Introduction
Eyelids are the protective barrier of eyeballs
against any trauma. They not only protect the eyes from light and
dust, but also moisturize and cleanse the cornea. The repair of
tarsal defects is crucial in terms of both function and aesthetic
quality. Transplantations of glycerine-preserved and liquid
nitrogen-cryopreserved tarsal plates for the repair of tarsal
defects have been demonstrated to yield certain clinical effects
(1–3). However, studies on combined tarsal
plate and palpebral conjunctival transplantation for such repair
have not been reported to date. We performed an experimental study
on tarsal plate and palpebral conjunctival transplantation for
repairing tarsal defects in rabbits using glycerine preservation,
alcohol preservation and liquid nitrogen cryopreservation between
May 2006 and July 2007. We compared the groups on their clinical
effects, histopathological rejection and apoptotic cells
postoperatively. We also completed 30 surgeries for tarsal defects
in patients caused by the excision of eyelid neoplasms using
cryopreservation between March 2001 and October 2009 and obtained
fairly favorable clinical outcomes.
Materials and methods
Animals
Twenty-four healthy Chinese white rabbits (weight,
2–3 kg) were sacrificed by air embolism. Forty-eight transplants
were made from both the superior tarsal plates and the palpebral
conjunctivae, and they were divided equally into three groups (with
16 implants per group): Group 1, liquid nitrogen cryopreservation;
Group 2, glycerine preservation; and Group 3, alcohol preservation.
All transplants were preserved for 6 months. The study was approved
by the ethics committee of Nanjing First Hospital Affiliated to
Nanjing Medical University, Nanjing, China. Written informed
consent was obtained from the patient.
Liquid nitrogen cryopreservation
Sixteen transplants were stored in a moist room
after sterilization, placed in a 4°C thermostatic water tank and
frozen for 20 min. Two concentrations of dimethyl sulfoxide
solution (5 and 7.5%) were used with 20% albumin as a solvent. The
transplants were placed in the two solutions, frozen for 10 min and
then kept in a liquid nitrogen container at −196°C. When required,
the transplants were taken out of the liquid nitrogen container and
then immersed in a 40°C thermostatic water bath. Approximately 120
sec later, when the cryoprotective solutions had finished thawing,
the samples were taken out using sterile tweezers and eventually
immersed in saline. The samples were ready for use after soaking
for 10 min.
Glycerine preservation
Sixteen transplants were placed in a pure sterile
glycerine bottle and refrigerated at 4°C. Twenty-four hours later,
they were moved into another pure glycerine bottle and kept under
sealed preserving conditions in a 4°C refrigerator. They were
washed with saline before use and then immersed in gentamicin
solution in the ratio of 1:3000. The transplants were ready for use
after 15–20 min of revival.
Alcohol preservation
Sixteen transplants were immersed in 75% alcohol.
Three days later, they were moved to 95% alcohol. Within a tight
container, they were refrigerated at 4°C and then washed with
saline before use. They were immersed in gentamicin solution in the
ratio of 1:3000. The transplants were ready for use after 15–20 min
of revival.
Animal models
Twenty-four healthy Chinese white rabbits weighing
between 2 and 3 kg were equally divided into three groups (with 8
rabbits per group). We reconstructed 16 pairs of superior tarsi by
tarsal plate and palpebral conjunctival transplantation in Groups
1–3. Pentobarbital sodium was injected into the vein of each rabbit
at 30–35 mg/kg. After general anesthesia, tarsal defects were
excised by more than half of the length of the upper eyelid
palpebral margin. The transplants were then trimmed into the right
size and shape. Tarsal and palpebral conjunctival stumps were
closed by interrupted sutures with 6-0 absorbed thread. The
subjects received topical application of tobramycin eye ointment
for the next 2 weeks. Based on daily observation of the
transplants, two animals in each group were sacrificed 1 week, 1
month and 3 months postoperatively; one animal in each group was
sacrificed ∼4 months postoperatively; and three animals died
without specific cause, one of the three were from Group 2, the
others were from Group 3.
