Introduction
Plant extracts containing isoflavones have been the
focus of numerous studies during the last decade due to their
protective effects against menopausal symptoms and a variety of
disorders, including cardiovascular disease, cancer,
hyper-lipidemia and osteoporosis (1,2). In
addition, the potential of dietary isoflavones in the prevention of
diabetes mellitus has attracted increased attention among the
public and in the medical community in recent years (2,3). Soy
isoflavones were reported to be beneficial for correcting
hyperglycemia and improving lipid profiles in streptozotocin
(STZ)-induced diabetic rats (4)
and obese Zucker rats (5). The
intervention studies of patients with type 2 diabetes have reported
favorable effects of soy isoflavones on glycated hemoglobin or
insulin resistance (6,7). However, other studies have reported
that soy isoflavone intake does not exert beneficial effects on
patients with type 2 diabetes (8,9).
Therefore, the effect of isoflavones on diabetes remains
inconclusive and more investigations need to be performed using
isoflavones from plants other than soy.
While soy isoflavones are the most studied
isoflavones used in studies on diabetes, few data are available for
red clover (Trifolium pratense) isoflavones. Isoflavones
from red clover differ from soy; the principal isoflavones in red
clover are biochanin A, formononetin, genistein and daidzein while
those in soy consist solely of genistein and daidzein. We
previously reported that red clover extract ameliorated
dyslipidemia in STZ-induced type 1 diabetic mice, but did not
correct hyperglycemia (10).
However, whether red clover extract exerts antidiabetic and
hypolipidemic effects in type 2 diabetic animals remains
unclear.
Among the mechanisms whereby isoflavones ameliorate
hyperglycemia and dyslipidemia, one may be the activation of
peroxisome-proliferator activated receptors (PPARs), nuclear
receptors that participate in cellular lipid homeostasis and
insulin action (11). PPARγ
ligands, like glitazones, are clinically used to treat type 2
diabetes as insulin-sensitizing drugs and PPARα ligands, like
fibrates, are used to manage elevated blood lipid levels and type 2
diabetes as hypolipidemic agents. Isoflavones from red clover were
demonstrated to be a potent PPARα/γ dual agonist, and among those
isoflavones, biochanin A is a more potent PPARα/γ agonist than its
metabolite genistein, and formononetin is also more potent than
daidzein (12). Thus, further
study on the antidiabetic effects and molecular mechanisms of red
clover extract in type 2 diabetes animal models needs to be
conducted. In the present study, using db/db mice as a model of
type 2 diabetes, we aimed to determine whether red clover extract
exerts antidiabetic and hypolipidemic effects in type 2 diabetic
animals. We also investigated whether the PPARα/γ agonist
mechanisms of red clover isoflavones are involved in the
hyperglycemia and dyslipidemia improvement.
Materials and methods
Materials
Red clover extract was purchased from a common
Chinese pharmacy and was standardized to 10% isoflavones
(consisting of 10.2% formononetin, 9.6% biochanin A, 0.32%
genistein and 0.08% daidzein).
Animal experiments
All experiments were conducted according to
protocols and guidelines approved by Longyan University
Institutional Animal Care and Use Committee. db/db
(BKS.Cg-m/Leprdb/J) mice were obtained from the Jackson
Laboratory (Bar Harbor, ME, USA). All animals were maintained on a
standard laboratory diet under a 12/12-h light/dark schedule. Male
db/db mice, 7–8 weeks of age, were randomly divided into 3
experimental groups (each containing 6 animals): db/db mice, db/db
+ 10 mg/kg/day red clover extract, db/db + 50 mg/kg/day red clover
extract. Red clover extract was administered orally in 0.5% sodium
carboxymethyl cellulose (CMC) suspension and continued for 35
days.
Determination of blood glucose and serum
lipids
The blood glucose level was measured periodically
throughout the experimental period using a glucometer (OneTouch
Ultra; LifeScan, Inc., Milpitas, CA, USA). At the end of the red
clover extract treatment, the mice were sacrificed and blood was
collected by orbital sinus puncture. Serum triglycerides (TG) and
total cholesterol (TC) were measured using commercial kits
(Jiancheng, Nanjing, China).
Liver lipid analyses
Liver lipid was extracted by chloroform/methanol.
Briefly, pulverized liver was homogenized in PBS, then extracted
with chloroform/methanol (2:1), dried overnight and resuspended in
a solution of 60% butanol 40% Triton X-114/methanol (2:1). Liver
total TG and cholesterol levels were measured using colorimetric
assays (Jiancheng).
