Introduction
Crohn’s disease (CD) and ulcerative colitis (UC) are
covered by the classification of inflammatory bowel disease (IBD).
IBD is an idiopathic disease resulting in intestinal mucosal
inflammation and loss of the barrier function. The exact mechanisms
of the induction and development of IBD are not yet fully
understood. Multiple factors may contribute to this condition. An
immunological disorder coupled with intolerance to intestinal flora
appear to be the most significant. In certain cases, they are
associated with gene mutations induced by environmental factors or
pathogenesis. The terminal ileum and colon have a high bacterial
content, which in IBD stimulates inflammation in the intestinal
lumen (1). A study of IBD patients
revealed that the loss of immune tolerance to symbiotic bacteria
involved increased T-cell and humoral immune responses (2). The chronic idiopathic IBDs, CD and
UC, appear to involve an over-active immune response to bacterial
antigens in genetically susceptible individuals (3). The therapy of IBDs typically
comprises immunosuppressive drugs, but clinicians tend also to use
antibiotics as certain symptoms, including fever, purulent stools,
abscesses and other signs of infection, have been considered to be
caused by bacteria (1).
Although scientists have debated whether bacteria
are the primary cause of CD, they cause only a superimposed
bacterial infection of the lesions while the disease is caused by
an overaggressive immune response to the bacteria. Several studies
have examined the effects of anti-tuberculosis treatment on IBD,
but these trials have generated conflicting results. Animal data
support the anti-inflammatory effects of a small number of
antibiotics, while in studies of CD, anti-inflammatory effects have
been shown in patients using metronidazole and ciprofloxacin
(4). However, there is no definite
proof of systemic antibiotics as the main treatment due to lack of
valid data on CD.
The objective of this meta-analysis was to perform a
systematic review and meta-analysis of randomized,
placebo-controlled clinical trials to assess the effectiveness of
antibiotic therapy in patients with CD.
Materials and methods
Literature search
Only randomized, controlled trials which compared
antibiotic therapy to placebo in patients with CD or UC were
included. We searched the Medline and Scopus databases from 1970 to
2010 using keywords that denote IBDs, antibacterial and
antimycobacterial drugs, antibacterial activity, metronidazole and
ciprofloxacin. The language used was English.
Data abstraction
The standardized data abstraction form and main
outcome data, including treatment programs, sample size and
results, were selected by two independent observers.
Ethambutol, isoniazid and rifamycins (including
rifampicin and rifabutin) were considered to be classic drugs
against infection with Mycobacterium tuberculosis, and
trials of antituberculosis drugs were included in the
meta-analysis. Similarly, nitroimidazoles (metronidazole),
macrolides (clarithromycin) and riminophenazines (clofazimine),
were also analyzed together (Tables
I and II).
 | Table ISummary of randomized, controlled
trials included in the meta-analysis of Crohn’s disease. |
Table I
Summary of randomized, controlled
trials included in the meta-analysis of Crohn’s disease.