Histological observations
Collected specimens were perfused with 10% neutral
formalin and then cut into paraffin-embedded sections. All slices
were stained with hematoxylin and eosin. Responses, including
inflammatory infiltration, transplant tissue necrosis, connective
tissue hyperplasia and glandular cells, were observed by light
microscopy. We marked the paraffin-embedded sections at the 3′-end
hydroxyl caused by apoptotic cell nuclear DNA cleavage. Apoptosis
was observed under a fluoroscope. Positive nuclei were detected by
double-blind observation. Using 10 randomly selected cells from
each specimen, we read the number of apoptotic cells under
high-power fields of vision and calculated the average value.
Statistical analysis
Statistical analysis was performed with SPSS 11.0.
Data were analyzed using the paired t-test. P<0.05 was
considered to indicate a statistically significant result.
Clinical observations
Twenty-nine patients (30 eyes; age range, 23–85
years) were admitted to our institution for tarsal defects caused
by the excision of eyelid neoplasms between March 2001 and October
2009. One patient presented with right superior eyelid neoplasm
recurrence after excision. This patient had undergone four
surgeries on the right eye, including two excisions of superior
eyelid neoplasms, allogeneic tarsal and palpebral conjunctival
transplantation by cryopreservation and reconstruction of the
superior eyelid in sequence. Of the 29 patients, 11 were male and
18 were female. Eleven patients had meibomian gland carcinoma, 9
had basal cell carcinoma, 6 had squamous cell carcinoma, 2 had
conjunctival carcinoma in situ and 1 had sebaceous nevus.
After neoplasm excision, 3 patients developed palpebral marginal
defects less than one-third of the length of the eyelid, 11
developed palpebral marginal defects from one-third to half in
length and 15 developed palpebral marginal defects more than half
in length. The course of disease ranged between 1 month and 20
years.
Preparation, preservation and thawing of
allo-tarsal plates and palpebral conjunctivae
Palpebral conjunctivae from both living bodies and
corpses (allo-tarsal plates and palpebral conjunctivae could not be
separated) were obtained from 18-to 60-year-old donors who were
selected according to EBAA standards. Within 6 h of the donors’
death, we prepared palpebral conjunctivae using the aseptic
technique. The palpebral conjunctivae were stored in a moist
chamber, refrigerated at 4°C and frozen for 20 min. Albumin (20%)
was used as a solvent and mixed with gradually increasing
concentrations of dimethyl sulfoxide solution (5 and 7.5%). The
palpebral conjunctivae were then immersed in the two solutions and
the mixtures were frozen for 10 min. A lower-temperature procedure
was used to maintain the specimens at −80°C. Finally, specimens
were stored in a −196°C liquid nitrogen container for 3 months to 5
years. When they were ready for use, the palpebral conjunctivae
were taken out from the liquid nitrogen container, immersed in a
40°C constant water bath and swung slightly. When the frozen
cryoprotective solutions had finished thawing, that is, only a thin
layer of ice appeared around the palpebral conjunctivae, they were
transferred to saline using sterile tweezers. The samples were
ready for use after soaking for 10 min (4).
Surgery
Eyelid subcutaneous tissues were collected and
fornices with tissue infiltration were injected with anesthesia (2%
lidocaine and 0.75% bupivacaine). Neoplasms were excised beyond 4–5
mm of their exterior. Rapidly frozen slices were subjected to
pathological examination. If the tissue of a slice margin was
considered normal, palpebral conjunctival transplantation was
initiated. The tarsal palpebral conjunctivae, along with the
eyelashes, were trimmed into the size and shape that were required.
They were then sutured with the original tarsal stump or internal
and external palpebral periorbital ligaments. If the case involved
a superior tarsal defect, we kept the levator palpebrae superioris
fixed on the upper eyelid margin. Palpebral conjunctivae were
closed with an interrupted suture. Transferring or sliding the
musculocutaneous flap can reconstruct skin defects. The correct
tension was applied to avoid entropion and ectropion of the eyelids
postoperatively. Dressings were postoperatively changed once to
twice a day and pressed for 5 days. Local and general anti-biotics
were used. Xibrom was applied to local sites. Stitches were removed
2 weeks postsurgery.
Evaluation criteria of therapeutic
effects
Cure refers to the morphological and functional
recovery of the eyelids. The length and height differences of the
palpebral fissure should both be <2 mm compared with normal
eyes. Eyelids should be able to close well, and entropion and
ectropion should not be observed. Improvement refers to a
morphologically and functionally improved state of the eyelids.
