Introduction
Depression is a common mental disorder, with main
characteristics including regular negative moods, decreased
physical activity, loss of interest in usual activities, feeling of
helplessness and suicidal tendencies (1). Although depression has been widely
studied, the pathogenesis of depression remains unknown. Until now,
antidepressants available on the pharmaceutical market mainly
include tricyclic antidepressants, monoamine oxidase inhibitors,
selective serotonin reuptake inhibitors and serotonin-noradrenergic
reuptake inhibitors (2). However,
numerous antidepressants frequently produce side-effects, including
sedation, sleep disturbance, cognitive impairment and sexual
dysfunction (3). Accordingly, the
development of more effective antidepressants without any (or with
fewer) adverse effects is required.
Heat shock protein 70 (Hsp70) functions as a
molecular chaperone that mediates a highly conserved system of
cellular responses to various stimuli (4). When the cell is exposed to stress,
Hsp70 is induced to maintain cellular homeostasis (5,6).
Several reports have shown that Hsp70 has a protective role in
various models of nervous system injury, including oxidative stress
and ischemic-reperfusion injury (6–8). In
recent years, a huge body of evidence has accumulated suggesting
that the activity of Hsp70 is associated with the pathogenesis of
depression. The activity of the glucocorticoid receptor, which
plays an important role in depression, is regulated by Hsp70
(9,10). The 162-base deletion in the
5′-flanking region of Hsp70 gene mRNA was observed in patients with
depression (11). It also has been
shown that antidepressants may increase Hsp70 expression, which is
a possible mechanism underlying the therapeutic efficacy of
antidepressants (12–14). As such, Hsp70 is a new therapeutic
target for depression.
Geranylgeranylacetone (GGA) is an acyclic isoprenoid
compound that has been widely used in clinic as an anti-ulcer drug.
Numerous studies have demonstrated that GGA is a non-toxic Hsp70
inducer, which safely induces Hsp70 expression in gastric mucosa,
intestine, liver, heart and retina (15–18).
GGA is a lipid-soluble reagent and easily crosses the blood-brain
barrier to exert neuroprotective activity. It has been reported
that GGA administration induced the expression of heat shock
proteins including Hsp70 and suppressed polyglutamine toxicity in
cell culture and mouse models of spinal and bulbar muscular atrophy
(19). GGA is also relevant to the
treatment and prevention of various neural diseases, including
ischemia-reperfusion injury and morphine addiction (20–22).
However, the effect of GGA on depression has not yet
been investigated. In the present study, we suggest that when GGA
is administered, it induces Hsp70, which may in turn alleviate
behavioral abnormalities in depression. The present study was
performed to investigate the possible antidepressant effects of GGA
in the chronic mild stress (CMS) model of depression in rats.
Materials and methods
Reagents
GGA was purchased from Eisai (Tokyo, Japan).
Antibodies [Hsp70, monoamine oxidase-A (MAO-A), caspase-3 and
β-actin] were obtained from Santa Cruz Biotechnology, Inc. (Santa
Cruz, CA, USA). DAB color developing reagent was purchased from
Boao Seng Company (Beijing, China). TRIzol reagent was obtained
from Molecular Research Center Company (Cincinnati, OH, USA).
RevertAid™ First Strand cDNA Synthesis kit and dNTPS were purchased
from Fermentas (Walldorf, Baden, Germany).
Animals
Male Sprague-Dawley rats (Chengdu Dashuo Biological
Technology Co., Ltd., Chengdu, China) weighing 180±20 g were used
in the experiments. The rats were allowed to habituate to the
housing facilities for 1 week before the experiments began. Rats
were maintained on a 12 h light-dark cycle and had free access to
food and water. The use of animals was performed in accordance with
the National Institutes of Health Guide for the Care and Use of
Laboratory Animals and approved by the Local Committee on Animal
Use and Protection.
CMS procedure
The method of stress was modified according to
previous studies and previous practice in our laboratory. The
stressed groups were subjected to the following stressors for three
weeks: water and food deprivation for 24 h; physical restraint for
30 min (rats were placed into small iron compartments, which were
15-cm high and hand-made; the diameter of this compartment could be
adjusted appropriately from 3–6 cm according to the size of rats);
rotation for 30 min (motor-driven rotator, the velocity of rotation
was 33 rpm); damp environment for 12 h (200 ml water was added to
100 g bedding); inclined cage for 12 h at 45°; the rats were
combined with new invader rats for 24 h; daytime reversed and night
reversed for 12 h, respectively. The order of each stress was
randomly arranged and one type of stress was performed daily. The
rats were randomly divided into 3 groups: the control group
(control, n=12); the stress group (stress, n=12); the stress +
1,000 mg/kg GGA group (stress + GGA, n=12). The rats of the stress
+ 1,000 mg/kg GGA group were administered with GGA (1,000 mg/kg).