| Author (ref) | Mean age | Gender (M/F) | Regimens | Concomitant
therapy | Duration | Clinical improvement
|
|---|
| Antibiotics | Placebo |
|---|
| Arnold et al,
2002 (9) | 45.2 | 28/19 | Cipro 500 mg
b.i.d. | Prednisone | 4 weeks | 21/28 | 5/19 |
| Prantera et
al, 2006 (8) | 38±12 | 16/11 | Rifaximin 800 mg
b.i.d. | Aminosalicylate | 12 weeks | 14/27 | 9/27 |
| West et al,
2004 (10) | 34 | 12/12 | Cipro 500 mg
b.i.d. | Infliximab | 6 weeks | 1/11 | 2/13 |
| Steinhart et
al, 2002 (7) | 32 | 57/77 | Cipro 500 mg b.i.d. +
metro 500 mg b.i.d. | Budesonide | 8 weeks | 22/66 | 21/64 |
| Leiper et al,
2008 (11) | 34 | 17/24 | Clari 1 g/day | | 12 weeks | 5/19 | 6/22 |
| Goodgame et
al, 2001 (12) | 39.4±9.2 | 18/13 | Clari 500 mg b.i.d. +
ethambutol 15 mg/kg | | 12 weeks | 5/9 | 6/9 |
| Blichfeldt et
al, 1978 (13) | 27.5 | 8/12 | Metro 250 mg
q.i.d. | Prednisone | 8 weeks | 11/20 | 10/20 |
| Ambrose et al,
1985 (14) | 36.5 | 13/22 | Metro 400 mg
b.i.d. | | 2 weeks | 12/18 | 6/17 |
| | | | | 4 weeks | 8/18 | 7/17 |
| | | | | 6 weeks | 10/16 | 7/14 |
| Ambrose et al,
1985 (14) | 37.0 | 12/21 | Sulfa 960 mg
b.i.d. | | 2 weeks | 10/16 | 6/17 |
| | | | | 4 weeks | 10/16 | 7/17 |
| Sutherland et
al, 1991 (6) | NA | NA | Metro 10–20
mg/kg/day | | 16 weeks | 18/63 | 6/36 |
| Selby et al,
2007 (5) | 36.5±11.3 | 101/112 | Clari 750 mg/day +
rifampicin 450 mg/day + clofa 50 mg/day | Prednisone | 16 weeks | 67/102 | 55/111 |
 | Table IICharacteristics of studies included in
the meta-analysis of ulcerative colitis. |
Table II
Characteristics of studies included in
the meta-analysis of ulcerative colitis.
| Author (ref) | Mean age | Gender (M/F) | Regimens | Concomitant
therapy | Duration | Clinical improvement
|
|---|
| Antibiotics | Placebo |
|---|
| Burke et al,
1990 (28) | 43.5 | 28/19 | Tobra 120 mg
t.i.d. |
Corticosteroids | 7 days | 31/42 | 18/42 |
| Ohkusa et
al, 2005 (31) | 39.5 | 12/8 | Amoxi 500 mg t.i.d.
+ Tetra 500 mg t.i.d. + metro 250 mg t.i.d. | Aminosalicylate +
corticosteroids | 14 days | 9/10 | 5/10 |
| Mantzaris et
al, 2001 (26) | 41.5 | 26/29 | Cipro 400 mg
b.i.d. | | 10 days | 23/29 | 20/26 |
| Mantzaris et
al, 1997 (25) | 41.5 | 33/37 | Rifaximin 400 mg
b.i.d. |
Corticosteroids | 14 days | 24/34 | 26/36 |
| Gionchetti et
al, 1999 (29) | | | Cipro 500–750 mg
b.i.d. | | 10 days | 9/14 | 5/12 |
| Turunen et
al, 1998 (30) | 34.2 | 58/25 | Cipro 500–750 mg
b.i.d. | Corticosteroids
Aminosalicylate | 180 days | 30/38 | 25/45 |
| Ohkusa et
al, 2010 (32) | NA | NA | Amoxi 500 mg t.i.d.
+ tetra 500 mg t.i.d. + metro 250 mg t.i.d. | | 90 days | 47/105 | 24/105 |
| Chapman et
al, 1986 (24) | 46.0 | 19/20 | Metro 500 mg t.i.d.
i.v. | Prednisone | 5 days | 14/19 | 14/20 |
| Mantzaris et
al, 1994 (27) | NA | NA | Metro 0.5 g t.i.d.
i.v. + tobra 4 mg/kg t.i.d. | Hydrocortisone | 10 days | 12/19 | 13/20 |
Statistical analysis
Homogeneity was assessed using a χ2 test
of homogeneity and the graphics were displayed simultaneously. A
pooled estimate of the odds ratio (OR) was calculated and applied
to a Mantel-Haenszel method test. Study results are presented as
ORs with 95% confidence intervals (CIs). For studies with
continuous outcome measures, results were converted to ORs. The log
OR corresponds to a constant multiplied by the standardized
difference between means.