Compared with normal eyes, the palpebral fissure length difference
should be no less than 2 mm, and its height difference should be no
less than 2 mm. Eyelids should be able to close well, and entropion
and ectropion should not be detected. A slight incision on the
tarsal margin would be present. Invalidity refers to cases in which
the eyelids do not exhibit any morphological and functional
improvement, and cases in which implants separated.
Results
Implants
All implants from the three experimental groups
exhibited responses including hyperemia and edema, among others, 1
day postoperatively. These responses reached a peak in the
following 7–10 days but gradually disappeared. Among the three
groups, the implants in Group 1 demonstrated milder responses.
During the observation period, all tarsal plates and palpebral
conjunctivae survived, and the surface of the palpebral
conjunctivae was smooth.
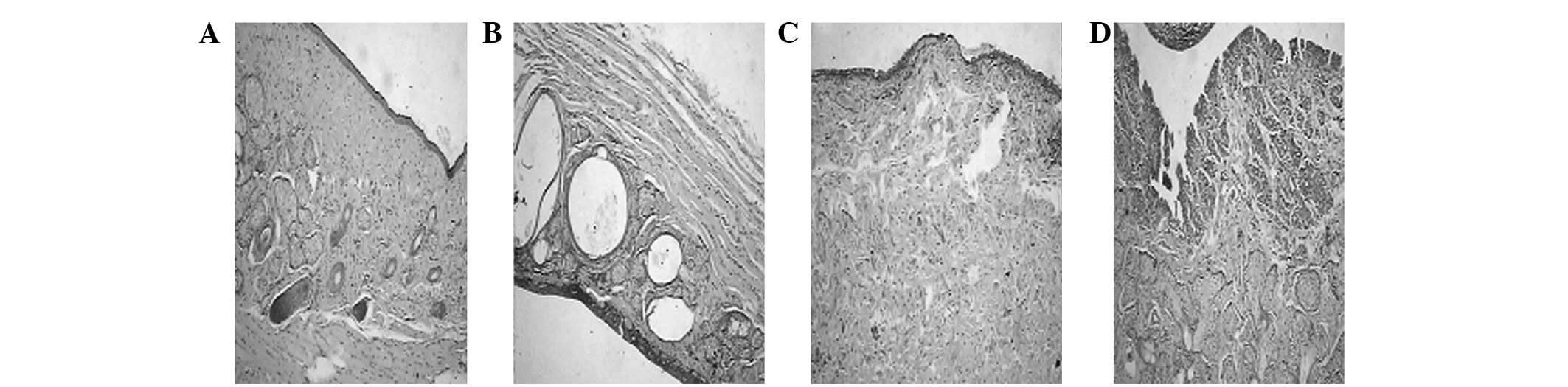
Morphological observations
Three groups of stained specimens were observed by
microscopy. During the first week, inflammatory infiltration,
necrosis and gland necrosis were detected among them. Inflammatory
infiltration disappeared within the first month. Collagen fiber and
connective tissue hyperplasia were eventually observed. Three
months later, inflammatory cells completely disappeared, but
collagen fiber hyperplasia emerged. Milder inflammatory
infiltration and less necrosis were observed in Group 1 in the
early stage compared with the other two groups, but a number of
gland cells did not survive. Group 1 also exhibited milder
connective hyperplasia during the late period (Fig. 1).
