Introduction
Fatty acids (FAs) are biological molecules with
important physiological roles in energy storage, membrane formation
and protein acylation (1). Animals
acquire FAs from the diet and via de novo biosynthesis
(2). In the latter, FAs are
predominantly generated by a 250- to 270-kDa multifunctional and
homodimeric enzyme, fatty acid synthase (FAS). Long-chain FAs, the
main product of FAS, are derived from acetyl-CoA, malonyl-CoA and
NADPH (3). FAs are essential
constituents of biological membranes and are important substrates
in energy metabolism. Although the mechanisms responsible for FAS
overexpression in tumors are not fully understood, the
PTEN/PI3K/AKT and RAS/RAF/MAPK/ERK1/2 pathways are known to
regulate FAS expression (3,4), and
these pathways are often hyperactive in tumors. Notably, in the
LNCaP prostate cancer cell line, pharmacological inhibition of PI3K
or reintroduction of wild-type PTEN was found to reduce FAS
expression (4).
Most tissues, except for the liver, adipose tissue,
cycling endometrium (5), fetal
lungs (6), lactating breast
(7,8) and embryos (3,9)
utilize dietary FAs to build new structural lipids. Therefore, FAS
is expressed at low levels in most normal tissues. By contrast, in
cancer tissues, the FA supply is highly dependent on de novo
biosynthesis via FAS. Indeed, several studies have shown that FAS
is overexpressed in many cancers, including breast (10,11),
prostate (12,13), ovarian (14) and colorectal carcinomas (15,16).
Furthermore, high FAS expression is associated with advanced
clinical stage, poor differentiation and poor prognosis of breast
(10), prostate (17) and ovarian carcinomas (15). Downregulation of FAS by RNAi was
found to inhibit growth and apoptosis in LnCaP cells but not in
normal fibroblasts (18).
Furthermore, pharmacological or RNAi-mediated downregulation of FAS
significantly sensitized the responsiveness of breast cancer cell
lines (SK-Br3, MCF-7 and MDA-MB-231) to paclitaxel or vinorelbine
(19,20). These results indicate that FAS is
an important prognostic factor in certain types of cancers and may
represent a potential therapeutic target for cancer
chemotherapy.
However, FAS expression in gastric carcinoma, one of
the most prevalent malignant tumors worldwide, particularly in
China, has not been established. To date, few clinical studies have
determined FAS expression in gastric carcinoma or compared its
expression with that in non-neoplastic adjacent tissue (21,22).
Since FAS expression varies at different ages and clinical
circumstances, determining FAS expression in tumor tissue alone is
insufficient to clarify the prognostic relevance of FAS expression
in cancer. Therefore, to provide insight into the clinical
relevance of FAS, we examined FAS expression in gastric carcinoma
and paired adjacent normal tissue samples collected from 90 Chinese
patients. We analyzed the associations between FAS expression and
clinicopathological characteristics, such as age, gender,
histological grade, American Joint Committee on Cancer (AJCC) tumor
stage, metastasis and tumor size, as well as molecular markers,
such as the loss of PTEN and pERK1/2 expression. Finally, we
determined the effects of FAS expression on prognosis.
Materials and methods
Patients and tissue samples
Ninety patients with gastric carcinoma who underwent
surgery between 2007 and 2008 were enrolled in this study. None of
the patients had received any treatment before surgery. We obtained
complete clinicopathological information for all patients,
including age, gender, tumor size, histological grade, AJCC tumor
stage, depth of invasion, lymph node metastasis and distant
metastasis. All of the patients included in this study had
adenocarcinoma. The median age of the patients at the time of
diagnosis was 65.5 years (range 34–83 years). The histological
grade of the tumor was evaluated based on the degree of tumor
differentiation, tumor necrosis and mitotic count, according to the
criteria of Enzinger and Weiss (23). Follow-up time was calculated as the
time from initial surgery to the death of the patient due to the
primary tumor or the date of last contact. Tumor tissue and paired
adjacent normal tissue samples were obtained at surgery. All
tissues were dissected in the operating room, immediately frozen,
and stored at −80°C. Informed consent for use of tissue samples in
future molecular studies was obtained from each patient. This study
was approved by the Ethics Committee of the Third Xiangya Hospital,
Central South University (Hunan, China). Clinical and treatment
information was extracted by chart review carried out by the
surgeon with approval from our institutional review board.