Quantitative analyses of the mRNA
expression by real-time PCR
Total RNA was isolated from tissues using the TRIzol
reagent (Invitrogen, Carlsbad, CA, USA) according to the
manufacturer’s instructions. Complementary DNA (cDNA) was
synthesized from hepatic mRNA using RevertAid™ First Strand cDNA
Synthesis kits (Fermentas, Vilnius, Lithuania). Hepatic acetyl CoA
oxidase (ACO), carnitine palmitoyl transferase-1 (CPT-1),
apolipoprotein A5 (APOA5), APOC3, sterol regulatory element binding
protein 1c (SREBP-1c) and fatty acid synthase (FAS) mRNA were
analyzed with the specific primers listed in Table I. Real-time polymerase chain
reactions were assayed using the FastStart Universal SYBR-Green
Master (Rox; Roche Applied Science, Mannheim, Germany). Each Ct
value was normalized to 18S rRNA.
 | Table IPrimer sequences used for
amplification of mRNA by real-time PCR. |
Table I
Primer sequences used for
amplification of mRNA by real-time PCR.
| Gene | Sequences |
|---|
| PPARα | Forward:
5′-AAGAGGGCTGAGCGTAGGT-3′ |
| Reverse:
5′-GGCCGGTTAAGACCAGACT-3′ |
| APOC3 | Forward:
5′-GTGTTGCAGATGTGCCTGTT-3′ |
| Reverse:
5′-GGAGGGGTGAAGACATGAGA-3′ |
| APOA5 | Forward:
5′-GAACGCTTGGTGACTGGAAT-3′ |
| Reverse:
5′-TCGCCTTACGTGTGAGTTTG-3′ |
| ACO | Forward:
5′-CCACATATGACCCCAAGACC-3′ |
| Reverse:
5′-AGGCATGTAACCCGTAGCAC-3′ |
| CPT-1 | Forward:
5′-GTCAAGCCAGACGAAGAACA-3′ |
| Reverse:
5′-CGAGAAGACCTTGACCATAG-3′ |
| FAS | Forward:
5′-TGCTCCCAGCTGCAGGC-3′ |
| Reverse:
5′-GCCCGGTAGCTCTGGGTGTA-3′ |
| SREBP-1c | Forward:
5′-ATCGGCGCGGAAGCTGTCGGGGTAGCGTC-3′ |
| Reverse:
5′-ACTGTCTTGGTTGTTGATGAGCTGGAGCAT-3′ |
| PPARγ | Forward:
5′-CAAACCCTTACCACGGTTGA-3′ |
| Reverse:
5′-CCATTGGGTCAGCTCTTGTGA-3′ |
| Glucokinase | Forward:
5′-TGAGATGGATGTGGTGGCAA-3′ |
| Reverse:
5′-CATGCCGACCTCACATTGG-3′ |
| CD36 | Forward:
5′-TGTTCCTCGCCATGAAATGA-3′ |
| Reverse:
5′-GCTAGGCAGCATGGAACTTGA-3′ |
| 18S rRNA | Forward:
5′-CGACGACCCATTCGAACGTCT-3′ |
| Reverse:
5′-CTCTCCGGAATCGAACCCTGA-3′ |
Statistical analysis
Quantitative data are expressed as mean ± SEM. The
Student’s t-test was used for pairwise comparisons and one-way
ANOVA with Newman-Keuls multiple comparison test for multigroup
analyses. P<0.05 was considered to indicate a statistically
significant result.
