Introduction
The endometrium undergoes cyclic regeneration during
the control of ovarian steroid hormones, and the blastocysts are
only able to implant in the uterus in the appropriate phase
(1). Blastocyst implantation is a
shared phenomenon in mammalians. At approximately 3–6 days after
fertilization (corresponding to D21–D24 in the natural cycle or
D5–D8 in the luteinizing hormone peak) which is also known as the
implantation window, the blastocysts enter the uterus, and then the
blastocysts and the uterus secrete related proteins and local
factors in a strict spatial and temporal order. These proteins and
factors recognize each other and interact to assist the
implantation (2). These
biologically active cytokines and proteins are also known as
markers for endometrial receptivity.
In females with infertility, tubal factors are the
most common and account for 40% of the causes of female
infertility. In addition, hydrosalpinx accounts for 10–30% of the
causes of tubal factor infertility. Initially, in vitro
fertilization-embryo transfer (IVF-ET) was used to treat
infertility due to tubal factors. However, numerous studies have
demonstrated that hydrosalpinx significantly reduces the
implantation and pregnancy rate (3). The mechanism underlying the influence
of hydrosalpinx on IVF-ET remains unclear. Previous studies have
revealed that the influence of hydrosalpinx on the endometrial
receptivity is one of these causes (4). In the present study, the expression
of leukemia inhibitory factor (LIF) and L-selectin in the
endometrium was compared between hydrosalpinx patients and tubal
obstruction patients, and in hydrosalpinx patients before and after
surgery during the implantation window. This study aimed to explore
the causes of a poor outcome of IVF-ET in hydrosalpinx
patients.
Patients and methods
Patients
A total of 60 patients with hydrosalpinx and 30
patients with tubal obstruction but without hydrosalpinx were
recruited from the Center for Reproductive Medicine of the First
Affiliated Hospital of Sun Yat-sen University, Guangzhou, China
from April to December 2010. This study has been approved by the
institutional review board of the First Affiliated Hospital of Sun
Yat sen University in March, 2010 and informed consent was obtained
from all participants.
All patients were aged below 40 years and had normal
menstrual cycles. Endocrine examinations were normal and
temperature monitoring revealed biphasic patterns. No patient had
been administered with corticosteroids in the past 6 months.
Exclusion criteria included endometriosis, uterine fibroids,
polycystic ovary syndrome, ovarian tumor, unexplained infertility,
immune infertility, chronic systemic diseases, sexually transmitted
diseases, trophoblastic disease, smoking and alcohol consumption
and male factor infertility.
Diagnostic methods
Hysterosalpingography (HSG)was employed for
hydrosalpinx diagnosis. Ultrasonography was carried out and it
revealed bilateral or unilateral hydrosalpinx. HSG or laparoscopy
(LAP) was employed to diagnose the tubal obstruction and
ultrasonography did not reveal hydrosalpinx.
Surgical treatment of hydrosalpinx
Under transvaginal ultrasound guidance, hydrosalpinx
aspiration was performed; salpingostomy, proximal tubal ligation or
salpingectomy were performed by laparoscopy.
Sample collection and processing
Since day 10 of the menstrual cycle, urine LH test
paper, transvaginal ultrasound and blood sex hormones were used to
detect the LH peak. At approximately 7–8 days after ovulation, the
endometrial tissue was collected at the fundus of the uterus and
washed with normal saline to avoid contamination with blood. The
samples were then fixed and embedded in paraffin, followed by
sectioning. Secretory phase endometrium was pathologically
confirmed. For patients with hydrosalpinx, sample collection was
carried out before and after surgery during the implantation
window, but sample collection was performed once in patients with
tubal obstruction.
Reagents for immunohistochemistry
Mouse anti-human LIF monoclonal antibody (1:80;
R&D Systems, Minneapolis, MN, USA) and mouse anti-human
L-selectin ligand monoclonal antibody (1:200; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) were used for
immunohistochemistry which was carried out according to the
manufacturer’s instructions.
Determination
Five fields at high magnification (x400) were
randomly selected from each section and Image-Pro Plus 5.1 software
(Media Cybernetics, Shanghai, China) was employed for the detection
of integrated optical density (IOD).