Tissue microarray (TMA) preparation
Core needle biopsies (1.5 mm diameter) were
extracted from paraffin-embedded tissue samples, and mounted into a
recipient paraffin block using a dedicated tissue array instrument
(Beecher Instruments, Sun Prairie, WI, USA). Then,
4-μm-thick sections of the TMA were cut, transferred to
glass slides and stained with hematoxylin and eosin.
Immunohistochemistry
TMA sections (1.5-mm diameter; 4-μm thick)
from archival, formalin-fixed, paraffin-embedded tissue specimens
were mounted on poly-L-lysine (Muto Chemicals, Tokyo, Japan)-coated
slides. The TMA sections were deparaffinized in xylene for 15 min,
rehydrated in an ethanol gradient and heated at 95°C for 5 min in
10 mM sodium citrate buffer (pH 6.0) in a microwave oven for
antigen retrieval. Endogenous peroxidase was inactivated by
incubating the sections in 3% H2O2 for 15 min
at room temperature. The sections were blocked in 3% normal donkey
serum and incubated at 4°C overnight with monoclonal anti-FAS
antibody (dilution, 1:50; no. 3180S, Cell Signaling Technology,
Danvers, MA, USA), anti-pERK1/2 antibody (dilution, 1:1000; no.
4370, Cell Signaling Technology) and anti-PTEN antibody (dilution,
1:50; no. 9559C, Cell Signaling Technology). Finally, the sections
were stained with horseradish peroxidase-conjugated donkey
anti-rabbit IgG (H+L) secondary antibody (711-035-152, Jackson
ImmunoResearch Europe, Newmarket, UK). Signal detection was carried
out using a Dako signaling amplification system (K346811; Dako,
Glostrup, Denmark). The TMA sections were counterstained with
hematoxylin, dehydrated, and mounted.
TMA score
Immunohistochemistry was scored based on staining
intensity and the percentage of positive cells. The staining
intensity was scored as follows: 0, negative; 1, weak; 2, moderate;
and 3, high intensity. The immunoreactive score was calculated as
staining intensity score x percentage of FAS-positive cells. We
also calculated S (T/A), immunore-active score of tumor
tissue/immunoreactive score of paired adjacent normal tissue, as an
index for the difference in expression between tumor and normal
tissue. FAS immunostaining was analyzed under a microscope (Nikon
Eclipse E600) and estimated independently by two pathologists.
Statistical analysis
Statistical analyses were performed using GraphPad
Prism 5 (GraphPad Software Inc., San Diego, CA, USA) for Windows.
The Student’s t-test was used to compare FAS expression between
cancer tissue and adjacent normal tissues. Contingency table
analysis and χ2 tests were used to investigate the
relationship between FAS expression and clinical variables. For
outcomes with a small number of cases, Fisher’s exact test was
used. Survival was estimated using the Kaplan-Meier method, and
differences in survival curves were determined using the log-rank
test. Values of P<0.05 were regarded as statistically
significant.
Results
Clinicopathological characteristics
Table I summarizes
the clinicopathological characteristics data of the patients with
previously untreated gastric carcinoma. The study cohort included
67 males and 23 females, ranging in age from 34 to 83 years (median
age, 65 years). The histological type of all patients was
adenocarcinoma. Tumor size ranged from 0 to 20 cm with a mean size
of 6.17 cm and a median size of 5.75 cm. The tumors were classified
as grade I in 1 patient, grade II in 23 patients and grade III in
66 patients. The median and mean duration of follow-up was 35 and
31.75 months, respectively, ranging from 1 to 51 months. Most of
the patients (77.8%, 70/90) developed distant or regional lymph
node metastasis.
 | Table ITMA clinical information. |
Table I
TMA clinical information.