Results
Red clover extract attenuates
hyperglycemia in db/db mice by activating PPARγ
To investigate the effect of red clover extract on
the development of diabetes, we treated two groups of mice with red
clover extract at the doses of 10 or 50 mg/kg/day, respectively,
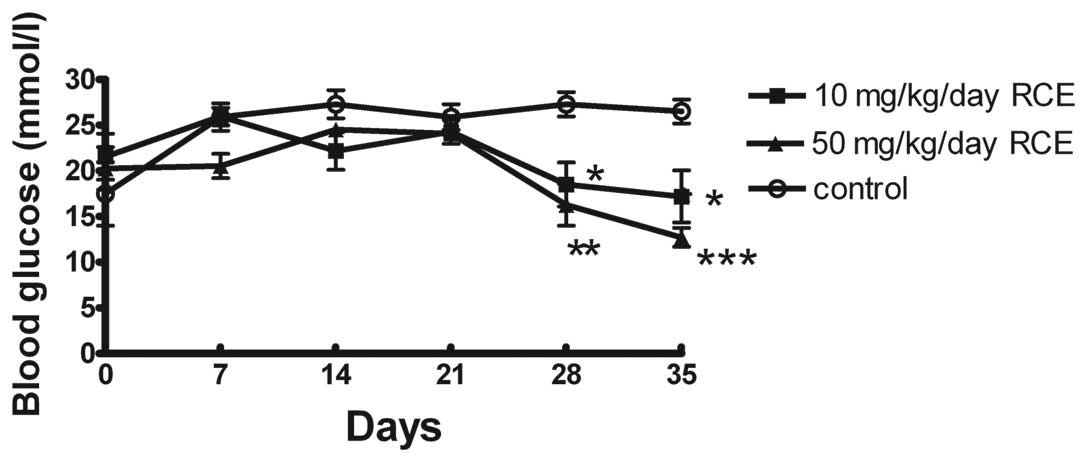
for 5 weeks. Fig. 1 shows the
effect of red clover extract treatment on blood glucose level in
db/db diabetic mice. After 4 weeks treatment with red clover
extract, the blood glucose levels of the 10 and 50 mg/kg/day red
clover extract-treated db/db mice were 18.5±2.4 and 16.3±2.3
mmol/l, respectively, compared with 27.3±1.3 mmol/l in the control
untreated db/db mice (P<0.05 and P<0.01, respectively). In
addition, after 5 weeks of treatment with red clover extract, the
blood glucose levels of the 10 and 50 mg/kg/day red clover
extract-treated db/db mice were 17.2±2.9 and 12.7±1.0 mmol/l,
respectively, compared with 26.5±1.3 mmol/l in the control
untreated db/db mice (P<0.05 and P<0.001, respectively). Our
data suggest that red clover extract treatment attenuates
hyperglycemia in type 2 diabetic animals.
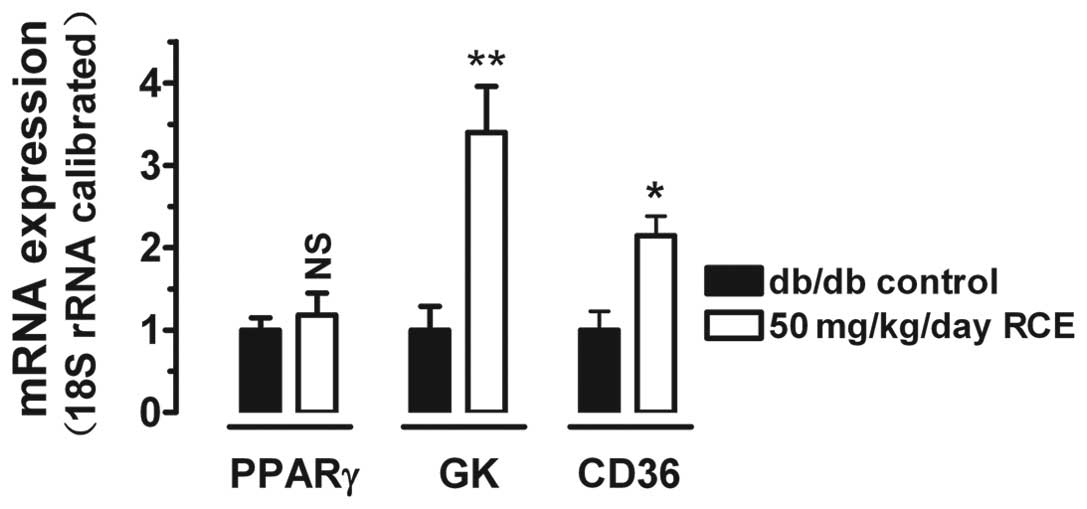
Glucokinase, a key enzyme involved in the regulation
of glucose metabolism, and CD36, a scavenger receptor involved in
hepatic fatty acid uptake, are two hepatic genes regulated by PPARγ
(13,14). To determine whether red clover
extract attenuates hyperglycemia in diabetic mice by activating
PPARγ, we analyzed the mRNA expression of PPARγ, glucokinase and
CD36. After 5 weeks of red clover extract treatment, expression of
glucokinase and CD36 was significantly upregulated (3.4-fold,
P<0.01 and 2.1-fold, P<0.05) in 50 mg/kg/day red clover
extract-treated diabetic mice (Fig.
2).