The rats of the stress and control groups were injected
intraperitoneally with saline (NS).
Open field test
The open-field test was conducted in a four-sided
100×100×50 cm box, divided into 25 equilateral smaller squares. The
test was conducted under a sedate environment. The rats were placed
onto the center square of the box, then their activity was measured
for 5 min. The number of crossings and rearings were scored
manually. The apparatus was cleaned between tests.
Immunohistochemistry analysis
The rats were sacrificed after the behavioral test
by deep anesthesia. The brain samples were placed into the 4%
paraformaldehyde for fixation overnight, followed by a wash with a
flow of water and dehydration by gradient alcohol. The paraffin
samples were sliced to a thickness of 5 μm. Endogenous peroxidase
was blocked with blocking solution (3% H2O2
in methanol) for 15 min, following PBS washing. The slices were
incubated in rabbit anti-mouse polyclonal Hsp70 (1:100 dilution)
for 1 h at room temperature, and then incubated with biotinylated
goat anti-rabbit IgG for 1 h. After rinsing with PBS, the slices
were incubated with albumin fluid labeled with horseradish
peroxidase for 1 h and then DAB solution was utilized for
coloration. The slices were counterstained with hematoxylin.
Reverse transcription-polymerase chain
reaction (RT-PCR)
The rats were sacrificed after the behavioral test
by deep anesthesia. The right hippocampus of rats was rapidly
dissected out, frozen and stored in a deep freezer at −80°C until
the assays. The total RNA of the hippocampus was isolated using
TRIzol reagent following the manufacturer’s instructions. The RNA
level was measured by an ultraviolet spectrophotometer by OD 260
measurements. cDNA was synthesized by reverse transcription. PCR
amplification was performed with primers designed for the genes of
interest. The primers used were as follows: HSP-70 sense,
5′-GCTGGTGAGCCACTTCGTG-3′ and antisense, 5′-TGGATCTGCGCCTTGTCC-3′
(Hsp70 PCR production, 288 bp); MAO-A sense, 5′-ATTGGAGGCGGCATC
TCAGGAT-3′ and antisense, 5′-AGGTGGGAATGCACC ACGGAAT-3′ (MAO PCR
production, 288 bp); caspase-3 sense, 5′-AACGAACGGACCTGTGG-3′ and
antisense, 5′-TTT GCATGGAAA GTGGC-3′ (caspase-3 PCR production, 390
bp); β-actin sense, 5′-CACTGCCGCATCCTCTTCCTC-3′ and antisense,
5′-CTCCTGCTTGCTGATCCACAT-3′ (β-actin PCR production, 400 bp). The
PCR products were separated by 2% agarose gel electrophoresis,
visualized with ethidium bromide, and quantified using ImageJ
software.
Western blot analysis
The rats were sacrificed after the behavioral test
by deep anesthesia. The right hippocampus of rats was rapidly
dissected out, frozen and stored in a deep freezer at −80°C until
the assays. Hippocampal tissue was homogenized in a solubilizing
solution [20 mM Tris-HCl (pH 7.0), 25 mM β-glycerophosphate, 2 mM
EGTA, 1% Triton X-100, 1 mM vanadate, 1% aprotinin, 1 mM
phenylmethylsulfonyl fluoride, 2 mM dithiothreitol] on ice for 40
min. The lysate was centrifuged at 15,000 rpm for 15 min. The
supernatant was denatured at 95°C for 5 min, then by 10 or 15%
SDS-polyacrylamide gel electrophoresis and transferred to a PVDF
membrance. Immunostaining was performed with anti-caspase-3
antibody, anti-MAO-A antibody, anti-Hsp70 antibody and β-actin
antibody. Immunoreactivity was detected with peroxidase-conjugated
secondary antibody in conjunction with chemiluminesence-based film
autoradiography. For quantification, ImageJ software was used.