| Characteristics | |
|---|
| Gender, n (%) | |
| Male | 67 (74.4) |
| Female | 23 (25.6) |
| Age (years) | |
| Median (range) | 65 (34–83) |
| Histological type, n
(%) | |
| Adenocacinoma | 90 (100.0) |
| Others | 0 (0.0) |
| Presentation, n
(%) | |
| Initial | 90 (100.0) |
| Recurrent | 0 (0.0) |
| Size (cm) | |
| Median (range) | 5.75 (0–20) |
| Grade, n (%) | |
| I | 1 (1.1) |
| II | 23 (25.6) |
| III | 66 (73.3) |
| Metastasis, n
(%) | |
| Negative | 20 (22.2) |
| Positive | 70 (77.8) |
FAS expression
We determined FAS expression in all 90 tumor tissue
and paired adjacent normal tissue samples by TMA and
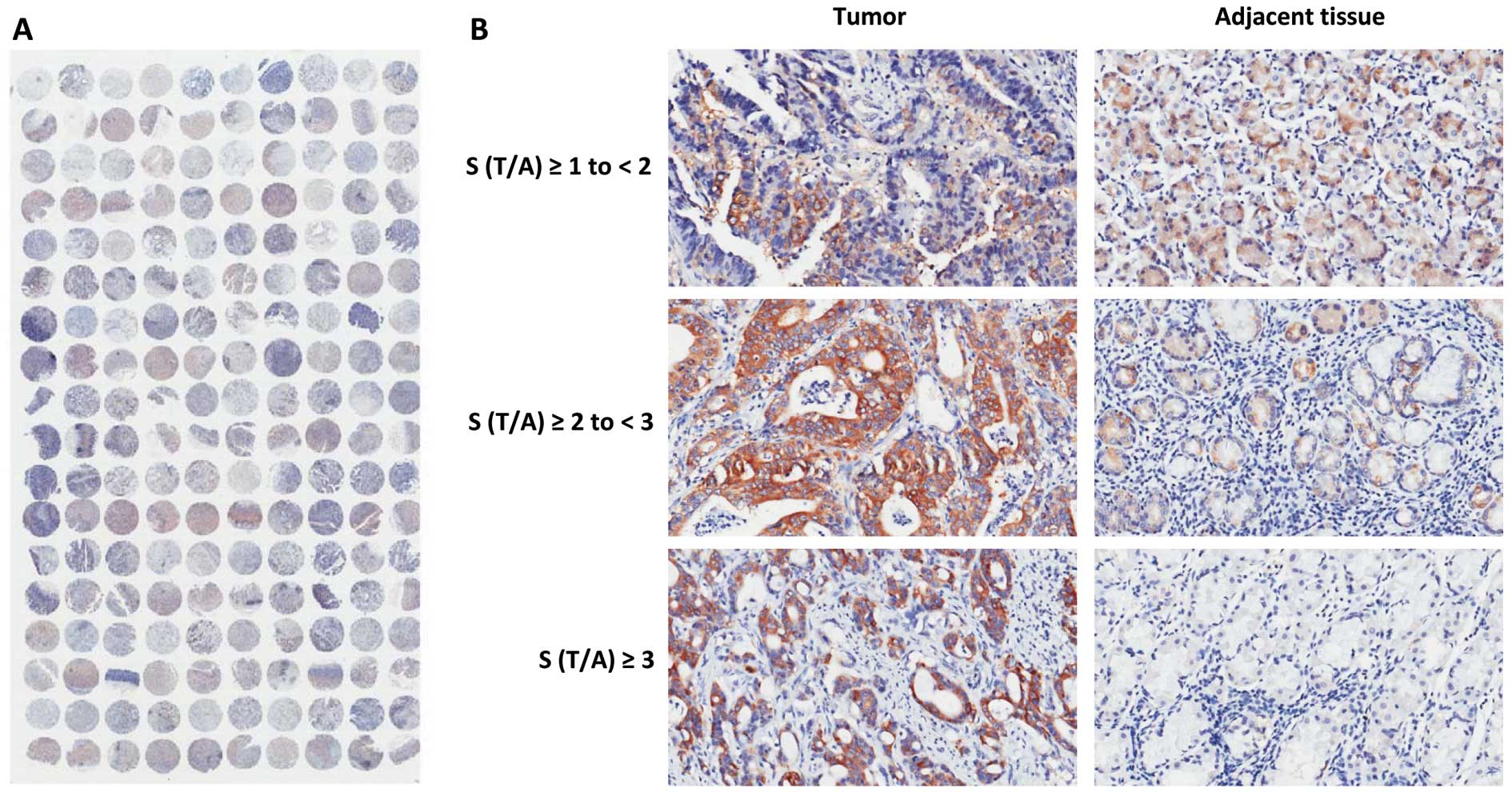
immunohistochemistry. A representative TMA stained for FAS is shown
in Fig. 1A. No signal was detected
in the nuclei or on the cell membrane, indicating that FAS was
mainly localized to the cytoplasm. The mean immunohistochemical