Statistical analysis
Data are expressed as the means ± standard
deviation. For patients with hydrosalpinx, data before and after
surgery were compared using a paired t-test and those between
hydrosalpinx patients and tubal obstruction patients were analyzed
with an independent sample t-test. SPSS version 13.0 software for
Windows was used for statistical analysis. A two-sided P-value of
<0.05 was considered to indicate a statistically significant
difference.
Results
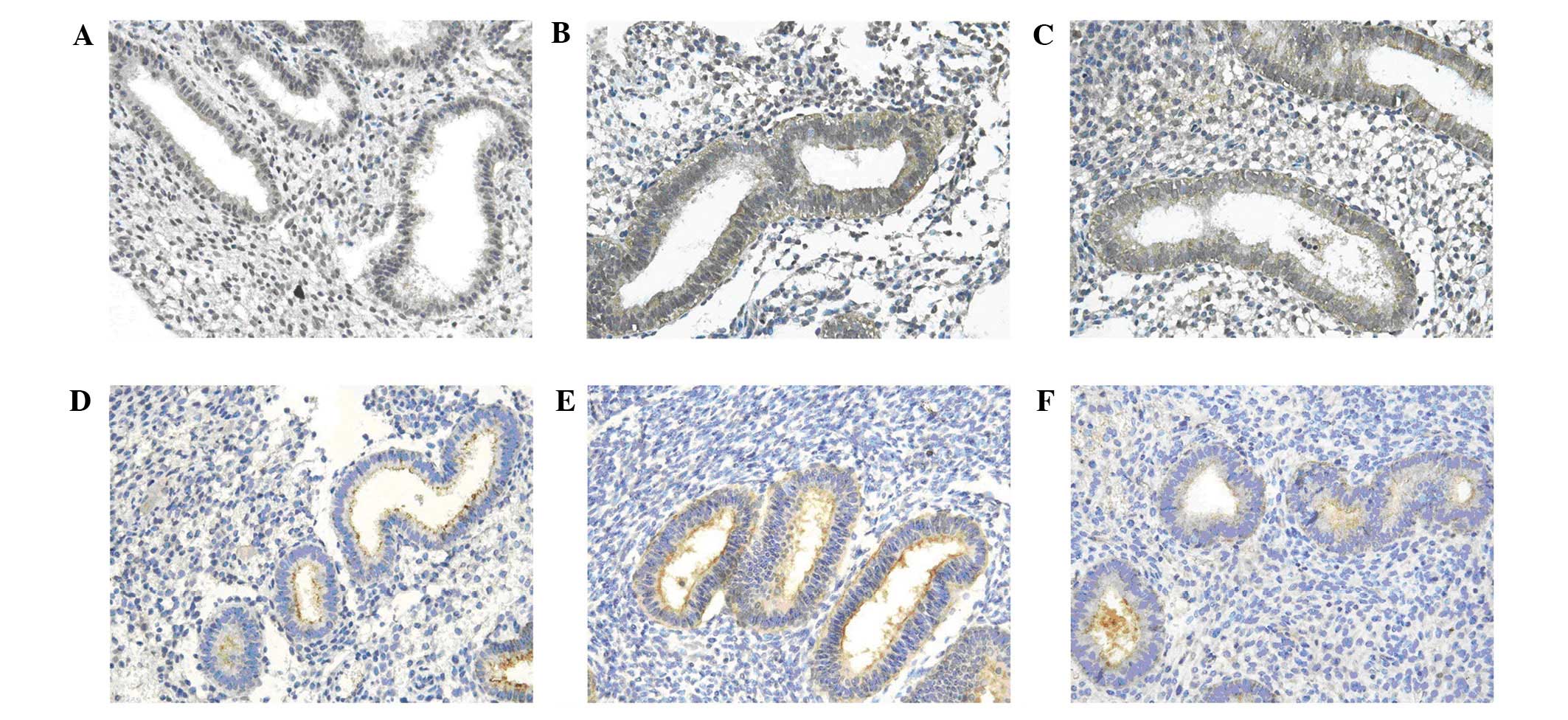
Under a light microscope, the expression of LIF and
L-selectin ligand was mainly found in the cytoplasm and the cell
membrane of endometrial epithelial cells, and less often found in
the interstitium. LIF expression in the endometrium of hydrosalpinx
patients was significantly different before and after surgery
(P<0.05) (Table I). Before
surgery, LIF expression in hydrosalpinx patients (Fig. 1A) was markedly different from that
of tubal obstruction patients (Table
II; Fig. 1C; P<0.05). After
surgery, LIF expression in hydrosalpinx patients (Fig. 1B) was similar to that of tubal
obstruction patients (P>0.05) (Table III). Before surgery, the integrin
αvβ3 expression in hydrosalpinx patients (Fig. 1D) was markedly different from that
of tubal obstruction patients (Table
II; Fig. 1F; P<0.05).