Statistical analysis
All values are presented as means ± SD. Data were
analyzed by ANOVA followed by a Tukey-Kramer test as the post hoc
test. Differences were considered statistically significant at a
level of P<0.05.
Results
Effects of GGA on the locomotor activity
in the open-field test
Three weeks of CMS led to a significant decrease in
locomotor activity compared with the control group, demonstrated by
a decreased number of crossings and rearings (Fig. 1). By repeated treatment with GGA
(1,000 mg/kg), changes in locomotor activity were almost completely
reversed.
Effects of GGA on MAO-A expression in the
hippocampus
It has been reported that there is an increased
level of MAO-A in depression (23). RT-PCR and western blot analysis
demonstrated that MAO-A mRNA and protein levels in the hippocampus
were increased by CMS (Fig. 2).
GGA treatment reversed these alterations, producing a significant
decrease in MAO-A mRNA and protein levels in the hippocampus.
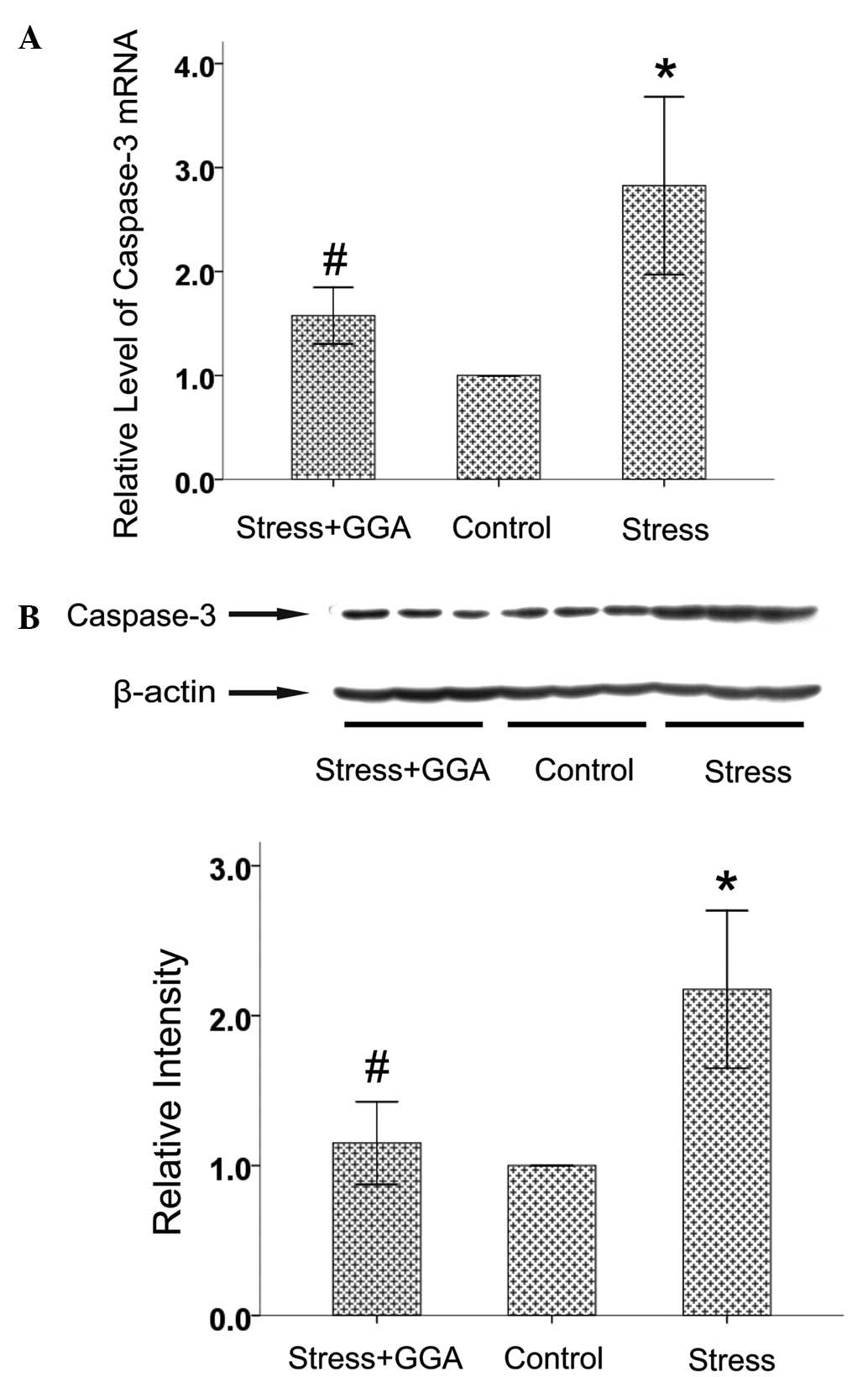
Effects of GGA on caspase-3 expression in
the hippocampus
Neuronal apoptosis is involved in the pathogenesis
of depression (24). Caspase-3 is
a common downstream effector in the apoptosis cascade. Compared
with the increased levels observed in the stress group, chronic GGA
treatment resulted in a significantly decreased level of
hippocampal caspase-3 mRNA and protein (Fig. 3). These results indicate that the
antidepressant effect of GGA might be partly due to the suppression
of neuronal apoptosis caused by CMS.
