Introduction
Bacterial infections, including Gram-negative
bacterial infections, have been revealed to be closely involved
with airway inflammatory injuries in acute exacerbations of chronic
obstructive pulmonary disease (AECOPD) and asthma exacerbations
(1).
Lipopolysaccharide (LPS) is a major component of the
outer membranes of Gram-negative bacteria (2). LPS is recognized as an important
determinant of the virulence of these organisms and the
symptomatology that accompanies a Gram-negative bacterial
infection. In rodent models, inhaled or instilled LPS causes acute
airway and pulmonary inflammation, characterized by the recruitment
of inflammatory leukocytes and the release of a variety of
inflammatory mediators (3,4).
The pattern of changes in inflammatory cell counts
and cytokine levels during the subacute phase of LPS-stimulated
airway inflammation has not been well described. In the present
study, an LPS-induced rat model was used to investigate time-course
changes in subacute airway inflammation, as evidenced by airway
histopathology, cell counts and proinflammatory cytokine levels in
the bronchoalveolar lavage fluid (BALF).
Materials and methods
Animals and reagents
Male Sprague-Dawley rats (220±20 g) were purchased
from the Experimental Animal Center of Sichuan University (Sichuan,
China). LPS from E. coli serotype 055:B5 was purchased from
Sigma (St. Louis, MO, USA).
Animal treatment
A total of 30 rats were divided into a
saline-treated experimental group (control group, n=15) and an
LPS-treated group (LPS group, n=15). Rats were anesthetized
intraperitoneally with chloral hydrate (3 ml/kg). LPS (200
μg/rat) in 100 μl of saline was administered by
intratracheal instillation to LPS rats and the same volume of
intratracheal saline was administered to control animals. Rats were
sacrificed 2, 4 or 7 days after LPS or saline administration. The
animal study was approved by the Committee of Laboratory Animal
Care of 363 Hospital (Sichuan, China).
Histopathology
The middle lobes of the rats’ right lungs were
embedded in paraffin, following fixation in 10% buffered formalin,
and then processed to obtain 4-μm sections for hematoxylin
and eosin (H&E) staining. The inflammatory score of
H&E-stained lung sections was graded according to a previously
described method (5). This scoring
method strictly adhered to the blinded principle.
BALF and cell counts
The left trachea was cannulated under deep
anesthesia and an aliquot of 5 ml saline (0.9% NaCl at room
temperature) was injected into the lung. Subsequently, 4.5 ml of
the total volume was recovered. The recovered fluid was centrifuged
at 1500 x g for 5 min to sediment the cells. After two washes with
PBS solution, cells were suspended in PBS containing 10%
heat-inactivated fetal calf serum and counted using a
hemocytometer. Differential cell counts were determined from cell
suspensions presented on slides using a cytocentrifuge (Cytospin 2;
Shandon, Sewickley, PA, USA). Cells on slides were dried, fixed and
then stained using the May-Giemsa method. A total of 200 cells were
identified under a photomicroscope.
Measurement of cytokines
The concentrations of tumor necrosis factor (TNF)-α,
interleukin (IL)-1β and cytokine-induced neutropil chemoattractant
(CINC)-1 in the BALF were measured using enzyme-linked
immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN,
USA). Samples were measured photometrically by an automated plate
reader (Microplate Reader Model 1680; Bio-Rad, Hercules, CA, USA).
All assays were performed in duplicate.