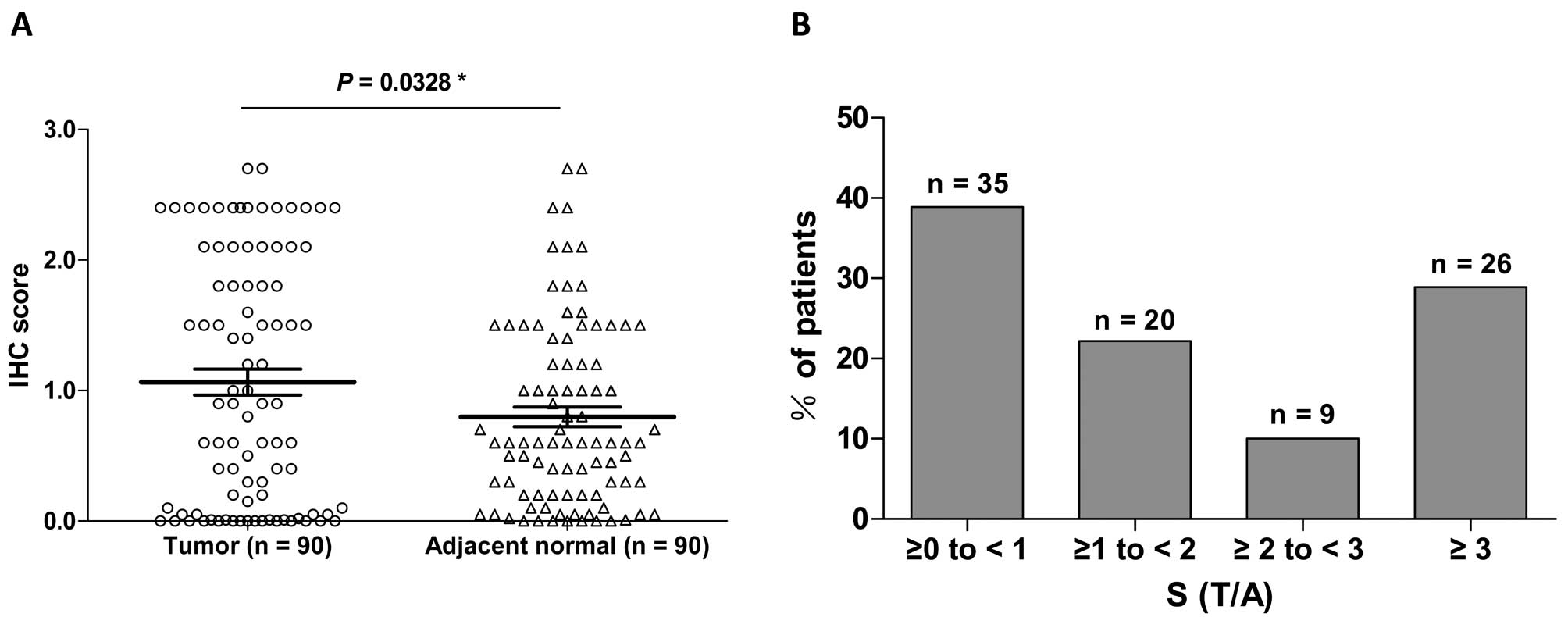
score of FAS expression was significantly higher in tumor tissue
than in adjacent normal tissue (1.065±0.099 vs. 0.798±0.074,
P<0.05, Fig. 2A). These data
indicate that FAS was overexpressed in the cytoplasm in tumor
tissue compared to the expression in the adjacent normal tissue. To
investigate the difference in expression between tumor tissues and
normal tissues, we calculated S (T/A) as described in Materials and
methods. Representative tissue sections corresponding to S (T/A) ≥1
to <2 (low), S (T/A) ≥2 to <3 (medium) and S (T/A) ≥3 (high
expression) are shown in Fig. 1B.
Overall, 38.9% (35/90), 22.2% (20/90), 10% (9/90) and 28.9% (26/90)
of the carcinoma tissue specimens were classified as S (T/A) ≥0 to
<1, S (T/A) ≥1 to <2, S (T/A) ≥2 to <3 and S (T/A) ≥3,
respectively (Fig. 2B).