Red clover extract regulates lipid
homeostasis in db/db mice by activating hepatic PPARα and
inhibiting hepatic FAS
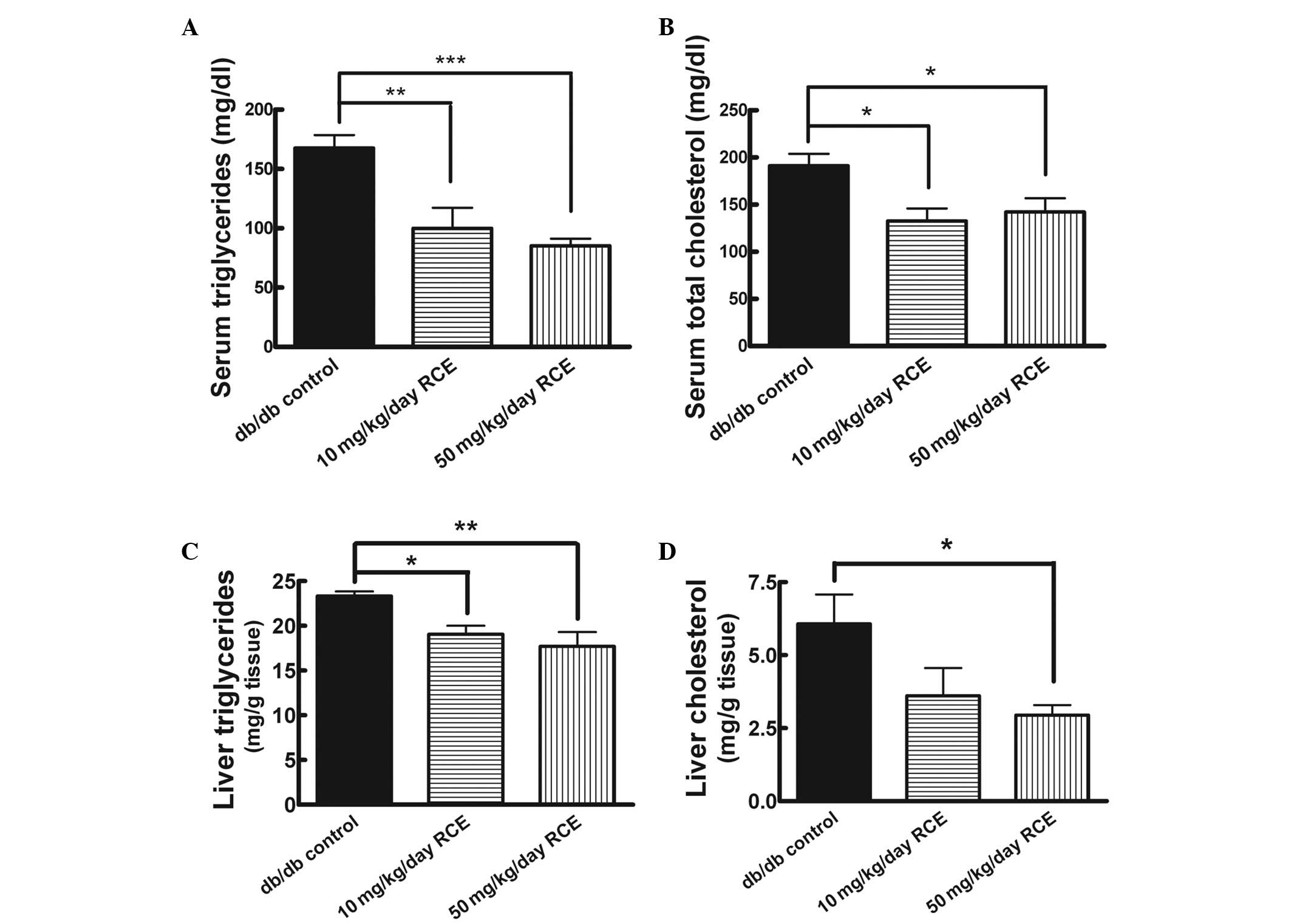
To determine the effects of red clover extract on
the overall lipid metabolism in the diabetic animals, we examined
the levels of serum TG and TC in db/db mice with or without red
clover extract treatment for a period of 5 weeks. Red clover
extract-treated db/db mice had significantly lower blood TG
(99.9±17.4 mg/dl for db/db + 10 mg/kg red clover extract vs.
167.5±11.0 mg/dl for db/db, P<0.01; 85.2±6.0 mg/dl for db/db +
50 mg/kg red clover extract vs. 167.5±11.0 mg/dl for db/db,
P<0.001) in comparison with the untreated db/db mice (Fig. 3A). In addition, both 10 and 50
mg/kg/day of red clover extract treatment caused significant
decreases in serum TC levels in db/db mice (132.7±13.3 mg/dl for
db/db + 10 mg/kg red clover extract vs. 191.1±12.8 mg/dl for db/db,
P<0.05; 142.2±14.7 mg/dl for db/db + 50 mg/kg red clover extract
vs. 191.1±12.8 mg/dl for db/db, P<0.05; Fig. 3B). However, the TC lowering effect
was not dose-dependent. Notably, no apparent difference in food
consumption was observed in all the experimental groups (data not
shown).