Statistical analysis
The SPSS 13.0 software package (SPSS Inc., Chicago,
IL, USA) was used for the statistical analyses. Values were
expressed as mean ± SD. A one-way ANOVA and the Student
Newman-Keuls test was used to compare the differences between the
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
Histologic changes in rat airways
following LPS stimulation
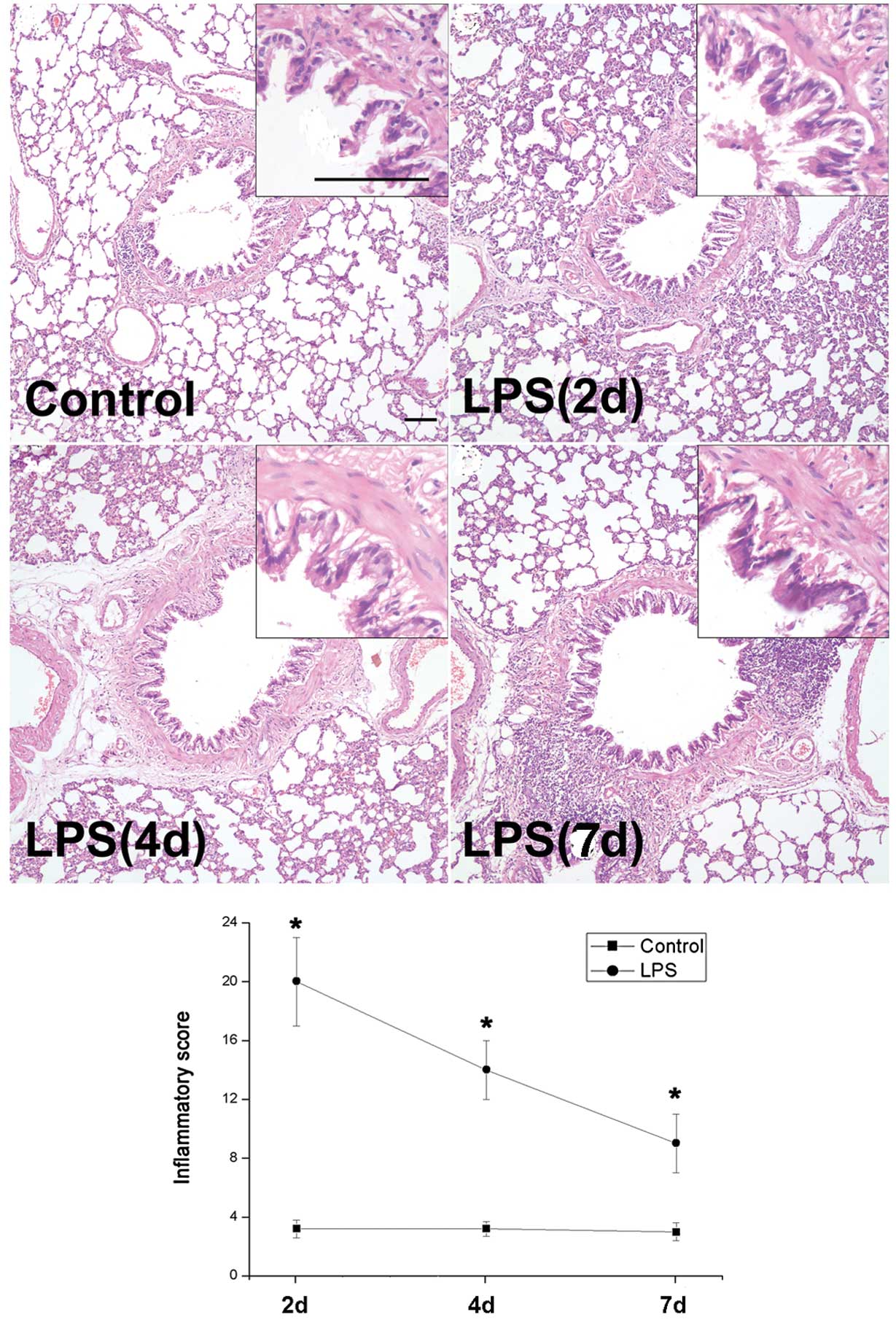
To assess the histomorphology of rat airways
following LPS stimulation, H&E staining and inflammatory
scoring were performed. Marked airway wall thickening with the
infiltration of inflammatory cells, which peaked on day 2, was
revealed in rat airways obtained from the LPS group and began to
decrease on day 4 (Fig. 1). The
inflammatory scores changed in a similar manner (Fig. 1).