Following surgery, the integrin αvβ3 expression in hydrosalpinx
patients (Fig. 1E) was comparable
to that of tubal obstruction patients (P>0.05) (Table III).
 | Table IExpression of LIF and L-selectin
ligand in hydrosalpinx patients before and after surgery. |
Table I
Expression of LIF and L-selectin
ligand in hydrosalpinx patients before and after surgery.
| Before surgery
(n=60) | After surgery
(n=60) | P-value |
|---|
| LIF | 0.42±0.17 | 0.58±0.21 | <0.05 |
| L-selectin
ligand | 0.37±0.11 | 0.54±0.15 | <0.05 |
 | Table IIExpression of LIF and L-selectin
ligand in hydrosalpinx patients before surgery and in tubal
obstruction patients. |
Table II
Expression of LIF and L-selectin
ligand in hydrosalpinx patients before surgery and in tubal
obstruction patients.
| Before surgery
(n=60) | Control group
(n=30) | P-value |
|---|
| LIF | 0.42±0.17 | 0.60±022 | 0.000 |
| L-selectin
ligand | 0.37±0.11 | 0.50±0.15 | 0.000 |
 | Table IIIExpression of LIF and L-selectin
ligand in hydrosalpinx patients after surgery and in tubal
obstruction patients. |
Table III
Expression of LIF and L-selectin
ligand in hydrosalpinx patients after surgery and in tubal
obstruction patients.
| After surgery
(n=60) | Control group
(n=30) | P-value |
|---|
| LIF | 0.58±0.21 | 0.60±022 | 0.662 |
| L-selectin
ligand | 0.54±0.15 | 0.50±0.15 | 0.302 |
Discussion
LIF is a multifunctional factor belonging to the
interleukin (IL)-6 family. The mature LIF is a secretory protein,
highly glycosylated at asparagine residues and is composed of 180
amino acids. LIF binds to the corresponding receptors exerting
biological effects, which may influence the reproductive activities
at different levels, including follicular development, embryonic
development, implantation and maintenance of pregnancy (5–7). LIF
has been regarded as one of the markers for endometrial
receptivity, having multiple biological activities. Studies have
confirmed that LIF expression is present in ovaries, follicular
fluid, fallopian tubes, trophoderm, endometrium and decidua.
LIF expression in the endometrium plays an important
regulatory role in the blastocyst implantation in mammalians. LIF
may strengthen the regulation and differentiation of the
trophoderm, and plays an important role in the adhesion and
invasion of blastocysts during implantation. LIF expression may be
found in the natural killer cells in the decidua, chorion and
endometrium during early pregnancy. There is evidence demonstrating
that LIF regulates the blastocyst implantation via the regulation
of the invasive ability of trophoblasts and by influencing the
immune tolerance, and that LIF expression is required for
blastocyst implantation (8–10).
In the detection of mRNA species with the RNAase protection method,
the results showed the protein and mRNA expression of LIF in the
endometrial epithelial cells during the whole menstrual cycle.
Notably, LIF expression is markedly increased in the endometrial
epithelial cells during blastocyst implantation (middle and late
secretory phase and early pregnancy) (11). Previous studies have shown that LIF
expression in the endometrial epithelial cells rapidly increases
during the middle and late luteal phase. However, during the middle
and late luteal phase, LIF expression in patients with infertility
from unknown causes was significantly lower than that in healthy
subjects. In the middle luteal phase, LIF expression in the
endometrium of patients with recurrent miscarriage was
significantly reduced when compared with healthy child-bearing
females (12). In addition, LIF
expression in the endometrium in the secretory phase was 22-fold
higher than that of the proliferative phase in females with a
history of pregnancy, but LIF expression in the secretory phase is
dramatically reduced in females with infertility (13). This suggests that LIF plays an
important role in the initiation of blastocyst implantation and
maintenance of pregnancy.
Our results showed that LIF expression in the
endometrium of hydrosalpinx patients during the implantation window
before surgery was markedly lower than that in the endometrium of
tubal obstruction patients without hydrosalpinx. After surgery, LIF
expression in the endometrium during the implantation window was
comparable to that of the control group. In addition, LIF
expression was significantly different before and after surgery.