Relationship between FAS overexpression
and clinicopathological parameters
To investigate the association between FAS
overexpression and clinicopathological parameters, three grades of
FAS overexpression (S (T/A) ≥1, ≥2 and ≥3) were established. FAS
overexpression was not significantly associated with any of the
clinicopathological variables recorded, including age, gender,
grade, tumor size and lymph node metastasis (Table II). As several reports have shown
that FAS expression is modulated by the PTEN/PI3K/AKT and
RAS/RAF/MEK/ERK pathways, we determined the expression of several
components in these two pathways (4,24).
Surprisingly, FAS overexpression was not significantly correlated
with total, cytoplasmic or nuclear loss of PTEN (Table II) or pERK expression (data not
shown) in gastric carcinomas.
 | Table IIFAS expression and clinicopathological
factors of the gastric carcinoma patients. |
Table II
FAS expression and clinicopathological
factors of the gastric carcinoma patients.
| S (T/A) <1 | S (T/A) ≥1 | aP-value | S (T/A) ≥2 | bP-value | S (T/A) ≥3 | cP-value |
|---|
| Age (years), n
(%) | | | | | | | |
| Median age | 65 | 65 | | 69 | | 65 | |
| Range | 45–83 | 34–83 | | 41–81 | | 41–81 | |
| <60 | 14 (48.3) | 15 (51.7) | 0.2078 | 7 (24.1) | 0.0645 | 6 (20.7) | 0.6086 |
| ≥60 | 21 (34.4) | 40 (65.6) | | 28 (45.9) | | 20 (32.8) | |
| Gender, n (%) | | | | | | | |
| Female | 6 (26.1) | 17 (73.9) | 0.1444 | 12 (52.2) | 0.1298 | 9 (39.1) | 0.286 |
| Male | 29 (43.3) | 38 (56.7) | | 23 (34.3) | | 17 (25.4) | |
| Histological grade,
n (%) | | | | | | | |
| I | 0 (0.0) | 1 (100.0) | 0.4328 | 0 (0.0) | 0.4524 | 0 (0.0) | 0.7564 |
| II | 7 (30.4) | 16 (69.6) | | 11 (47.8) | | 6 (26.1) | |
| III | 28 (42.4) | 38 (57.6) | | 24 (36.4) | | 20 (30.3) | |
| AJCC tumor stage, n
(%) | | | | | | | |
| I | 1 (16.7) | 5 (83.3) | 0.5244 | 2 (33.3) | 0.4868 | 1 (16.7) | 0.2082 |
| II | 12 (40.0) | 18 (60.0) | | 11 (36.7) | | 6 (20.0) | |
| III | 20 (39.2) | 31 (60.8) | | 22 (43.1) | | 19 (37.3) | |
| IV | 2 (66.7) | 1 (33.3) | | 0 (0.0) | | 0 (0.0) | |
| Metastasis, n
(%) | | | | | | | |
| Negative | 5 (25) | 15 (75.0) | 0.1485 | 8 (40.0) | 0.908 | 5 (25.0) | 0.6635 |
| Positive | 30 (42.9) | 40 (57.1) | | 27 (38.6) | | 21 (30.0) | |
| Tumor size (cm), n
(%) | | | | | | | |
| <5 | 14 (45.2) | 17 (54.8) | 0.3763 | 11 (35.5) | 0.631 | 7 (22.6) | 0.3385 |