Results
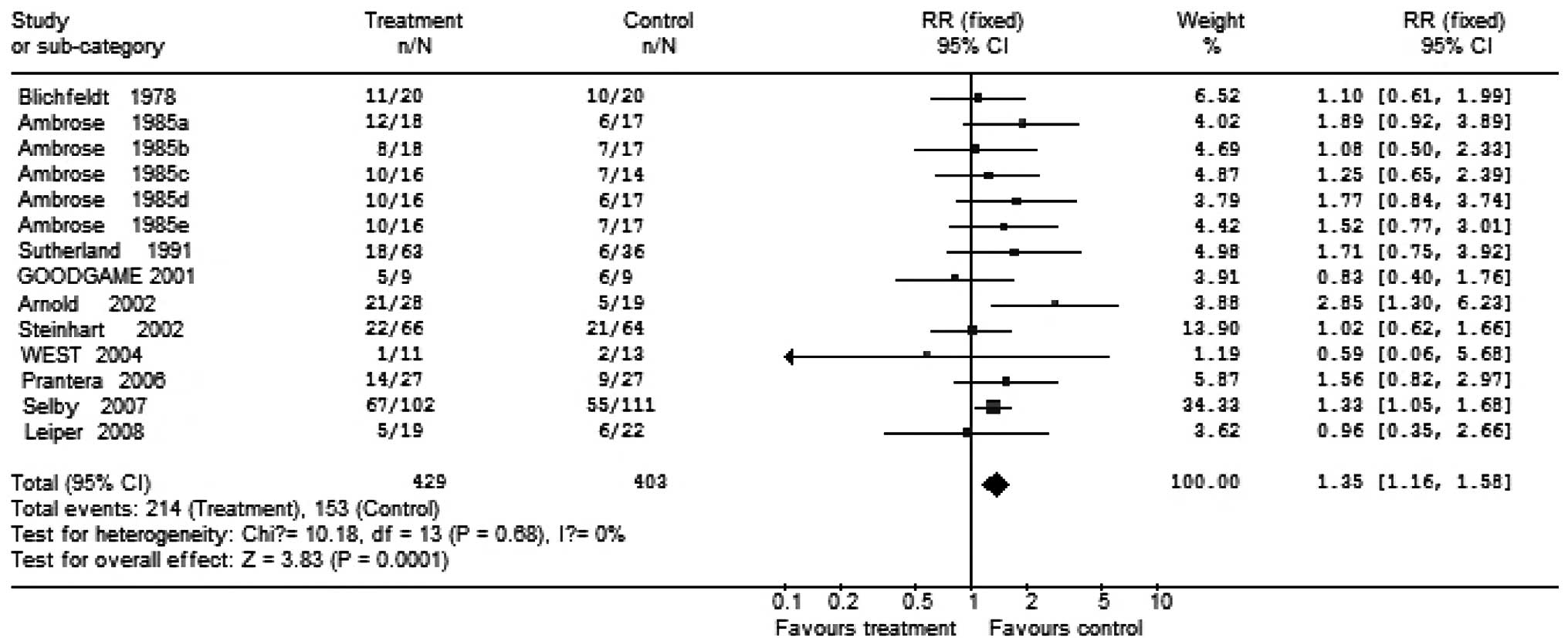
All included studies had a double-blind design. The
trials involved a total of 832 patients with CD who were randomized
to receive broad-spectrum antibiotic therapy; 429 patients were
treated with antimicrobials while 403 patients received placebo. In
the antibiotic group, 39 (9.1%) received ciprofloxacin, 135 (31%)
patients received metronidazole alone, 32 (7.4%) received
cotrimoxazole alone, 19 (4.4%) patients received clarithromycin
alone and 66 (15.3%) patients received metronidazole plus
ciprofloxacin. In these trials, some patients received concomitant
therapy and some did not. Clinical improvement occurred in 56.1%
(214/429) of patients in the antibiotic group and 37.9% (153/403)
of patients in the placebo group. The summary OR for clinical
improvement with any antibiotic therapy in the trial was 1.35 (95%
CI, 1.16–1.58), The Breslow-Day test for heterogeneity indicated
that the studies were not significantly heterogeneous (Figs. 1 and 2).