This indicates that hydrosalpinx influences LIF expression in the
endometrium during the implantation window and that LIF expression
increases after the surgical treatment of hydrosalpinx. These
findings were consistent with those from previous studies. Seli
et al (4) found that LIF
expression in the endometrium of hydrosalpinx patients during the
implantation window was markedly lower than that in the endometrium
of healthy females, and that LIF expression increased after
salpingectomy. These findings demonstrate that hydrosalpinx reduces
LIF expression in the endometrium during the implantation window.
Hydrosalpinx is a manifestation of chronic pelvic inflammation and
the cytotoxic factors are at a high level in the affected fallopian
tube (14). These factors might
reflux into the uterus, influencing the endometrium. Copperman
et al (15) compared the
endometrium of hydrosalpinx patients and subjects with normal
fallopian tubes. Their results showed that the number of
inflammatory cells in the endometrium of hydrosalpinx patients was
markedly higher than that in the endometrium of the controls, and
that the expression of IL-2, a representative inflammatory
cytokine, was also markedly increased in the endometrium of
hydrosalpinx patients as compared with the controls. IL-2 is a
specific cytokine secreted by the Th1 lymphocytes. Piccinni et
al (16) found that Th1
cytokines can downregulate LIF expression. Thus, the increased IL-2
expression in the endometrium of hydrosalpinx patients may reduce
LIF expression.
L-selectin is a member of the selectin family, which
mediates the binding of white blood cells to endothelial cells and
is involved in the migration of white blood cells through the
vascular endothelial cells into inflammatory tissues, and the
homing and recycling of lymphocytes (17,18).
Previous studies have shown that reproduction, immunity and
vascular functions have similarities at the molecular level
(19,20).
The selectin family is a group of cellular adhesion
molecules and consists of 3 molecules of similar structure:
L-selectin, P-selectin and E-selectin, which were named as they
were initially identified in the white blood cells, platelets and
endothelial cells. To date, a total of 5 ligands of L-selectin have
been identified: i) Glycosylation-dependent cell adhesion
molecule-1 (GlyCAM-1) is a 50-kDa, secretory, salivary mucin that
binds to selectins of more than 3 subtypes. The sulfated
oligosaccharide chains of GlyCAM-1 bind to L-selectin. In addition,
GlyCAM-1 binds to the lymphocytes, resulting in the activation of
integrin β1 and β2; ii) CD34 is a 90-kDa glycoprotein and a type I
transmembrane salivary mucin. CD34 is expressed in the endothelial
cells of the vascular system, hematopoietic precursor cells, brain
and embryonic fibroblasts; iii) mucosal addressin cell adhesion
molecule-1 (MAdCAM-1) is a protein identified using MECA-367
monoclonal antibody. MAdCAM-1 is also a ligand of integrin α4β7 in
lymphocytes; iv) Sgp200 is a sulphated glycoprotein which was
identified in the separation of mouse high endothelial venules
(HEVs) in the lymph nodes with chimera of L-selectin. Sgp200 is 200
kD in molecular weight and is secreted. The molecular
characteristics of Sgp200 remain unclear; v) P-selectin
glycoprotein ligand 1 also binds to L-selectin, exerting biological
effects.
L-selectin is constitutively expressed in the
majority of white blood cells. It mediates the adhesion between
white blood cells and endothelial cells, and is involved in the
migration of white blood cells through the endothelial cells into
inflammatory tissues and the homing and recycling of lymphocytes.
In addition, it mediates the adhesion between white blood cells. At
the inflammatory sites, L-selectin initiates the adhesion between
white blood cells and endothelial cells, which is mediated by the
regulation of the expression of ligands on the endothelial cells.
Genbacev et al (21) found
that the migration of white blood cells across the blood vessels
was morphologically similar to the adhesion of blastocysts to the
uterus, and the blastocysts were in the liquid environment
containing mucins secreted by the uterus during the adhesion of
blastocysts to the uterus. Subsequently, Genbacev et al
(21) found that the binding of
L-selectin on the trophoblasts to its ligand could initiate the
blastocyst implantation. During the implantation window, the
expression of L-selectin on the blastocysts and that of its ligand
in the endometrium have an increasing tendency. Their study not
only confirmed the binding of L-selectin to its ligand, but it also
demonstrated that L-selectin is an important regulator of
pregnancy. Other studies have also revealed L-selectin expression
in sperm, demonstrating its involvement in fertilization.