Effects of GGA on Hsp70 expression in the
hippocampus
To investigate the role of Hsp70 in the
antidepressant effect of GGA, we examined the Hsp70 level in the
hippocampus after an open-field test by RT-PCR, western blot
analysis and immunohistochemical analysis. GGA treatment
significantly increased the levels of hippocampal Hsp70 mRNA and
protein (Figs. 4 and 5). These data suggest that GGA induces
Hsp70 expression, which may be involved in the antidepressant
effect of GGA.
Discussion
The current study demonstrates that GGA, an Hsp70
inducer, possesses an antidepressant effect. CMS in rats caused a
reduction in locomotor activity and an increase in levels of
monoamine oxidase-A and caspase-3 in hippocampus. GGA treatment
(1,000 mg/kg) reversed stress-induced alteration in locomotor
activity and levels of MAO-A and caspase-3. Previous animal studies
hava reported that LD50 values of GGA is 15,000 mg/kg in
oral dose and approximately 4,000 mg/kg in intraperitoneal
injection in rats and mice (21).
As such, although the dose used in the present study is high, it is
not acutely toxic. Therefore, GGA may be a safe and potent agent
for treating depression.
It has been widely reported that depression is a
consequence of diminished neurotransmission due to a decrease in
neurotransmitter concentrations (25,26).
Among various neurotransmitters, monoamines (including
noradrenaline, dopamine and serotonin) play crucial roles in the
pathology of depression (27).
Levels of monoamines are generally low in patients with depression
(23). Monoamine oxidase (MAO),
the most significant enzyme that catabolizes monoamines, is a
trait-dependent indicator of vulnerability to depression (28). There are two forms of MAO: MAO-A
and MAO-B. MAO-A activity is believed to be associated with
depression, while MAO-B activity is believed to be associated with
neurodegenerative diseases such as Parkinson’s disease (29,30).
An elevated level of MAO-A is considered as the primary
monoamine-lowering process in depression (23). In accordance with these reports,
the present study observed that the levels of MAO-A mRNA and
protein were increased in the hippocampus of CMS rats. In the
present study, we also observed that GGA possessed an
antidepressant effect and that the increased level of MAO-A caused
by CMS was suppressed by GGA. These results suggest that the
inhibitory effect of GGA on MAO-A expression may be a mechanism
underlying antidepressant effects of GGA. However, further study is
necessary to determine how GGA suppresses MAO-A expression. It has
been reported that GGA increases thioredoxin-1, Hsp70 and
prostaglandin expression in various cells, and exerts
cytoprotection (15,20,31,32).
In the present study, we observed that GGA induced Hsp70 expression
in the hippocampus. However, further studies are required to
investigate whether the inhibitory effect of GGA on MAO-A
expression is due to the induction of Hsp70 and/or other inducing
proteins.
Although the monoamine hypothesis provides a
satisfactory explanation of the mechanism underlying the pathology
of depression, alternative mechanisms are also involved in the
pathology of depression. Recent reports have shown an association
between depression, atrophy and cell loss in the brain (33–35).
Loss of neuronal and glial density is observed in post-mortem
brains of patients with depression (36–39).
Chronic unpredictable stress leads to neuronal apoptosis in the
cerebral cortex (40). The
hippocampus is particularly sensitive to stress. It has been
reported that there is a selective loss of hippocampal volume in
depression (41,42). Loss of neurons is also observed in
animal models of depression (41).
It has been proposed that apoptosis is a contributing factor to the
decrease in hippocampal volume and cell loss (43). Caspase-3 plays a crucial role in
the execution of apoptosis (44).