In addition, we investigated the effects of red
clover extract on the liver lipid levels in db/db mice. As shown in
Fig. 3C and D, 10 mg/kg/day and 50
mg/kg/day red clover extract treatment in db/db mice significantly
reduced hepatic TG by ∼18.3 and 24.1%, respectively, (19.1±1.0 mg/g
tissue for db/db + 10 mg/kg red clover extract vs. 23.3±0.5 mg/g
tissue for db/db, P<0.05; 17.7±1.6 mg/g tissue for db/db + 50
mg/kg red clover extract vs. 23.3±0.5 mg/g tissue for db/db,
P<0.01) and reduced hepatic cholesterol by ∼40.5 and 51.5%,
respectively (3.6±0.9 mg/g tissue for db/db + 10 mg/kg red clover
extract vs. 6.1±1.0 mg/g tissue for db/db, P>0.05; 2.9±0.3 mg/g
tissue for db/db + 50 mg/kg red clover extract vs. 6.1±1.0 mg/g
tissue for db/db, P<0.05).
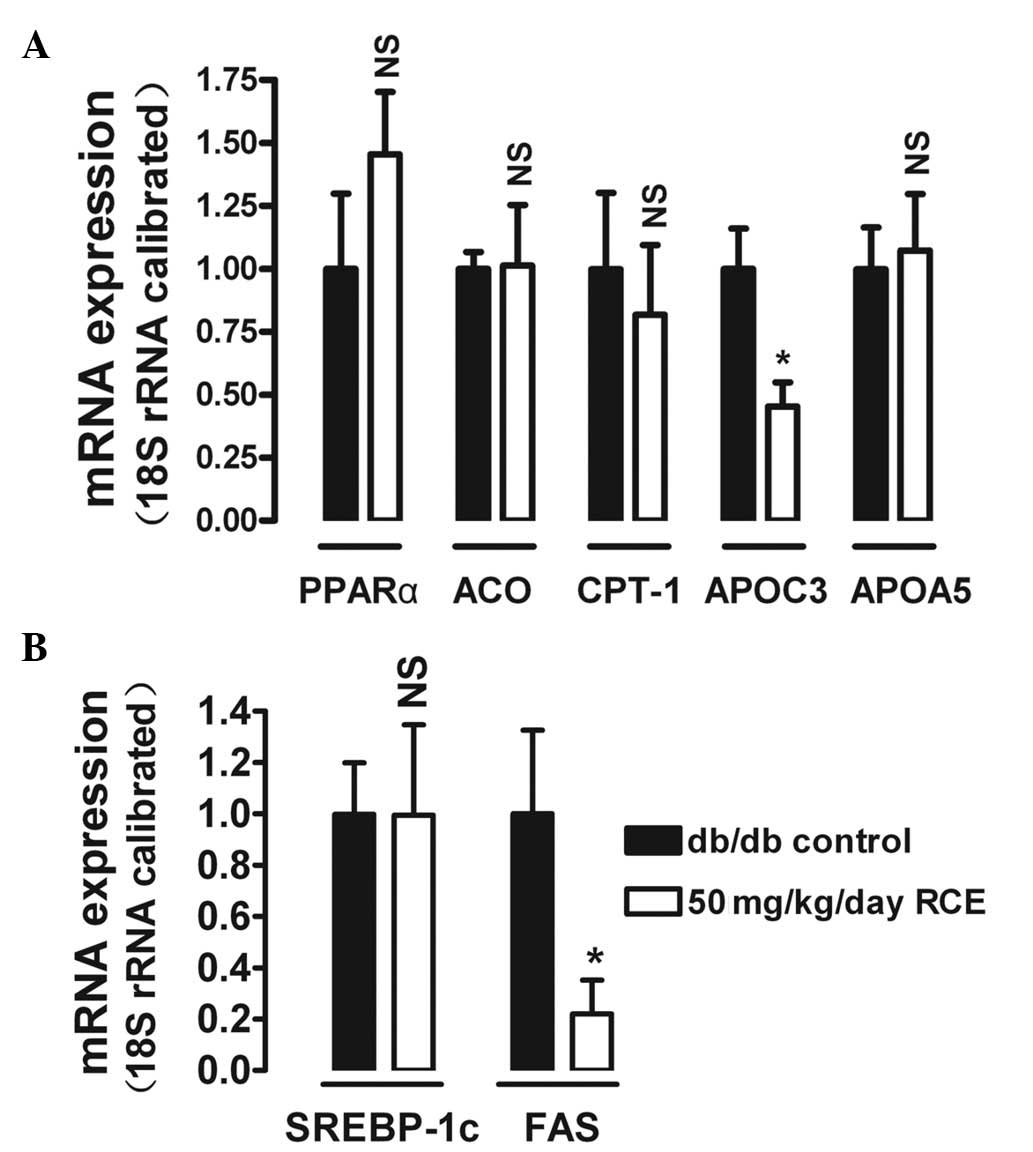
Isoflavones like biochanin A and formononetin are
reported to be potent agonists of PPARα (12,15,16).