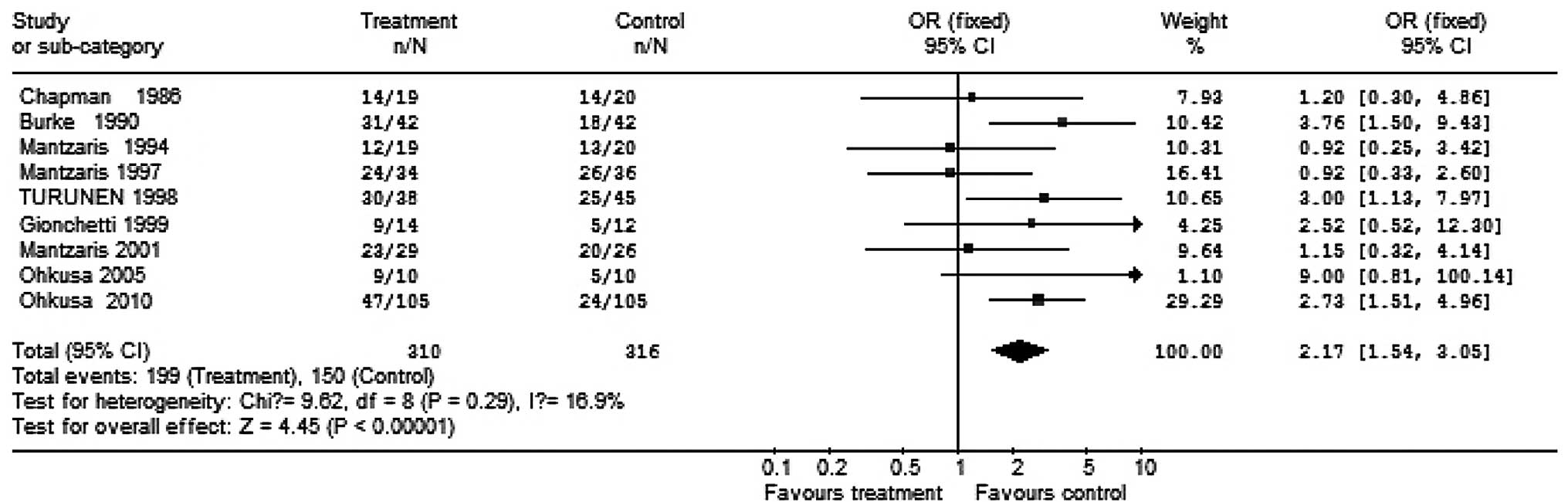
A total of 626 patients taking part in trials for UC
were randomized to receive antibiotics; 310 patients were treated
with antibiotics and 316 patients received placebo. In those who
received antibiotics, 101 (32.6%) received ciprofloxacin, 115
(37.1%) received amoxicillin plus tetracycline plus metronidazole,
42 (13.5%) received tobramycin alone and 19 (6.1%) received
metronidazole alone. Remission was induced in 64.2% of the patients
treated with antibiotics, compared with 47.5% of the placebo group.
The pooling of these trials yielded an OR of 2.17 (95% CI,
1.54–3.05) in favor of antibiotic therapy. The Breslow-day test for
heterogeneity revealed that the studies were statistically
homogeneous and may be combined (Figs.
3 and 4).
Discussion
Crohn’s disease
The majority of this work has been conducted using
metronidazole, ciprofloxacin or clarithromycin alone or in
combination. The two antibiotics most commonly used in the
treatment of CD are metronidazole and ciprofloxacin. These
antibiotics are active against two of the bacterial species
suspected to be involved in the pathogenesis and, at least, relieve
the symptoms of CD. Their use is widely accepted for the treatment
of perianal fistula and colitis, although there is incontrovertible
evidence supporting their use for these conditions.