Changes in cell counts and
proinflammatory cytokine levels in the BALF following LPS
stimulation
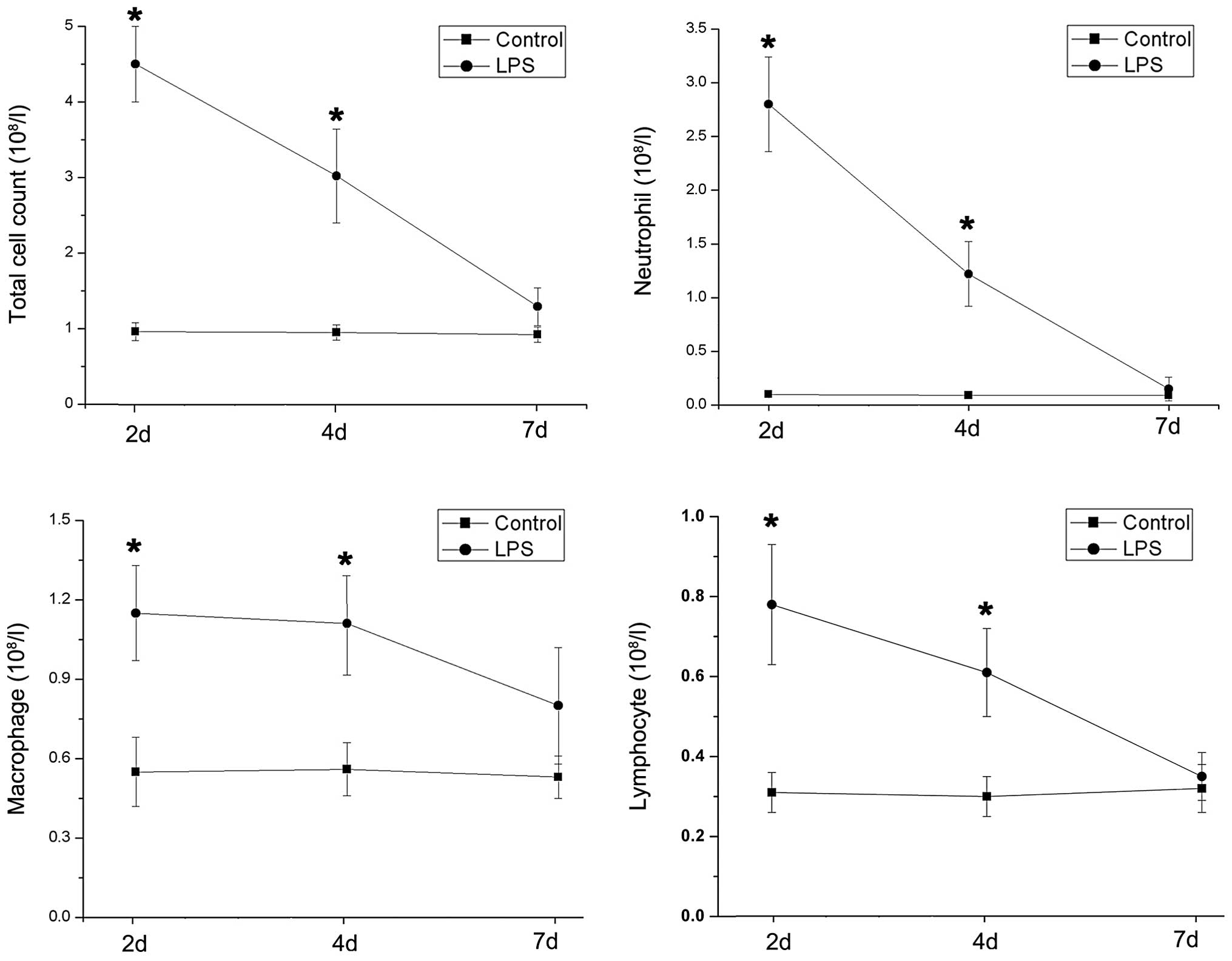
The inflammatory level of rat airways was further
evaluated using cell counts and proinflammatory cytokine levels in
the BALF. The total and differential counts of inflammatory cells
increased significantly following LPS treatment, although the
patterns of change were not all alike (Fig. 2). Macrophages, but not neutrophils
and lymphocytes, maintained a relatively stable level from day 2 to
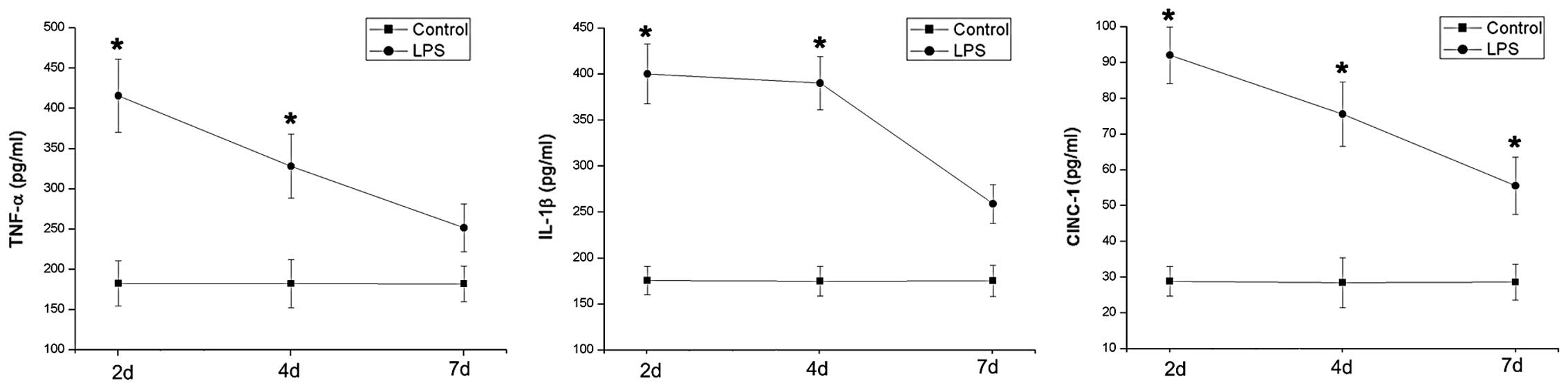
day 4 following LPS stimulation. Notably, inflammatory cytokine
levels peaked on day 2 and sharply decreased until the endpoint of
the study. However, the concentrations of proinflammatroy cytokines
decreased less rapidly than the neutrophil count after peaking
(Fig. 3).
Discussion
In the present study, the time-course changes in
leukocyte counts and cytokine levels of the BALF in LPS-induced
subacute airway inflammation were described in detail. Following
LPS stimulation, the proinflammatory cytokine levels and cell
counts in the BALF peaked on day 2 and subsequently decreased on
days 4 and 7, in accordance with the alterations to the airway
histomorphology.
Following activation by stimuli (e.g., LPS),
leukocytes, particularly neutrophils and macrophages, are recruited
into airway lumens where they generate inflammatory mediators
(6). Macrophages and neutrophils
appear to have significant roles in the early airway inflammation
response. Although neutrophils are considered to be the main source
of proinflammatory cytokines in acute airway inflammation,
macrophages are the predominant cells in the airway defence system
which is the key determinant of the severity of airway inflammation
(7,8). Airway macrophages not only constitute
a potentially powerful source of pronflammatory and
anti-inflammatory cytokines and tissue-degrading proteinases and
antiproteinases, but are also involved in the removal of cells
undergoing apoptosis (9–11). Notably, in the present study,
macrophages maintained a relatively stable level from day 2 to day
4. By contrast, neutrophil and lymphocyte counts, decreased
markedly after peaking on day 2. Similarly, proinflammatory
cytokine levels decreased after peaking on day 2, but the evaluated
cytokine levels did not decline as sharply as the neutrophil
counts. IL-1β levels changed in a similar manner to macrophage
counts. Together, these findings suggest that macrophages may
contribute more to the maintenance of the subacute phase of
LPS-stimulated airway inflammation than neutrophils.