In the present study, we demonstrated that caspase-3 expression was
increased in the hippocampus after CMS, suggesting the induction of
apoptosis. Caspase-3 could be activated in the apoptotic cell by
extrinsic (death ligand) and intrinsic (mitochondrial or
endoplasmic reticulum) pathways (45–48).
Further studies are required to determine which apoptosis pathway
is involved in the CMS-induced apoptosis. A number of studies have
shown that GGA protects against various stress injuries, including
acoustic injury, ischemia, age-related hearing loss and
3-nitropropionic acid-induced cochlear damage, which involves Hsp70
induction (21,49,50).
GGA is a lipid-soluble reagent that easily crosses the blood-brain
barrier (50,51). In the present study, we observed
that GGA had antidepressant effects. In addition, GGA induced Hsp70
expression in the hippocampus. The activation of caspase-3 caused
by CMS was suppressed by GGA treatment. Hsp70 has been shown to
inhibit the immediate apoptosis of cells exposed to numerous
stresses in numerous tissues (52). The induction of Hsp70 expression by
GGA may be associated with the inhibition of reactive oxygen
species generation, toxicity of excitatory amino acid and apoptosis
(8,53,54).
Accordingly, the protective effect of GGA against the apoptosis
cascade by inducing Hsp70 expression may be a mechanism underlying
the antidepressant effects of GGA.
In conclusion, GGA exerted an antidepressant effect
in the CMS model of depression in rats and this effect may be
mediated by inducing Hsp70 expression to suppress MAO-A expression
and the apoptosis cascade. In addition, these results suggest that
attempts to develop Hsp70 inducers are beneficial for protection
against depression.
Acknowledgements
This study was supported by the
Science and Technology Joint Special Fund of Yunnan Province (no.
2010CD197).
References
|
1
|
Kessler RC, Berglund P, Demler O, et al:
The epidemiology of major depressive disorder: results from the
National Comorbidity Survey Replication (NCS-R). JAMA.
289:3095–3105. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nemeroff CB: The burden of severe
depression: a review of diagnostic challenges and treatment
alternatives. J Psychiatr Res. 41:189–206. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rosen RC and Marin H: Prevalence of
antidepressant-associated erectile dysfunction. J Clin Psychiatry.
64(Suppl 10): 5–10. 2003.
|
|
4
|
Evans CG, Chang L and Gestwicki JE: Heat
shock protein 70 (hsp70) as an emerging drug target. J Med Chem.
53:4585–4602. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Parsell DA and Lindquist S: The function
of heat-shock proteins in stress tolerance: degradation and
reactivation of damaged proteins. Annu Rev Genet. 27:437–496. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sharp FR, Massa SM and Swanson RA:
Heat-shock protein protection. Trends Neurosci. 22:97–99. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ooie T, Takahashi N, Saikawa T, et al:
Single oral dose of geranylgeranylacetone induces heat-shock
protein 72 and renders protection against ischemia/reperfusion
injury in rat heart. Circulation. 104:1837–1843. 2001. View Article : Google Scholar
|
|
8
|
Guo S, Wharton W, Moseley P and Shi H:
Heat shock protein 70 regulates cellular redox status by modulating
glutathione-related enzyme activities. Cell Stress Chaperones.
12:245–254. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kimmins S and MacRae TH: Maturation of
steroid receptors: an example of functional cooperation among
molecular chaperones and their associated proteins. Cell Stress
Chaperones. 5:76–86. 2000. View Article : Google Scholar
|
|
10
|
Rajapandi T, Greene LE and Eisenberg E:
The molecular chaperones Hsp90 and Hsc70 are both necessary and
sufficient to activate hormone binding by glucocorticoid receptor.
J Biol Chem. 275:22597–22604. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shimizu S, Nomura K, Ujihara M, et al: An
allel-specific abnormal transcript of the heat shock protein 70
gene in patients with major depression. Biochem Biophys Res Commun.
219:745–752. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Martini F, Fernández C, Segundo LS,
Tarazona JV and Pablos MV: Assessment of potential immunotoxic
effects caused by cypermethrin, fluoxetine, and thiabendazole using
heat shock protein 70 and interleukin-1beta mRNA expression in the
anuran Xenopus laevis. Environ Toxicol Chem. 29:2536–2543.