The few randomized controlled trials to study
metronidazole and/or ciprofloxacin have mostly presented negative
results (5), although the
treatment has been reported to be more effective in those patients
whose disease involved the colon (6,7).
One study made use of rifaximin, a non-absorbed
rifamycin drug with a broad spectrum of activity against
Gram-positive, Gram-negative and even colonic anaerobic bacteria.
Owing to the fact that it is not absorbed, systemic adverse effects
are rare. In a double-blind, placebo-controlled trial reported by
Prantera et al (8), 83 CD
patients were divided into 3 groups: group A received rifaximin,
800 mg once a day; group B received rifaximin, 400 mg twice daily;
and group C received placebo. Remission rates were significantly
higher (52%) in group B than in group A (32%) and in the placebo
group (33%) (8). Thus, the
antibiotics with cell activity, including the macrolide antibiotics
azithromycin and clarithromycin, may provide a more effective
chemotherapy. Based on the role of intestinal microflora in the
pathogenesis of CD, the use of antibiotics or combined therapy,
appears to be a rational strategy. In a previously published study,
ciprofloxacin was shown to be effective when added to the existing
medium-active treatment in therapy-resistant CD patients (9). Ciprofloxacin, a quinolone drug, is
clinically effective in preventing the growth of intestinal
bacteria and appears to have immunomodulatory properties.
Accumulating evidence supports its role as the primary treatment
for CD. For example, in a prospective study by Colombel et
al (16), ciprofloxacin (500
mg twice daily) was as effective as mesalazine (4 g/day) and
induced remission in patients with mild to moderate CD.
Two important observations have strengthened the
bacterial hypothesis for CD. Firstly, genetic studies have
identified mutations in the NOD2 gene and the IRGM and ATG16L1
autophagy genes (17). These
mutations mean that cells do not contain intracellular bacterial
replication, and may have defects in their ability to eliminate
bacteria (18). Secondly, an
increased number of mucosa-associated E. coli have been
identified in patients with IBD (19).
In addition to additional data being required to
confirm the effectiveness of antibiotics in the treatment of CD,
there is the emergence of resistant bacterial strains and the
possible infection by Clostridium difficile to be
considered. A study has reported the previous use of antibiotics by
the majority of a group of IBD patients with Clostridium
difficile infection (20). In
an uncontrolled trial, antibiotic therapy resulted in clinical
remission in 49 cases (68%) (21).
In the same study, 55 patients (76%) showed a clinical response,
which was higher in those patients who were also taking steroids
(26/29) than those individuals who were not (29/43).
A large amount of data support the use of
antibiotics in patients with CD and with diverticulitis.
Antibiotics may act via different pathways in patients with CD.
They may inhibit the bacteria linked to the pathological processes
of the disease or simply lower the luminal bacterial overgrowth.
The suppression of intestinal flora may reduce the strength of
certain symptoms, including pain and diarrhea.
In our analysis, CD patients undergoing
antibacterial therapy are 1.35-fold more likely to experience
clinical remission than patients who are not undergoing
antibacterial therapy. Homogeneity among the trials was established
based on a graphic display and statistical tests. In addition, the
meta-analysis of short-term antimicrobial use also revealed that
treatment with antibiotics was effective in the induction of
clinical remission. These results were clinically and statistically
significant.
Whether antibiotics are of actual benefit remains to
be determined since only a few controlled trials have been
completed. Many used a small number of volunteers or used subgroups
which were not always clearly defined. Antibiotic use has
predominantly involved metronidazole or ciprofloxacin, and many
patients cannot tolerate these drugs for a long duration.
Therefore, these antibiotics are used in the treatment of ileal,
ileocolonic and colonic CD (22).
Ulcerative colitis
Several different antibiotics, alone and in
combination, have been evaluated in the primary and adjuvant
treatment of active UC. The routine use of antibiotics is not
recommended in mild or moderate UC. In a prospective, double-blind,
randomized controlled trial reported by Gilat et al
(23), oral metronidazole and
sulfasalazine were used for the outpatient management of mild to
moderate UC. This revealed that metronidazole was significantly
less effective than sulfasalazine by endoscopic and clinical
criteria.