To determine the effects of red clover extract treatment on certain
target genes of PPARα in the liver, we analyzed hepatic ACO, CPT-1,
APOC3 and APOA5 mRNA expression in db/db diabetic mice. After 5
weeks of red clover extract treatment, APOC3, a protein capable of
inhibiting TG hydrolysis by lipoprotein lipase (LPL) was
downregulated by 54.8% (P<0.05) in the 50 mg/kg red clover
extract-treated diabetic mice (Fig.
4A). In contrast to APOC3, only slight and insignificant
alterations were observed for APOA5, ACO and CPT-1.
The effect of red clover extract on fatty acid
synthesis was also investigated. After 5 weeks of red clover
extract treatment, the hepatic mRNA expression of FAS was
significantly downregulated by 78% (P<0.05) in the 50 mg/kg red
clover extract-treated diabetic mice (Fig. 4B). However, mRNA expression of
SREBP-1c, a protein that regulates FAS expression, was not changed
by red clover extract treatment.
Discussion
In vitro studies have demonstrated that
biochanin A, formononetin and genistein, three of the isoflavones
from red clover are effective PPARγ agonists (12,15,16).
In this study, we demonstrated that red clover extract treatment
resulted in activation of known PPARγ-regulated genes in the liver
in vivo. The activation of PPARγ improves insulin
sensitivity, which maintains the blood glucose homeostasis
(17). The glucokinase activation
resulting from PPARγ activation also maintains the blood glucose
homeostasis since glucokinase is a glucose sensor (14). Therefore, the PPARγ agonist
property of red clover extract could partially explain its
hypoglycemic mechanism. However, certain studies reported that
purified isoflavones were ineffective at activating PPARγ whereas
soy protein isolate was effective in vivo (18), which suggests that nonisoflavone
phytochemicals or their metabolites are responsible for the
activation of PPARγ. Therefore, whether biochanin A and
formononetin or other nonisoflavone phyto-chemicals from red clover
extract activate hepatic PPARγ in diabetic mice remains to be
investigated.
The hypoglycemic effects of isoflavones remain
controversial. Certain studies have reported that isoflavones are
beneficial for correcting hyperglycemia in STZ-induced diabetic
rats (4), obese Zucker rats
(5) and in patients with type 2
diabetes (6,7). Certain other studies have reported
that isoflavone intake does not exert anti-hyperglycemic effects on
STZ-induced diabetic mice (10)
and type 2 diabetes patients (8,9). In
addition, there are certain debates upon the hypolipidemic effect
of red clover isoflavones under various disease conditions. Several
studies reported the effect of red clover extract on improving
lipid profile (10,19–22)
whereas other studies did not identify an effect (23,24).
Our current data is consistent with certain previous studies
(10) suggesting that red clover
extract ameliorates dyslipidemia in diabetic animals. Furthermore,
we demonstrated that red clover extract downregulated the hepatic
mRNA expression of APOC3, a target gene transcriptionally regulated
by PPARα. PPARα is an important metabolic nuclear receptor that
regulates lipid metabolism through direct transcriptional control
of genes involved in peroxisomal and mitochondrial β-oxidation
pathways, fatty acid uptake, and TG catabolism (25). Hepatic activation of PPARα by its
agonists, such as WY-14643, decreases blood TG levels by
upregulating the expression of LPL and APOA5 and downregulating
APOC3 (26,27). In the present study, however, the
mRNA expression of APOA5, ACO and CPT-1, three other
PPARα-regulated genes, was not altered by red clover extract
treatment. The diversity of PPARα target gene expression following
PPARα activation was also reported in certain other studies
(10,28), yet the underlying molecular
mechanism remains unclear.