2010. View
Article : Google Scholar
|
|
13
|
Yu J, Roh S, Lee JS, et al: The effects of
venlafaxine and dexamethasone on the expression of HSP70 in rat C6
glioma cells. Psychiatry Investig. 7:43–48. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Allagui MS, Nciri R, Rouhaud MF, et al:
Long-term exposure to low lithium concentrations stimulates
proliferation, modifies stress protein expression pattern and
enhances resistance to oxidative stress in SH-SY5Y cells. Neurochem
Res. 34:453–462. 2009. View Article : Google Scholar
|
|
15
|
Hirakawa T, Rokutan K, Nikawa T and Kishi
K: Geranylgeranylacetone induces heat shock proteins in cultured
guinea pig gastric mucosal cells and rat gastric mucosa.
Gastroenterology. 111:345–357. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ishii Y, Kwong JM and Caprioli J: Retinal
ganglion cell protection with geranylgeranylacetone, a heat shock
protein inducer, in a rat glaucoma model. Invest Ophthalmol Vis
Sci. 44:1982–1992. 2003. View Article : Google Scholar
|
|
17
|
Latchman DS: Heat shock proteins and
cardiac protection. Cardiovasc Res. 51:637–646. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamagami K, Yamamoto Y, Ishikawa Y,
Yonezawa K, Toyokuni S and Yamaoka Y: Effects of
geranyl-geranyl-acetone administration before heat shock
preconditioning for conferring tolerance against
ischemia-reperfusion injury in rat livers. J Lab Clin Med.
135:465–475. 2000. View Article : Google Scholar
|
|
19
|
Katsuno M, Sang C, Adachi H, et al:
Pharmacological induction of heat-shock proteins alleviates
polyglutamine-mediated motor neuron disease. Proc Natl Acad Sci
USA. 102:16801–16806. 2005. View Article : Google Scholar
|
|
20
|
Tanito M, Kwon YW, Kondo N, et al:
Cytoprotective effects of geranylgeranylacetone against retinal
photooxidative damage. J Neurosci. 25:2396–2404. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yasuda H, Shichinohe H, Kuroda S, Ishikawa
T and Iwasaki Y: Neuroprotective effect of a heat shock protein
inducer, geranylgeranylacetone in permanent focal cerebral
ischemia. Brain Res. 1032:176–182. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Luo FC, Qi L, Lv T, et al:
Geranylgeranylacetone protects mice against morphine-induced
hyperlocomotion, rewarding effect, and withdrawal syndrome. Free
Radic Biol Med. 52:1218–1227. 2012. View Article : Google Scholar
|
|
23
|
Meyer JH, Ginovart N, Boovariwala A, et
al: Elevated monoamine oxidase a levels in the brain: an
explanation for the monoamine imbalance of major depression. Arch
Gen Psychiatry. 63:1209–1216. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Duman RS: Depression: a case of neuronal
life and death? Biol Psychiatry. 56:140–145. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Manji HK, Drevets WC and Charney DS: The
cellular neurobiology of depression. Nat Med. 7:541–547. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mathew SJ, Manji HK and Charney DS: Novel
drugs and therapeutic targets for severe mood disorders.
Neuropsychopharmacology. 33:2080–2092. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Elhwuegi AS: Central monoamines and their
role in major depression. Prog Neuropsychopharmacol Biol
Psychiatry. 28:435–451. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Du L, Faludi G, Palkovits M, Sotonyi P,
Bakish D and Hrdina PD: High activity-related allele of MAO-A gene
associated with depressed suicide in males. Neuroreport.
13:1195–1198. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wouters J: Structural aspects of monoamine
oxidase and its reversible inhibition. Curr Med Chem. 5:137–162.
1998.PubMed/NCBI
|
|
30
|
Riederer P, Lachenmayer L and Laux G:
Clinical applications of MAO-inhibitors. Curr Med Chem.
11:2033–2043. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Terano A, Shiga J, Hiraishi H, Ota S and
Sugimoto T: Protective action of tetraprenylacetone against
ethanol-induced damage in rat gastric mucosa. Digestion.
35:182–188. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hirota K, Nakamura H, Arai T, et al:
Geranylgeranylacetone enhances expression of thioredoxin and
suppresses ethanol-induced cytotoxicity in cultured hepatocytes.
Biochem Biophys Res Commun. 275:825–830. 2000. View Article : Google Scholar
|
|
33
|
Bremner JD: Does stress damage the brain?
