated
efficient gene transfection. The co-stimulatory molecules CD80,
CD86 and HLA-DR were upregulated significantly following Ad
modification of the DCs compared with immature DCs, which
demonstrated that the adenovirus transfection and gene expression
did not affect on the DC maturation and antigen-presenting
function.
AFP is a transcriptionally regulated protein
expressed by most HCCs. Murine and human T-cell repertoires are
reportedly able to recognize AFP despite being exposed to high
plasma levels of this oncofetal protein during embryonic
development (28–30). Therefore, AFP may be a target for
the adjuvant immunotherapy of patients with HCC. In a previous
study (5), we used AFP-DC as a
cancer vaccine to evaluate the antitumor response. Despite the
induction of specific CTL responses, the data demonstrated that the
AFP-DCs elicited only limited antitumor immunity against HCC. Our
results, together with those of other authors, suggest that the
AFP-DC vaccine had to be improved to increase the antitumor
efficacy.
IL-2 is important in the activation, differentiation
and growth of hematopoietic cells, particularly T lymphocytes and
NK cells. In several animal models, vaccination with such
cytokine-transfected DCs induced rejection of the tumors and, in
certain cases, protection against re-challenge with the parental
tumor. In this study, we investigated the therapeutic effects of
DCs co-transfected with AdIL-2 and AdAFP. We observed only small
amounts of IL-2 in the supernatants of unmodified DCs, GFP-DCs or
AFP-DCs which may be insufficient for the stimulation of T-cell
proliferation and this may explain the failure of the AFP-DC
vaccine to effectively control tumor growth compared with
AFP/IL-2-DC, while a higher level of IL-2 production by the DCs was
detected following IL-2 transfection. In vitro results
suggested that AFP/IL-2-DCs enhance antigen-specific antitumor
efficacy more potently than IL-2-DCs or AFP-DCs. In the animal
experiment, AFP-DC and IL-2-DC vaccines inhibited the tumor growth
significantly compared with the DC vaccine; however, the tumor
inhibition by AFP/IL-2-DC vaccine was the most potent.
To conclude, the co-transfection of DCs with AFP and
IL-2 genes may be further developed into a potential combination
therapy strategy for adoptive cellular immunotherapy.
Genetically-modified DCs offer a great opportunity for the
immunotherapy of patients with HCC. This study therefore provides a
promising strategy for a novel gene therapy for the treatment of
HCC.
Abbreviations:
|
Ad
|
adenovirus;
|
|
APCs
|
antigen-presenting cells;
|
|
CTLs
|
cytotoxic T lymphocytes;
|
|
DCs
|
dendritic cells;
|
|
GM-CSF
|
granulocyte macrophage colony
stimulating factor;
|
|
IL-2
|
inter-leukin-2;
|
|
HCC
|
hepatocellular carcinoma;
|
|
IL-4
|
interleukin-4;
|
|
MOI
|
multiplicity of infection;
|
|
TAA
|
tumor-associated antigen
|
Acknowledgements
The authors thank Professor Li Fan and
Technician XiaoMing Si for help in collecting peripheral blood from
HCC patients and Dr ZengHui Teng and Dr LongYang Ma for technical
assistance. This study was supported by the National Natural
Science Foundation of China (No.30901763).
References
|
1
|
Nakamoto Y and Kaneko S: Dendritic
cell-based immunotherapy for hepatocellular carcinoma. Gan To
Kagaku Ryoho. 37:413–416. 2010.(In Japanese).
|
|
2
|
Cohen S, Haimovich J and Hollander N:
Dendritic cell-based therapeutic vaccination against myeloma:
vaccine formulation determines efficacy against light chain
myeloma. J Immunol. 182:1667–1673. 2009. View Article : Google Scholar
|
|
3
|
Di Nicola M, Carlo-Stella C, Anichini A,
et al: Clinical protocol. Immunization of patients with malignant
melanoma with autologous CD34+ cell-derived dendritic
cells transduced ex vivo with a recombinant replication-deficient
vaccinia vector encoding the human tyrosinase gene: a phase I
trial. Hum Gene Ther. 14:1347–1360. 2003.PubMed/NCBI
|
|
4
|
Dubsky P, Ueno H, Piqueras B, et al: Human
dendritic cell subsets for vaccination (Review). J Clin Immunol.
25:551–572. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cao DY, Yang JY, Dou KF, Ma LY and Teng
ZH: α-fetoprotein and interleukin-18 gene-modified dendritic cells
effectively stimulate specific type-1 CD4- and CD8-mediated T-cell
response from hepatocellular carcinoma patients in vitro. Hum
Immunol. 68:334–341. 2007.
|
|
6
|
Lapteva N, Aldrich M, Weksberg D, et al:
Targeting the intratumoral dendritic cells by the oncolytic
adenoviral vaccine expressing RANTES elicits potent antitumor
immunity. J Immunother. 32:145–156. 2009. View Article : Google Scholar
|
|
7
|
Chen L, Tang XD, Yu ST, et al: Induction
of anti-tumour immunity by dendritic cells transduced with hTERT
recombinant adenovirus in mice. J Pathol. 217:685–692. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Buchsel PC and DeMeyer ES: Dendritic
cells: Emerging roles in tumor immunotherapy (Review). Clin J Oncol
Nurs. 10:629–640. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Butterfield LH, Ribas A, Potter DM and
Economou JS: Spontaneous and vaccine induced AFP-specific T cell
phenotypes in subjects with AFP-positive hepatocellular cancer.