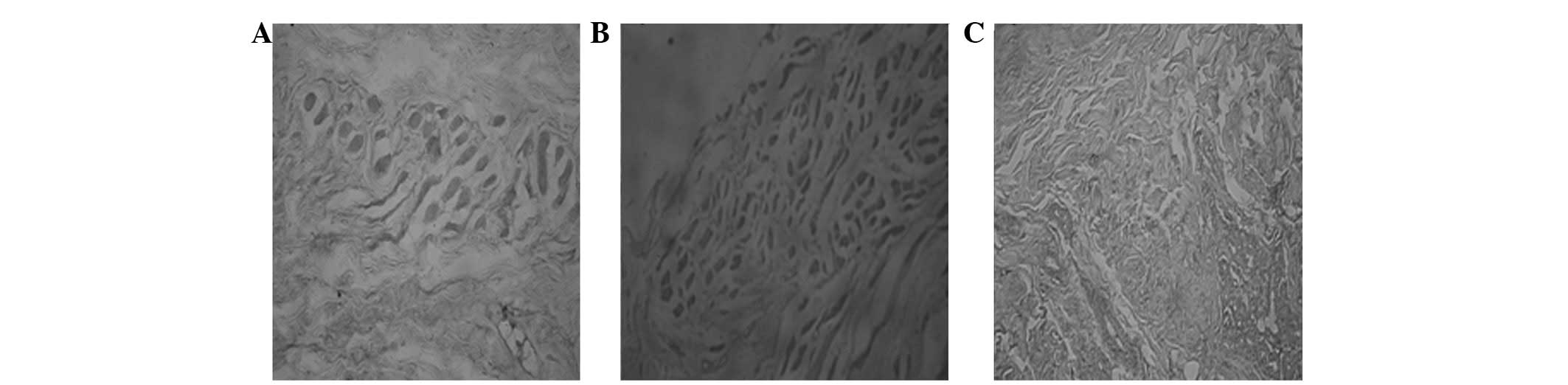
Terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL) assay
Paraffin-embedded sections were specifically marked
at the 3′-end hydroxyl caused by apoptotic cell nuclear DNA
cleavage using TUNEL to detect DNA. Cells that had brown and yellow
granules in the nucleus were considered positive (i.e., apoptotic
cells). Apoptosis of all the transplants of tarsal plates and
palpebral conjunctivae was observed in the three groups 1 week, 1
month and 3 months (Fig. 2)
postoperatively. However, during the same period, the number of
apoptotic cells in Group 1 was markedly lower compared with the
numbers in Groups 2 and 3. Statistically significant (P<0.05)
and insignificant (P>0.05) differences were noted (Table I).
 | Table IComparison of the number of apoptotic
cells in the rabbits’ tarsal plate and palpebral conjunctival
transplantation. |
Table I
Comparison of the number of apoptotic
cells in the rabbits’ tarsal plate and palpebral conjunctival
transplantation.
| Group | n | 1st week | 1st month | ≥3rd month |
|---|
| Cryopreserved group
(A) | 16 | 10.69±2.31 | 17.63±2.00 | 16.19±2.63 |
| Glycerine-preserved
group (B) | 16 | 18.50±3.38 | 24.69±3.19 | 26.00±3.19 |
| Alcohol-preserved
group (C) | 16 | 19.00±2.19 | 26.81±2.69 | 26.63±2.50 |
| P-value | | | | |
| A/B | | <0.01 | 0.03 | 0.03 |
| A/C | | <0.01 | 0.03 | 0.02 |
| B/C | | 0.14 | 0.26 | 0.46 |
Clinical observations
Patients were followed for 6 to 90 months
postoperatively (53.3 months on average) and none presented with
recurrence. In 15 eyes, mild notching remained and light
conjunctiva became shallow, whereas the rest of the eyelids
recovered morphologically and functionally. Furthermore, the
eyelids were able to close well, and postoperative entropion and
ectropion were not observed. Transplantation failed in only one
patient (1 eye) due to several reasons: First, the incisal edge
residue from the initial surgery had not been completely excised.