Ligands of L-selectin were first identified in mouse
HEVs of lymph nodes, using MECA-79 monoclonal antibody. Thus the
corresponding antigen was named peripheral lymph node addressin
(PNAd) which includes G1yCAM-1, CD34 and Sgp200, for example.
In a previous study, the antibody against the ligand
of L-selectin was used in the immunofluorescence detection of
endometrium in the follicular and luteal phase (22). The results showed that the
endometrium was weakly positive for MECA-79 in the follicular phase
and the MECA-79 positive cells were found in the endometrial glands
and epithelial cells of the uterine cavity. In the luteal phase,
the endometrium was markedly positive for MECA-79, especially in
cells of the uterine cavity. Continuous biopsy of the endometrium
and subsequent western blot assay for MECA-79 showed that MECA-79
expression on days 3 and 6 was markedly increased when compared
with that on days 0 and 2. This suggested that the endometrium
became receptive and the expression of the ligand on L-selectin
increased. In females with normal menstrual cycles, MECA-79
expression in the endometrium has a similar tendency.
To date, few studies have been conducted to
investigate L-selectin and its ligands in the endometrium and
little is known regarding the effect of hydrosalpinx on the
expression of L-selectin and its ligands.
In the present study, L-selectin ligand expression
in the endometrium during the implantation window in hydrosalpinx
patients before surgery was markedly lower than that of patients
with tubal obstruction. After surgery, L-selectin ligand expression
during the implantation window in hydrosalpinx patients was
comparable to that of tubal obstruction patients. In addition,
L-selectin ligand expression was significantly different before and
after surgery. This suggests that hydrosalpinx influences the
L-selectin ligand expression in the endometrium during the
implantation window and that L-selectin ligand expression increases
after surgery.
Hydrosalpinx is a manifestation of chronic pelvic
inflammation. Copperman et al (15) compared the endometrium of
hydrosalpinx patients and subjects with normal fallopian tubes.
Their results demonstrated that the number of inflammatory cells in
the endometrium of hydrosalpinx patients was markedly higher than
that of the controls, and the expression of IL-2, a representative
inflammatory cytokine, was also significantly increased in the
endometrium of hydrosalpinx patients as compared with controls.
IL-2 is a specific cytokine secreted by the Th1 lymphocytes.
Piccinni et al (16) found
that Th1 cytokines can downregulate LIF expression. Thus, the
increased IL-2 expression in the endometriun of hydrosalpinx
patients may reduce the LIF expression. The cytotoxic factors in
the affected fallopian tube were at a high level (14). These factors might reflux into the
uterus, influencing the L-selectin ligand expression in the
endometrium. Findings in the present study and previous studies
demonstrated that hydrosalpinx could reduce the expression of
well-known markers for endometrial receptivity, including LIF. Our
findings also revealed hydrosalpinx reduced the expression of
L-selectin ligand in the endometrium. On the basis that the change
in LIF expression was consistent with that of L-selectin ligand in
the endometrium of hydrosalpinx patients, we postulated that
L-selectin and its ligand could be used as markers for endometrial
receptivity. However, more multicenter, prospective, randomized
controlled studies with large sample sizes would be required to
confirm our findings.
Overall, the endometrium undergoes cyclic
regeneration that varies in different individuals. Accurate
evaluation of endometrial receptivity is the basis for the
improvement of endometrial receptivity. In the present study, the
effect of hydrosalpinx on the expression of LIF and L-selectin
ligand in the endometrium was investigated. Our findings revealed
that hydrosalpinx influenced the expression of LIF and L-selectin
ligand in the endometrium, which compromised the endome-trial
receptivity of blastocyst implantation and the ability to maintain
pregnancy. The expression of LIF and L-selectin ligand in the
endometrium was increased after surgical intervention of
hydrosalpinx.
Acknowledgements
The study was supported by the Science
and Technology Project of Guangdong Province (2009B030801155) and
the Research Project of Population and Family Planning Commission
of Guangdong Province (2010243).