| ≥5 | 21 (35.6) | 38 (64.4) | | 24 (40.7) | | 19 (32.2) | |
| PTEN (total), n
(%) | | | | | | | |
| Negative | 12 (36.4) | 21 (63.6) | 0.8234 | 13 (39.4) | 1.0 | 10 (30.3) | 0.8145 |
| Positive | 23 (40.4) | 34 (59.6) | | 22 (38.6) | | 16 (28.1) | |
| PTEN (cytopasmic),
n (%) | | | | | | | |
| Negative | 24 (39.3) | 37 (60.7) | 1.0 | 23 (37.7) | 0.8184 | 16 (26.2) | 0.4612 |
| Positive | 11 (37.9) | 18 (62.1) | | 12 (41.4) | | 10 (36.7) | |
| PTEN (nuclear), n
(%) | | | | | | | |
| Negative | 14 (35.0) | 26 (65.0) | 0.5224 | 16 (40.0) | 1.0 | 12 (30.0) | 1.0 |
| Positive | 21 (42.0) | 29 (58.0) | | 19 (38.0) | | 14 (28.0) | |
Survival analysis
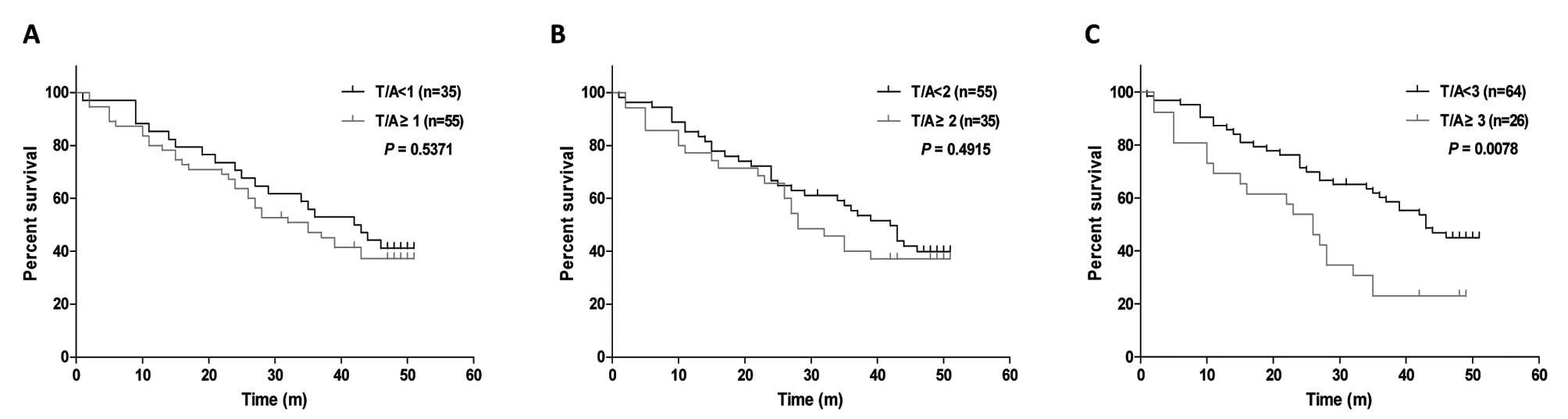
Finally, we conducted survival analysis to determine
whether FAS overexpression in tumors was associated with survival
by plotting Kaplan-Meier survival curves and calculating the 3-year
survival rate for all three grades of FAS overexpression. For cases
with S (T/A) ≥1 or ≥2, FAS overexpression was not associated with
overall survival or 3-year survival rate (Table III; Fig. 3A and B). However, among cases with
S (T/A) ≥3, FAS overexpression was significantly associated with
poor survival (Fig. 3C, log-rank
test, P=0.0078) and with a decreased 3-year survival rate (Table III; χ2 test,
P=0.0023).
 | Table IIIFAS expression and 3-year overall
survival of the gastric carcinoma patients. |
Table III
FAS expression and 3-year overall
survival of the gastric carcinoma patients.
| Overall
survival | S (T/A) <1 | S (T/A) ≥1 | S (T/A) <2 | S (T/A) ≥2 | S (T/A) <3 | S (T/A) ≥3 |
|---|
| ≥3 years | 18 | 26 | 29 | 15 | 38 | 6 |
| <3 years | 17 | 29 | 26 | 20 | 26 | 20 |
| P-value | 0.8292 | 0.3939 | 0.0023 |
Discussion
FAS protein, also known as oncogenic antigen 519
(OA-519), is overexpressed and hyperactivated in the majority of
human malignancies. It plays a central role in the maintenance of
the malignant phenotype by enhancing cancer cell survival and
proliferation (3). Overexpression
of FAS has been reported in human carcinomas including prostate,
ovary, breast, colon, endometrium, thyroid gland, squamous cell
carcinoma of the lung, and gastric carcinomas. In this study, FAS
overexpression was detected in 22.2% (20/90), 10% (9/90) and 28.9%
(26/90) of the carcinoma tissue specimens graded as S (T/A) ≥1 to
<2, S (T/A) ≥2 to <3 and S (T/A) ≥3, respectively.