FAS is the multifunctional protein that plays a
central role in de novo fatty acid synthesis and in the
long-term regulation of lipogenesis (29). The promoter region of FAS contains
binding sites for the transcription factor called SREBP-1c
(30). In the present study, we
demonstrated that the hepatic mRNA expression of FAS in diabetic
db/db mice was significantly downregulated by red clover extract
treatment. However, SREBP-1c mRNA expression was not altered
simultaneously. SREBP is synthesized as inactive precursors and the
NH2 terminal domain of SREBP must be cleaved in a 2-step
proteolytic process by site-1 (S1P) and site-2 (S2P) proteases to
act as a transcription factor. This proteolytic release of SREBP
stimulates lipid synthesis in hepatocytes and other cells (31). Notably, genistein treatment of
HepG2 cells was found to decrease the expression of FAS but did not
change the expression of SREBP-1 mRNA, likely via the
downregulation of S1P expression and subsequent SREBP-1 proteolytic
cleavage (32). Thus, whether red
clover isoflavones reduce FAS expression by the same pathway
remains to be elucidated.
To the best of our knowledge, the present study is
the first to demonstrate the antidiabetic effect of red clover
extract in type 2 diabetic animals. Red clover extract improves
lipid homeostasis in type 2 diabetic animals. In the present study,
we demonstrated the PPARα/γ agonist activities in vivo and
elucidated the antidiabetic and hypolipidemic mechanism of red
clover extract. We also demonstrated that red clover extract
suppressed lipogenesis by inhibiting FAS activity. Our data
indicate the benefit of a dietary supplement of red clover extract
for diabetes patients.
Acknowledgements
This study was supported by the
Science and Technology Planning Project of Fujian Province, China
(grant no. 2010N0023) and the Educational Commission of Fujian
Province, China (grant no. JA10258).
References
|
1
|
Wong MC, Emery PW, Preedy VR and Wiseman
H: Health benefits of isoflavones in functional foods? Proteomic
and meta-bonomic advances. Inflammopharmacology. 16:235–239. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Usui T: Pharmaceutical prospects of
phytoestrogens. Endocr J. 53:7–20. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bhathena SJ and Velasquez MT: Beneficial
role of dietary phytoestrogens in obesity and diabetes. Am J Clin
Nutr. 76:1191–1201. 2002.PubMed/NCBI
|
|
4
|
Lee JS: Effects of soy protein and
genistein on blood glucose, antioxidant enzyme activities, and
lipid profile in streptozotocin-induced diabetic rats. Life Sci.
79:1578–1584. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mezei O, Banz WJ, Steger RW, Peluso MR,
Winters TA and Shay N: Soy isoflavones exert antidiabetic and
hypolipidemic effects through the PPAR pathways in obese Zucker
rats and murine RAW 264.7 cells. J Nutr. 133:1238–1243.
2003.PubMed/NCBI
|
|
6
|
Jayagopal V, Albertazzi P, Kilpatrick ES,
et al: Beneficial effects of soy phytoestrogen intake in
postmenopausal women with type 2 diabetes. Diabetes Care.
25:1709–1714. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Z, Hong K, Saltsman P, et al: Long-term
efficacy of soy-based meal replacements vs an individualized diet
plan in obese type II DM patients: relative effects on weight loss,
metabolic parameters, and C-reactive protein. Eur J Clin Nutr.
59:411–418. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gobert CP, Pipe EA, Capes SE, Darlington
GA, Lampe JW and Duncan AM: Soya protein does not affect glycaemic
control in adults with type 2 diabetes. Br J Nutr. 103:412–421.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
González S, Jayagopal V, Kilpatrick ES,
Chapman T and Atkin SL: Effects of isoflavone dietary
supplementation on cardiovascular risk factors in type 2 diabetes.
Diabetes Care. 30:1871–1873. 2007.PubMed/NCBI
|
|
10
|
Qiu L, Ye H, Chen L, Hong Y, Zhong F and
Zhang T: Red clover extract ameliorates dyslipidemia in
streptozotocin-induced diabetic C57BL/6 mice by activating hepatic
PPARα. Phytother Res. 26:860–864. 2012.PubMed/NCBI
|
|
11
|
Kota BP, Huang TH and Roufogalis BD: An
overview on biological mechanisms of PPARs. Pharmacol Res.