References
|
1
|
Lessey BA: Assessment of endometrial
receptivity. Fertil Steril. 96:522–529. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Taylor E and Gomel V: The uterus and
fertility. Fertil Steril. 89:1–16. 2008. View Article : Google Scholar
|
|
3
|
Mijatovic V, Veersema S, Emanuel MH, et
al: Essure hysteroscopic tubal occlusion device for the treatment
of hydrosalpinx prior to in vitro fertilization-embryo transfer in
patients with a contraindication for laparoscopy. Fertil Steril.
93:1338–1342. 2010. View Article : Google Scholar
|
|
4
|
Seli E, Kayisli UA, Cakmak H, et al:
Removal of hydrosalpinges increases endometrial leukaemia
inhibitory factor (LIF) expression at the time of the implantation
window. Hum Reprod. 20:3012–3017. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Paiva P, Menkhorst E, Salamonsen L and
Dimitriadis E: Leukemia inhibitory factor and interleukin-11:
critical regulators in the establishment of pregnancy. Cytokine
Growth Factor Rev. 20:319–328. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aghajanova L, Altmäe S, Bjuresten K, et
al: Disturbances in the LIF pathway in the endometrium among women
with unexplained infertility. Fertil Steril. 91:2602–2610. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Aghajanova L: Update on the role of
leukemia inhibitory factor in assisted reproduction. Curr Opin
Obstet Gynecol. 22:213–219. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dimitriadis E, Nie G, Hannan NJ, et al:
Local regulation of implantation at the human fetal-maternal
interface. Int J Dev Biol. 54:313–322. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
van der Gaast MH, Macklon NS,
Beier-Hellwig K, et al: The feasibility of a less invasive method
to assess endometrial maturation - comparison of simultaneously
obtained uterine secretion and tissue biopsy. BJOG. 116:304–312.
2009.
|
|
10
|
Mikolajczyk M, Wirstlein P and Skrzypczak
J: The impact of leukemia inhibitory factor in uterine flushing on
the reproductive potential of infertile women-a prospective study.
Am J Reprod Immunol. 58:65–74. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Perrier d’Hauterive S, Charlet-Renard C,
Berndt S, et al: Human chorionic gonadotropin and growth factors at
the embryonic-endometrial interface control leukemia inhibitory
factor (LIF) and interleukin 6 (IL-6) secretion by human
endometrial epithelium. Hum Reprod. 19:2633–2643. 2004.
|
|
12
|
Yang ZM, Chen DB, Le SP, et al:
Differential hormonal regulation of leukemia inhibitory factor
(LIF) in rabbit and mouse uterus. Mol Reprod Dev. 43:470–476. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Altmäe S, Martinez-Conejero JA, Salumets
A, et al: Endometrial gene expression analysis at the time of
embryo implantation in women with unexplained infertility. Mol Hum
Reprod. 16:178–187. 2010.PubMed/NCBI
|
|
14
|
Strandell A, Thorburn J and Wallin A: The
presence of cytokines and growth factors in hydrosalpingeal fluid.
J Assist Reprod Genet. 21:241–247. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Copperman AB, Wells V, Luna M, et al:
Presence of hydrosalpinx correlated to endometrial inflammatory
response in vivo. Fertil Steril. 86:972–976. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Piccinni MP, Beloni L, Livi C, et al:
Defective production of both leukaemia inhibitory factor and type 2
T-helper cytokines by decidual T cells in unexplained recurrent
abortions. Nat Med. 4:1020–1024. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rosen SD: Ligands for L-selectin: homing,
inflammation, and beyond. Annu Rev Immunol. 22:129–156. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
McEver RP: Selectins: lectins that
initiate cell adhesion under flow. Curr Opin Cell Biol. 14:581–586.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Thiery JP: Cell adhesion in development: a
complex signaling network. Curr Opin Genet Dev. 13:365–371. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou Y, Genbacev O and Fisher SJ: The
human placenta remodels the uterus by using a combination of
molecules that govern vasculogenesis or leukocyte extravasation.
Ann N Y Acad Sci. 995:73–83. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Genbacev OD, Prakobphol A, Foulk RA, et
al: Trophoblast L-selectin-mediated adhesion at the maternal-fetal
interface. Science. 299:405–408. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shamonki MI, Kligman I, Shamonki JM, et
al: Immunohistochemical expression of endometrial L-selectin ligand
is higher in donor egg recipients with embryonic implantation.
Fertil Steril. 86:1365–1375. 2006. View Article : Google Scholar : PubMed/NCBI
|