Many studies have revealed that FAS overexpression
and hyperactivity is regulated by the MAPK/ERK1/2 and PTEN/PI3K/AKT
signaling pathways. Therefore, we examined PTEN and pERK expression
levels in the same TMA samples. However, FAS overexpression was not
correlated with pERK expression or the loss of PTEN in these
gastric carcinoma specimens. These findings suggest that there are
signaling pathways independent of the PTEN/PI3K/AKT and
RAS/RAF/MEK/ERK pathways, such as the ubiquitin-protease pathway,
that regulate FAS expression (25).
Although FAS overexpression at any of the three
levels defined in this study was not associated with any of the
clinicopathological parameters assessed (age, gender, AJCC stage
and histological grade), high FAS overexpression [S (T/A) ≥3] was
significantly associated with poor survival and with a reduced
3-year survival rate. Although several studies have reported that
FAS is overexpressed in gastric cancer (21,22),
to our knowledge, our study is the first to show that high FAS
overexpression could be a prognostic marker in gastric
carcinoma.
In addition to its potential utility as a prognostic
marker for cancer patients, FAS shows some promise as a
chemotherapeutic target (3,26–28).
For example, several studies have shown that cerulenin, a specific
noncompetitive inhibitor of the β-ketoacyl synthase activity of
FAS, is selectively cytotoxic to breast and ovarian cancer cells
exhibiting enhanced fatty acid synthesis, but not to normal cells
with constitutively low FAS expression (29,30).
Based on these and other results, more research into the clinical
relevance of FAS overexpression is necessary, particularly because
FAS may represent an excellent target for treating gastric
carcinoma, and other tumors.
References
|
1
|
Kuhajda FP: Fatty-acid synthase and human
cancer: new perspectives on its role in tumor biology. Nutrition.
16:202–208. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Takahiro T, Shinichi K and Toshimitsu S:
Expression of fatty acid synthase as a prognostic indicator in soft
tissue sarcomas. Clin Cancer Res. 9:2204–2212. 2003.PubMed/NCBI
|
|
3
|
Menendez JA and Lupu R: Fatty acid
synthase and the lipogenic phenotype in cancer pathogenesis. Nat
Rev Cancer. 7:763–777. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Van de Sande T, De SE, Heyns W, Verhoeven
G and Swinnen JV: Role of the phosphatidylinositol
3′-kinase/PTEN/Akt kinase pathway in the overexpression of fatty
acid synthase in LNCaP prostate cancer cells. Cancer Res.
62:642–646. 2002.
|
|
5
|
Escot C, Joyeux C, Mathieu M, Maudelonde
T, Pages A, Rochefort H and Chalbos D: Regulation of fatty acid
synthetase ribonucleic acid in the human endometrium during the
menstrual cycle. J Clin Endocrinol Metab. 70:1319–1324. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rooney SA: Fatty acid biosynthesis in
developing fetal lung. Am J Physiol. 257:L195–L201. 1989.PubMed/NCBI
|
|
7
|
Joyeux C, Chalbos D and Rochefort H:
Effects of progestins and menstrual cycle on fatty acid synthetase
and progesterone receptor in human mammary glands. J Clin
Endocrinol Metab. 70:1438–1444. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Smith S and Ryan P: Asynchronous
appearance of two enzymes concerned with medium chain fatty acid
synthesis in developing rat mammary gland. J Biol Chem.
254:8932–8936. 1979.PubMed/NCBI
|
|
9
|
Chirala SS, Chang H, Matzuk M, et al:
Fatty acid synthesis is essential in embryonic development: fatty
acid synthase null mutants and most of the heterozygotes die in
utero. Proc Natl Acad Sci USA. 100:6358–6363. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Alo PL, Visca P, Trombetta G, et al: Fatty
acid synthase (FAS) predictive strength in poorly differentiated
early breast carcinomas. Tumori. 85:35–40. 1999.PubMed/NCBI
|
|
11
|
Shurbaji MS, Pasternack GR and Kuhajda FP:
Expression of haptoglobin-related protein in primary and metastatic
breast cancers. A longitudinal study of 48 fatal tumors. Am J Clin
Pathol. 96:238–242. 1991.PubMed/NCBI
|
|
12
|
Nguyen PL, Ma J, Chavarro JE, et al: Fatty
acid synthase polymorphisms, tumor expression, body mass index,
prostate cancer risk, and survival. J Clin Oncol. 28:3958–3964.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shurbaji MS, Kuhajda FP, Pasternack GR and
Thurmond TS: Expression of oncogenic antigen 519 (OA-519) in
prostate cancer is a potential prognostic indicator. Am J Clin
Pathol. 97:686–691. 1992.PubMed/NCBI
|
|
14
|
Gansler TS, Hardman W III, Hunt DA,
Schaffel S and Hennigar RA: Increased expression of fatty acid
synthase (OA-519) in ovarian neoplasms predicts shorter survival.