In a randomized controlled trial reported by Chapman
et al (24), 39 patients
with severe UC were treated with either intravenous metronidazole
(500 mg every 8 h) or placebo as an adjunct to intravenous steroids
for 5 days. No significant difference was observed in clinical
improvement between the two groups. In two randomized controlled
trials reported by Mantzaris et al (25,26),
patients with mild to severe acute UC received either oral or
intravenous ciprofloxacin for 2 weeks as an adjunct to corticoid
therapy. No significant difference in clinical improvement was
observed, with 71% (24/34) and 79% (23/29) of the patients in the
ciprofloxacin-treated group and 72% (26/36) and 77% (20/26) of the
patients in the placebo-treated group achieving remission. The
authors concluded that a short course of oral ciprofloxacin
treatment did not appear to increase the proportion of patients
with active UC going into remission. In another study, the
combination of intravenous metronidazole and tobramycin as an
adjunct to corticosteroids was not found to be effective in causing
clinical improvement compared with placebo after 10 days of therapy
in the treatment of severe UC (27).
However, antibiotics may have a certain benefit as
adjuncts to standard anti-inflammatory treatment. Burke et
al (28) reported a study in
which 84 cases of acute UC were randomized to receive oral
tobramycin or placebo for 7 days as an adjunct to steroidal
therapy. When evaluated 18–21 days after the end of treatment,
31/42 patients (74%) in the tobramycin group and 18/42 patients
(43%) in the placebo group had achieved clinical remission
(p=0.008).
Despite considerable evidence for the involvement of
bacteria in UC, broad-spectrum antibiotics are usually not used for
the treatment of this disease; severe cases are the exception. Only
a few antibiotics have been used in the treatment of UC, whether
individually or in combination. One trial has shown that rifaxamin
is beneficial in the treatment of UC (29). Turunen et al (30) performed a randomized, controlled
trial which suggested that ciprofloxacin is beneficial as an
adjunctive treatment for active UC during the first 6-month period
of administration. At 6 months, the treatment-failure rate in the
ciprofloxacin-treated group was 21% (8/38 patients), which was
significantly lower than the placebo group rate of 44% (20/45
patients). The authors concluded that the use of ciprofloxacin
therapy for 6 months in UC improved the results of conventional
treatment with mesalazine and prednisone.
In our study, UC patients undergoing antibacterial
therapy have been determined to be 2.17-fold more likely to
experience clinical remission than patients receiving no
antibacterial therapy. Homogeneity among the trials was established
based on a graphic display (Fig.
2) and statistical tests. In addition, meta-analysis of
short-term antibacterial trials revealed that 5–180 days of
antibiotic therapy is effective for clinical remission. These
results were clinically and statistical significant.
However, a lack of well-designed, placebo-controlled
trials has made the efficacy of antibiotics as the primary
treatment for IBD questionable. Poor study design, high drop-out
rates and insufficient numbers of subjects in the current studies
have led to negative or equivocal results causing further
controversy.
To date, these studies have provided sufficient
evidence that antibiotics have been useful in the treatment of this
disease. The use of antibiotics in patients with IBD, however, is
controversial. The treatment of experimental IBD with antibiotics
may have contradictory results, since it remains unclear which
bacteria cause the disease. Moreover, many organisms which grow in
the gut mucosa, where they are more resistant to standard
antibiotic treatment, may not be able to reach the mucosal surface.
Susceptibility testing of organisms has not been carried out in a
systematic manner, and antibiotic treatment is not aimed at
specific bacteria. In fact, other clinical infections may be
present. It would be a difficult and time-consuming task to
complete in clinical practice if multiple varieties of bacteria
were involved. This is due to the requirement for the mucosal
microbial flora of each patient to be characterized and a drug
sensitivity test on each patient to be carried out.
Therefore, to determine the effect of antibiotics in
the treatment of IBD, larger randomized clinical trials of
antibiotics should be carried out, either in solo or combined with
other antibiotics or therapies.