Cancer Immunol Immunother. 56:1931–1943. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Evdokimova VN and Butterfield LH:
Alpha-fetoprotein and other tumour associated antigens for
immunotherapy of hepatocellular cancer (Review). Expert Opin Biol
Ther. 8:325–336. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gardini A, Ercolani G, Riccobon A, et al:
Adjuvant, adoptive immunotherapy with tumor infiltrating
lymphocytes plus interleukin-2 after radical hepatic resection for
colorectal liver metastases: 5-year analysis. J Surg Oncol.
87:46–52. 2004.
|
|
12
|
Dillman R, Schiltz P, DePriest C, et al:
Tumor-infiltrating lymphocytes and interleukin-2: dose and
schedules of administration in the treatment of metastatic cancer.
Cancer Biother Radiopharm. 19:730–737. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xian J, Yang H, Lin Y and Liu S:
Combination nonviral murine interleukin 2 and interleukin 12 gene
therapy and radiotherapy for head and neck squamous cell carcinoma.
Arch Otolaryngol Head Neck Surg. 131:1079–1085. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bray D, Yu SZ, Koprowski H II, Rhee J,
Kumar S, Pericle F, Suntharalingam M, Van Echo DA, Li D and
O’Malley BW Jr: Combination nonviral interleukin 2 gene therapy and
external-beam radiation therapy for head and neck cancer. Arch
Otolaryngol Head Neck Surg. 129:618–622. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li D, Zeiders JW, Liu S, Guo M, Xu Y,
Bishop JS and O’Malley BW Jr: Combination nonviral cytokine gene
therapy for head and neck cancer. Laryngoscope. 111:815–820. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kimura Y, Mizuno H, Satake K, Tahara H and
Tsukuda M: Effects of combined therapy with interleukin 2 and
interleukin 12 gene-transfected tumor vaccine for head and neck
carcinoma. Arch Otolaryngol Head Neck Surg. 129:1181–1185. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
O’Malley BW Jr, Li D, McQuone SJ and
Ralston R: Combination nonviral interleukin-2 gene immunotherapy
for head and neck cancer: from bench top to bedside. Laryngoscope.
115:391–404. 2005.PubMed/NCBI
|
|
18
|
Li D, Ronson B, Guo M, Liu S, Bishop JS,
Van Echo DA and O’Malley BW Jr: Interleukin 2 gene transfer
prevents NKG2D suppression and enhances antitumor efficacy in
combination with cisplatin for head and neck squamous cell cancer.
Cancer Res. 62:4023–4028. 2002.PubMed/NCBI
|
|
19
|
Ovali E, Dikmen T, Sonmez M, Yilmaz M,
Unal A, Dalbasti T, et al: Active immunotherapy for cancer patients
using tumor lysate pulsed dendritic cell vaccine: a safety study. J
Exp Clin Cancer Res. 26:209–214. 2007.PubMed/NCBI
|
|
20
|
Figdor CG, de Vries IJ, Lesterhuis WJ and
Melief CJ: Dendritic cell immunotherapy: mapping the way. Nat Med.
10:475–480. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Koya RC, Weber JS, Kasahara N, et al:
Making dendritic cells from the inside out: Lentiviral
vector-mediated gene delivery of granulocyte-macrophage
colony-stimulating factor and interleukin 4 into CD14+
monocytes generates dendritic cells in vitro. Hum Gene Ther.
15:733–748. 2004. View Article : Google Scholar
|
|
22
|
Liu Y, Chiriva-Internati M, Grizzi F, et
al: Rapid induction of cytotoxic T-cell response against cervical
cancer cells by human papillomavirus type 16 E6 antigen gene
delivery into human dendritic cells by an adeno-associated virus
vector. Cancer Gene Ther. 8:948–957. 2001. View Article : Google Scholar
|
|
23
|
Asemissen AM and Brossart P: Vaccination
strategies in patients with renal cell carcinoma. Cancer Immunol
Immunother. 58:1169–1174. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Timares L, Douglas JT, Tillman BW, et al:
Adenovirus-mediated gene delivery to dendritic cells. Methods Mol
Biol. 246:139–154. 2004.PubMed/NCBI
|
|
25
|
Chiriva-Internati M, Liu Y, Salati E, et
al: Efficient generation of cytotoxic T lymphocytes against
cervical cancer cells by adeno-associated virus/human
papillomavirus type 16 E7 antigen gene transduction into dendritic
cells. Eur J Immunol. 32:30–38. 2002. View Article : Google Scholar
|
|
26
|
Motta I, André F, Lim A, et al:
Cross-presentation by dendritic cells of tumor antigen expressed in
apoptotic recombinant canarypox virus-infected dendritic cells. J
Immunol. 167:1795–1802. 2001. View Article : Google Scholar
|
|
27
|
Diebold SS, Cotton M, Koch N and Zenke M:
MHC class II presentation of endogenously expressed antigens by
transfected dendritic cells. Gene Ther. 8:487–493. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Moll H: Antigen delivery by dendritic
cells (Review). Int J Med Microbiol. 294:337–344. 2004. View Article : Google Scholar
|
|
29
|
Alisa A, Ives A, Pathan AA, et al:
Analysis of CD4+ T-cell responses to a novel
alpha-fetoprotein-derived epitope in hepatocellular carcinoma
patients. Clin Cancer Res. 11:686–6694. 2005.
|
|
30
|
Mizukoshi E, Nakamoto Y, Tsuji H, et al:
Identification of alpha-fetoprotein-derived peptides recognized by
cytotoxic T lymphocytes in HLA-A24+ patients with
hepatocellular carcinoma. Int J Cancer. 118:1194–1204. 2006.
View Article : Google Scholar : PubMed/NCBI
|