Hum Pathol. 28:686–692. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rashid A, Pizer ES, Moga M, et al:
Elevated expression of fatty acid synthase and fatty acid synthetic
activity in colorectal neoplasia. Am J Pathol. 150:201–208.
1997.PubMed/NCBI
|
|
16
|
Uddin S, Hussain AR, Ahmed M, et al: High
prevalence of fatty acid synthase expression in colorectal cancers
in Middle Eastern patients and its potential role as a therapeutic
target. Am J Gastroenterol. 104:1790–1801. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Epstein JI, Carmichael M and Partin AW:
OA-519 (fatty acid synthase) as an independent predictor of
pathologic state in adenocarcinoma of the prostate. Urology.
45:81–86. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
De SE, Brusselmans K, Heyns W, Verhoeven G
and Swinnen JV: RNA interference-mediated silencing of the fatty
acid synthase gene attenuates growth and induces morphological
changes and apoptosis of LNCaP prostate cancer cells. Cancer Res.
63:3799–3804. 2003.
|
|
19
|
Menendez JA, Colomer R and Lupu R:
Inhibition of tumor-associated fatty acid synthase activity
enhances vinorelbine (Navelbine)-induced cytotoxicity and apoptotic
cell death in human breast cancer cells. Oncol Rep. 12:411–422.
2004.
|
|
20
|
Menendez JA, Vellon L, Colomer R and Lupu
R: Pharmacological and small interference RNA-mediated inhibition
of breast cancer-associated fatty acid synthase (oncogenic
antigen-519) synergistically enhances Taxol (paclitaxel)-induced
cytotoxicity. Int J Cancer. 115:19–35. 2005. View Article : Google Scholar
|
|
21
|
Hashimoto T, Kusakabe T, Sugino T, et al:
Expression of heart-type fatty acid-binding protein in human
gastric carcinoma and its association with tumor aggressiveness,
metastasis and poor prognosis. Pathobiology. 71:267–273. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kusakabe T, Nashimoto A, Honma K and
Suzuki T: Fatty acid synthase is highly expressed in carcinoma,
adenoma and in regenerative epithelium and intestinal metaplasia of
the stomach. Histopathology. 40:71–79. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Enzinger FM and Weiss SW: Soft Tissue
Tumors. 3rd edition. Mosby-Year Book, Inc; St. Louis, MO: pp. 4–12.
1995
|
|
24
|
Bandyopadhyay S, Pai SK, Watabe M, et al:
FAS expression inversely correlates with PTEN level in prostate
cancer and a PI 3-kinase inhibitor synergizes with FAS siRNA to
induce apoptosis. Oncogene. 24:5389–5395. 2005. View Article : Google Scholar
|
|
25
|
Graner E, Tang D, Rossi S, et al: The
isopeptidase USP2a regulates the stability of fatty acid synthase
in prostate cancer. Cancer Cell. 5:253–261. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Flavin R, Peluso S, Nguyen PL and Loda M:
Fatty acid synthase as a potential therapeutic target in cancer.
Future Oncol. 6:551–562. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kridel SJ, Lowther WT and Pemble CW: Fatty
acid synthase inhibitors: new directions for oncology. Expert Opin
Investig Drugs. 16:1817–1829. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kuhajda FP: Fatty acid synthase and
cancer: new application of an old pathway. Cancer Res.
66:5977–5980. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pizer ES, Wood FD, Heine HS, Romantsev FE,
Pasternack GR and Kuhajda FP: Inhibition of fatty acid synthesis
delays disease progression in a xenograft model of ovarian cancer.
Cancer Res. 56:1189–1193. 1996.PubMed/NCBI
|
|
30
|
Pizer ES, Jackisch C, Wood FD, Pasternack
GR, Davidson NE and Kuhajda FP: Inhibition of fatty acid synthesis
induces programmed cell death in human breast cancer cells. Cancer
Res. 56:2745–2747. 1996.PubMed/NCBI
|