In this process, proinflammatory cytokines play a
critical role in LPS-associated subacute inflammatory reactions. An
increasing number of studies have underlined the potential
importance of TNF-α and IL-1β as pivotal cytokines in the
initiation of the early inflammatory response to LPS exposure,
although Moreland et al reported that a blockade of TNF-α
and/or IL-1β expression was unable to protect mouse airways from
acute inflammatory injury after a 4-h aerosolized LPS exposure
(11). Furthermore, CINC-1 (the
rat homolog of human IL-8) is one of the most important chemotactic
cytokines (chemokines), inducible by proinflammatory cytokines,
such as TNF-α and IL-1β. CINC-1 is involved in leukocyte
transmigration into tissues, a process derived from not only
leukocytic cells (monocytes, neutrophils), but nonleukocytic cells
(endothelial cells, fibroblasts, epithelial cells) following LPS
stimulation (12,13). Our data indicate that the levels of
evaluated proinflammatory cytokines participating in the
inflammatory process accurately reflect the extent of LPS-induced
subacute airway inflammation. Notably, the relatively stable level
of proinflammatory cytokines, particularly IL-1β, contributed to
the maintanence of the subacute phase of the inflammatory
response.
In summary, our data present further experimental
evidence of a potentially important role for macrophages in
maintaining the subacute response of LPS-induced airway
inflammation which is closely involved with the release of and
mediation by proinflammatory cytokines.
References
|
1
|
Toews GB: Impact of bacterial infections
on airway diseases. Eur Respir Rev. 14:62–68. 2005. View Article : Google Scholar
|
|
2
|
Bishop RE: Fundamentals of endotoxin
structure and function. Contrib Microbiol. 12:1–27. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brass DM, Hollingsworth JW,
McElvania-Tekippe E, Garantziotis S, Hossain I and Schwartz DA:
CD14 is an essential mediator of LPS-induced airway disease. Am J
Physiol Lung Cell Mol Physiol. 293:L77–L83. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen L, Wang T, Zhang JY, Zhang SF, Liu
DS, Xu D, Wang X, Chen YJ and Wen FQ: Toll-like receptor 4 relates
to lipopolysaccharide-induced mucus hypersecretion in rat airway.
Arch Med Res. 40:10–17. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cimolai N, Taylor GP, Mah D and Morrison
BJ: Definition and application of a histopathological scoring
scheme for an animal model of acute Mycoplasma pneumoniae
pulmonary infection. Microbiol Immunol. 36:465–478. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dinarello CA: Proinflammatory cytokines.
Chest. 118:503–508. 2000. View Article : Google Scholar
|
|
7
|
Peters-Golden M: The alveolar macrophage:
the forgotten cell in asthma. Am J Respir Cell Mol Biol. 31:3–7.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mangan DF and Wahl SM: Differential
regulation of human monocyte programmed cell death (apoptosis) by
chemotactic factors and pro-inflammatory cytokines. J Immunol.
147:3408–3412. 1991.PubMed/NCBI
|
|
9
|
Welgus HG, Campbell EJ, Bar-Shavit Z,
Senior RM and Teitelbaum SL: Human alveolar macrophages produce a
fibroblast-like collagenase and collagenase inhibitor. J Clin
Invest. 76:219–224. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Linden A and Adachi M: Neutrophilic airway
inflammation and IL-17. Allergy. 57:769–775. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moreland JG, Fuhrman RM, Wohlford-Lenane
CL, Quinn TJ, Benda E, Pruessner JA and Schwartz DA: TNF-alpha and
IL-1 beta are not essential to the inflammatory response in
LPS-induced airway disease. Am J Physiol Lung Cell Mol Physiol.
280:L173–L180. 2001.PubMed/NCBI
|
|
12
|
Mukaida N: Pathophysiological roles of
interleukin-8/CXCL8 in pulmonary diseases. Am J Physiol Lung Cell
Mol Physiol. 284:L566–L577. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kuwahara I, Lillehoj EP, Lu W, Singh IS,
Isohama Y, Miyata T and Kim KC: Neutrophil elastase induces IL-8
gene transcription and protein release through p38/NF-(kappa)B
activation via EGFR transactivation in a lung epithelial cell line.
Am J Physiol Lung Cell Mol Physiol. 291:L407–L416. 2006. View Article : Google Scholar : PubMed/NCBI
|