Biol Psychiatry. 45:797–805. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bremner JD, Narayan M, Anderson ER, Staib
LH, Miller HL and Charney DS: Hippocampal volume reduction in major
depression. Am J Psychiatry. 157:115–118. 2000.
|
|
35
|
Colla M, Kronenberg G, Deuschle M, et al:
Hippocampal volume reduction and HPA-system activity in major
depression. J Psychiatr Res. 41:553–560. 2007.PubMed/NCBI
|
|
36
|
Eastwood SL and Harrison PJ: Synaptic
pathology in the anterior cingulate cortex in schizophrenia and
mood disorders. A review and a western blot study of synaptophysin,
GAP-43 and the complexins. Brain Res Bull. 55:569–578. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lenze E, Cross D, McKeel D, Neuman RJ and
Sheline YI: White matter hyperintensities and gray matter lesions
in physically healthy depressed subjects. Am J Psychiatry.
156:1602–1607. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nolan CL, Moore GJ, Madden R, et al:
Prefrontal cortical volume in childhood-onset major depression:
preliminary findings. Arch Gen Psychiatry. 59:173–179.
2002.PubMed/NCBI
|
|
39
|
Ongür D, Drevets WC and Price JL: Glial
reduction in the subgenual prefrontal cortex in mood disorders.
Proc Natl Acad Sci USA. 95:13290–13295. 1998.PubMed/NCBI
|
|
40
|
Bachis A, Cruz MI, Nosheny RL and
Mocchetti I: Chronic unpredictable stress promotes neuronal
apoptosis in the cerebral cortex. Neurosci Lett. 442:104–108. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gould E and Tanapat P: Stress and
hippocampal neurogenesis. Biol Psychiatry. 46:1472–1479. 1999.
View Article : Google Scholar
|
|
42
|
McEwen BS: Physiology and neurobiology of
stress and adaptation: central role of the brain. Physiol Rev.
87:873–904. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lee AL, Ogle WO and Sapolsky RM: Stress
and depression: possible links to neuron death in the hippocampus.
Bipolar Disord. 4:117–128. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Woo M, Hakem R, Soengas MS, et al:
Essential contribution of caspase 3/CPP32 to apoptosis and its
associated nuclear changes. Genes Dev. 12:806–819. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Salvesen GS: Caspases: opening the boxes
and interpreting the arrows. Cell Death Differ. 9:3–5. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ghavami S, Hashemi M, Ande SR, et al:
Apoptosis and cancer: mutations within caspase genes. J Med Genet.
46:497–510. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ghribi O, Herman MM and Savory J: The
endoplasmic reticulum is the main site for caspase-3 activation
following aluminum-induced neurotoxicity in rabbit hippocampus.
Neurosci Lett. 324:217–221. 2002. View Article : Google Scholar
|
|
48
|
Shiraishi H, Okamoto H, Yoshimura A and
Yoshida H: ER stress-induced apoptosis and caspase-12 activation
occurs downstream of mitochondrial apoptosis involving Apaf-1. J
Cell Sci. 119:3958–3966. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kim YH, Song JJ, Kim YC, et al:
Geranylgeranylacetone ameliorates acute cochlear damage caused by
3-nitropropionic acid. Neurotoxicology. 31:317–325. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Mikuriya T, Sugahara K, Sugimoto K, et al:
Attenuation of progressive hearing loss in a model of age-related
hearing loss by a heat shock protein inducer,
geranylgeranylacetone. Brain Res. 1212:9–17. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Fujiki M, Kobayashi H, Abe T and Ishii K:
Astroglial activation accompanies heat shock protein upregulation
in rat brain following single oral dose of geranylgeranylacetone.
Brain Res. 991:254–257. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Mosser DD, Caron AW, Bourget L, et al: The
chaperone function of hsp70 is required for protection against
stress-induced apoptosis. Mol Cell Biol. 20:7146–7159. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yin HY, Ma XF, Liu F, Xia M and Xu AT:
Protective effect of geranylgeranylacetone on cisplatin
ototoxicity. Chemotherapy. 55:1–5. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Li CY, Lee JS, Ko YG, Kim JI and Seo JS:
Heat shock protein 70 inhibits apoptosis downstream of cytochrome c
release and upstream of caspase-3 activation. J Biol Chem.
275:25665–25671. 2000. View Article : Google Scholar : PubMed/NCBI
|