References
|
1
|
Sartor RB: Therapeutic manipulation of the
enteric microflora in inflammatory bowel diseases: antibiotics,
probiotics, and prebiotics. Gastroenterology. 126:1620–1633. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Duchman R, Kaiser I, Hermann E, et al:
Tolerance exists towards resident intestinal flora but is broken in
active inflammatory bowel disease (IBD). Clin Exp Immunol.
102:448–455. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Podolsky DK: Inflammatory bowel disease. N
Engl J Med. 347:417–429. 2002. View Article : Google Scholar
|
|
4
|
Prantera C and Scribano ML: Antibiotics
and probiotics in inflammatory bowel disease: why, when, and how.
Curr Opin Gastroenterol. 25:329–333. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sandborn WJ and Feagan BG: Review article:
mild to moderate Crohn’s disease – defining the basis for a new
treatment algorithm. Aliment Pharmacol Ther. 18:263–277. 2003.
|
|
6
|
Sutherland L, Singleton J, Sessions J,
Hanauer S, Krawitt E, Rankin G, Summers R, Mekhjian H, Greenberger
N and Kelly M: Double blind, placebo controlled trial of
metronidazole in Crohn’s disease. Gut. 32:1071–1075. 1991.
|
|
7
|
Steinhart AH, Feagan BG, Wong CJ,
Vandervoort M, Mikolainis S, Croitoru K, Seidman E, Leddin DJ,
Bitton A, Drouin E, et al: Combined budesonide and antibiotic
therapy for active Crohn’s disease: a randomized controlled trial.
Gastroenterology. 123:33–40. 2002.
|
|
8
|
Prantera C, Lochs H, Campieri M, Scribano
ML, Sturniolo GC, Castiglione F and Cottone M: Antibiotic treatment
of Crohn’s disease: results of a multicentre, double blind,
randomized, placebo-controlled trial with rifaximin. Aliment
Pharmacol Ther. 23:1117–1125. 2006.
|
|
9
|
Arnold GL, Beaves MR, Pryjdun VO and Mook
WJ: Preliminary study of ciprofloxacin in active Crohn’s disease.
Inflamm Bowel Dis. 8:10–15. 2002.PubMed/NCBI
|
|
10
|
West RL, van der Woude CJ, Hansen BE, et
al: Clinical and endosonographic effect of ciprofloxacin on the
treatment of perianal fistulae in Crohn’s disease with infliximab:
a double-blind placebo-controlled study. Aliment Pharmacol Ther.
20:1329–1336. 2004.PubMed/NCBI
|
|
11
|
Leiper K, Martin K, Ellis A, et al:
Clinical trial: randomized study of clarithromycin versus placebo
in active Crohn’s disease. Aliment Pharmacol Ther. 27:1233–1239.
2008.PubMed/NCBI
|
|
12
|
Goodgame RW, Kimball K, Akram S, Ike E, Ou
CN, Sutton F and Graham D: Randomized controlled trial of
clarithromycin and ethambutol in the treatment of Crohn’s disease.
Aliment Pharmacol Ther. 15:1861–1866. 2001.PubMed/NCBI
|
|
13
|
Blichfeldt P, Blomhoff JP, Myhre E and
Gjone E: Metronidazole in Crohn’s disease. A double blind
cross-over clinical trial. Scand J Gastroenterol. 13:123–127.
1978.
|
|
14
|
Ambrose NS, Allan RN, Keighley MR, et al:
Antibiotic therapy for treatment in relapse of intestinal Crohn’s
disease. A prospective randomized study. Dis Colon Rectum.
28:81–85. 1985.
|
|
15
|
Selby W, Pavli P, Crotty B, et al:
Two-year combination antibiotic therapy with clarithromycin,
rifabutin, and clofazimine for Crohn’s disease. Gastroenterology.