51:85–94. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shen P, Liu MH, Ng TY, Chan YH and Yong
EL: Differential effects of isoflavones, from Astragalus
membranaceus and Pueraria thomsonii, on the activation
of PPARalpha, PPARgamma, and adipocyte differentiation in vitro. J
Nutr. 136:899–905. 2006.PubMed/NCBI
|
|
13
|
Inoue M, Ohtake T, Motomura W, et al:
Increased expression of PPARgamma in high fat diet-induced liver
steatosis in mice. Biochem Biophys Res Commun. 336:215–222. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim SY, Kim HI, Park SK, et al: Liver
glucokinase can be activated by peroxisome proliferator-activated
receptor-gamma. Diabetes. 53(Suppl 1): S66–S70. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mueller M, Hobiger S and Jungbauer A: Red
clover extract: a source for substances that activate peroxisome
proliferator-activated receptor alpha and ameliorate the cytokine
secretion profile of lipopolysaccharide-stimulated macrophages.
Menopause. 17:379–387. 2010.
|
|
16
|
Qiu L, Lin B, Lin Z, Lin Y, Lin M and Yang
X: Biochanin A ameliorates the cytokine secretion profile of
lipopolysaccharide-stimulated macrophages by a PPARγ-dependent
pathway. Mol Med Report. 5:217–222. 2012.PubMed/NCBI
|
|
17
|
Guo L and Tabrizchi R: Peroxisome
proliferator-activated receptor gamma as a drug target in the
pathogenesis of insulin resistance. Pharmacol Ther. 111:145–173.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ronis MJ, Chen Y, Badeaux J and Badger TM:
Dietary soy protein isolate attenuates metabolic syndrome in rats
via effects on PPAR, LXR, and SREBP signaling. J Nutr.
139:1431–1438. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Asgary S, Moshtaghian J, Naderi G, et al:
Effects of dietary red clover on blood factors and cardiovascular
fatty streak formation in hypercholesterolemic rabbits. Phytother
Res. 21:768–770. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Geller SE and Studee L: Soy and red clover
for mid-life and aging. Climacteric. 9:245–263. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lukaczer D, Darland G, Tripp M, et al:
Clinical effects of a proprietary combination isoflavone
nutritional supplement in menopausal women: a pilot trial. Altern
Ther Health Med. 11:60–65. 2005.PubMed/NCBI
|
|
22
|
Schult TM, Ensrud KE, Blackwell T,
Ettinger B, Wallace R and Tice JA: Effect of isoflavones on lipids
and bone turnover markers in menopausal women. Maturitas.
48:209–218. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Haines C, James A, Sahota D, et al:
Comparison between phytoestrogens and estradiol in the prevention
of atheroma in ovariectomized cholesterol-fed rabbits. Climacteric.
9:430–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Howes JB, Sullivan D, Lai N, et al: The
effects of dietary supplementation with isoflavones from red clover
on the lipoprotein profiles of post menopausal women with mild to
moderate hypercholesterolaemia. Atherosclerosis. 152:143–147. 2000.
View Article : Google Scholar
|
|
25
|
Lefebvre P, Chinetti G, Fruchart JC and
Staels B: Sorting out the roles of PPAR alpha in energy metabolism
and vascular homeostasis. J Clin Invest. 116:571–580. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Auwerx J, Schoonjans K, Fruchart JC and
Staels B: Transcriptional control of triglyceride metabolism:
fibrates and fatty acids change the expression of the LPL and apo
C-III genes by activating the nuclear receptor PPAR.
Atherosclerosis. 124(Suppl): S29–S37. 1996. View Article : Google Scholar
|
|
27
|
Vu-Dac N, Gervois P, Jakel H, et al:
Apolipoprotein A5, a crucial determinant of plasma triglyceride
levels, is highly responsive to peroxisome proliferator-activated
receptor alpha activators. J Biol Chem. 278:17982–17985. 2003.
View Article : Google Scholar
|
|
28
|
Qiu L, Wu X, Chau JF, et al: Aldose
reductase regulates hepatic peroxisome proliferator-activated
receptor alpha phosphorylation and activity to impact lipid
homeostasis. J Biol Chem. 283:17175–17183. 2008. View Article : Google Scholar
|
|
29
|
Semenkovich CF: Regulation of fatty acid
synthase (FAS). Prog Lipid Res. 36:43–53. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Horton JD and Shimomura I: Sterol
regulatory element-binding proteins: activators of cholesterol and
fatty acid biosynthesis. Curr Opin Lipidol. 10:143–150. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Horton JD, Goldstein JL and Brown MS:
SREBPs: activators of the complete program of cholesterol and fatty
acid synthesis in the liver. J Clin Invest. 109:1125–1131. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shin ES, Lee HH, Cho SY, Park HW, Lee SJ
and Lee TR: Genistein downregulates SREBP-1 regulated gene
expression by inhibiting site-1 protease expression in HepG2 cells.
J Nutr. 137:1127–1131. 2007.PubMed/NCBI
|