132:2313–2319. 2007.PubMed/NCBI
|
|
16
|
Colombel JF, Lémann M, Cassagnou M, et al:
A controlled trial comparing ciprofloxacin and mesalamine for the
treatment of active Crohn’s disease. Groupe d’Etudes Therapeutiques
des Affections Inflammatoires Digestives (GETAID). Am J
Gastroenterol. 94:674–678. 1999.PubMed/NCBI
|
|
17
|
Cho JH and Weaver CT: The genetics of
inflammatory bowel disease. Gastroenterology. 133:1327–1339. 2007.
View Article : Google Scholar
|
|
18
|
Kobayashi KS, Chamaillard M, Ogura Y, et
al: Nod2-dependent regulation of innate and adaptive immunity in
the intestinal tract. Science. 307:731–734. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Barnich N and Darfeuille-Michaud A:
Adherent-invasive Escherichia coli and Crohn’s disease. Curr
Opin Gastroenterol. 23:16–20. 2007.
|
|
20
|
Issa M, Vijayapal A, Graham MB, et al:
Impact of Clostridium difficile on inflammatory bowel
disease. Clin Gastronterol Hepatol. 5:345–351. 2007.
|
|
21
|
Greenbloom SL, Steinhart AH and Greenberg
GR: Combination ciprofloxacin and metronidazole for active Crohn’s
disease. Can J Gastroenterol. 12:53–56. 1998.
|
|
22
|
Carter MJ, Lobo AJ, Travis SP, et al:
Guidelines for the management of inflammatory bowel disease in
adults. Gut. 53(Suppl 5): V1–V16. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gilat T, Suissa A, Leichtman G, et al: A
comparative study of metronidazole and sulfasalazine in active, not
severe, ulcerative colitis. An Israeli multicenter trial. J Clin
Gastroenterol. 9:415–417. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chapman RW, Selby WS and Jewell DP:
Controlled trial of intravenous metronidazole as an adjunct to
corticosteroids in severe ulcerative colitis. Gut. 27:1210–1212.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mantzaris GJ, Archavlis E, Christoforidis
P, et al: A prospective randomized controlled trial of oral
ciprofloxacin in acute ulcerative colitis. Am J Gastroenterol.
92:454–456. 1997.PubMed/NCBI
|
|
26
|
Mantzaris GJ, Petraki K, Archavlis E, et
al: A prospective randomized controlled trial of intravenous
ciprofloxacin as an adjunct to corticosteroids in acute, severe
ulcerative colitis. Scand J Gastroenterol. 36:971–974. 2001.
View Article : Google Scholar
|
|
27
|
Mantzaris GJ, Hatzis A, Kontogiannis P and
Triadaphyllou G: Intravenous tobramycin and metronidazole as an
adjunct to corticosteroids in acute, severe ulcerative colitis. Am
J Gastroenterol. 89:43–46. 1994.PubMed/NCBI
|
|
28
|
Burke DA, Axon AT, Clayden SA, et al: The
efficacy of tobramycin in the treatment of ulcerative colitis.
Aliment Pharmacol Ther. 4:123–129. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gionchetti P, Rizzello F, Ferrieri A, et
al: Rifaximin in patients with moderate or severe ulcerative
colitis refractory to steroid-treatment: a double-blind,
placebo-controlled trial. Dig Dis Sci. 44:1220–1221. 1999.
View Article : Google Scholar
|
|
30
|
Turunen U, Färkkilä MA, Hakala K, et al:
Long-term treatment of ulcerative colitis with ciprofloxacin: a
prospective, double-blind, placebo-controlled study.
Gastroenterology. 115:1072–1078. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ohkusa T, Nomura T, Terai T, et al:
Effectiveness of antibiotic combination therapy in patients with
active ulcerative colitis: a randomized, controlled pilot trial
with long-term follow-up. Scand J Gastroenterol. 40:1334–1342.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ohkusa T, Kato K, Terao S, et al: Newly
developed antibiotic combination therapy for ulcerative colitis: a
double-blind placebo-controlled multicenter trial. Am J
Gastroenterol. 105:1820–1829. 2010. View Article : Google Scholar
|