Next, when the tumor that expanded again was excised, we did not
replace the tarsal plate and palpebral conjunctival transplant, as
a result of which the original transplant separated in the
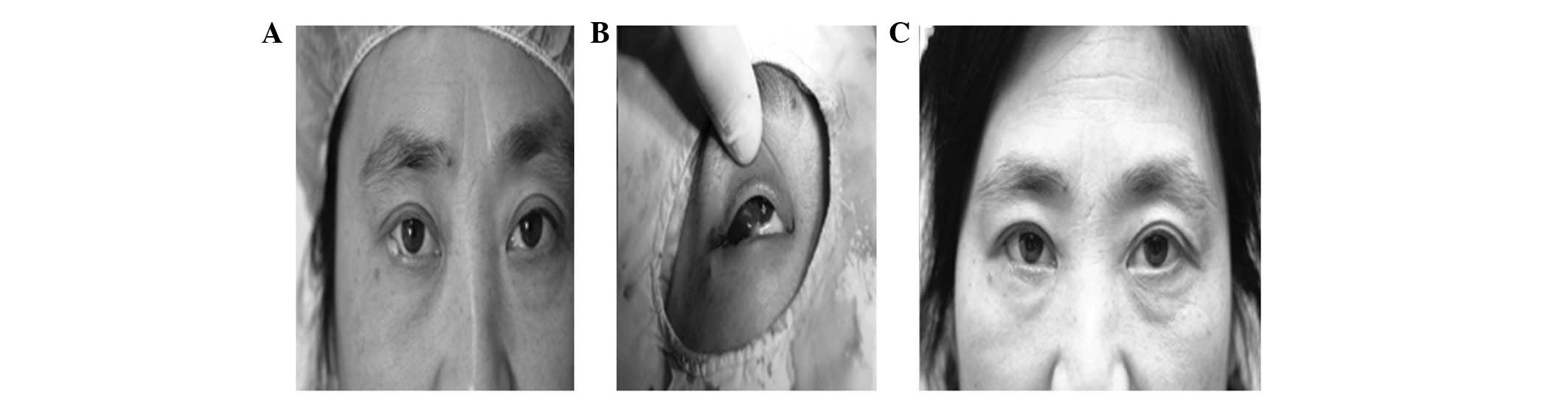
following half month of the second surgery. Overall, 14 eyes were
cured and 15 eyes improved in this group. The eyes of 3 patients in
whom palpebral marginal defects measured less than one-third in
length were cured. Of 11 cases of palpebral marginal defects
measuring one-third to half in length, 8 eyes were cured and 3
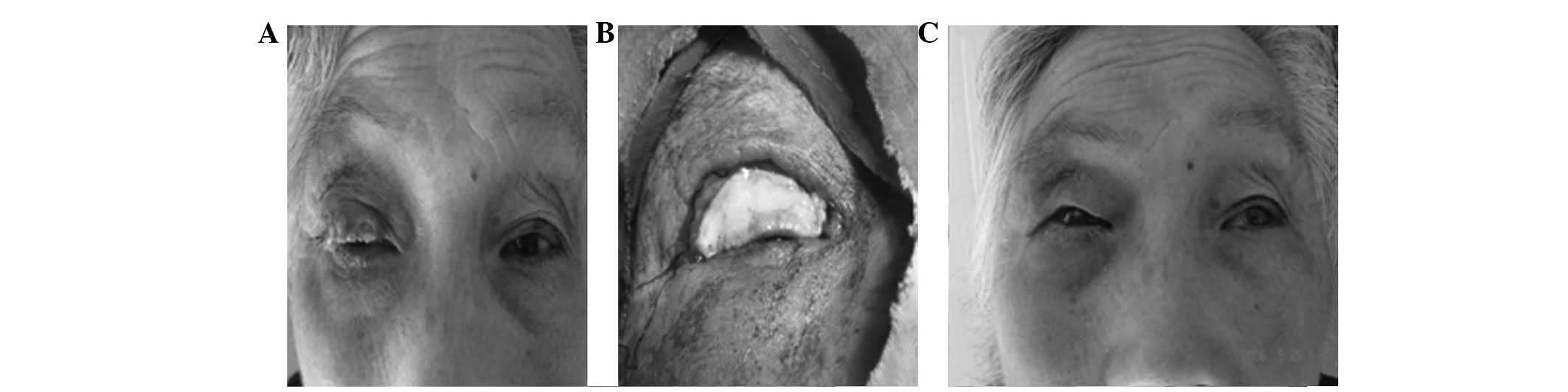
improved (Fig. 3). Finally, of 15
cases of palpebral marginal defects measuring more than half in
length, 3 eyes were cured and 12 improved (Fig. 4).
Discussion
Procedures to repair tarsal defects can be selected
and designed based on several factors, including patient age, the
characteristics of the eyelids, the size and depth of the defect
and different relationships with eyelid margins. Either sliding or
repair of the transposition flap can be selected for cases with
larger defects of the anterior eyelid (5). The first choice would be to use an
interior chin myocutaneous flap (6). For significantly larger defects,
thickness skin grafting may be adopted (7). The primary methods of tarsal repair
include free graft transplantation (8), sliding, indexable palpebral
conjunctival flap (9) and use of
various tarsus substitutes, such as composite transplants of the
nasal septal chondromucosal cartilage (10), allogeneic sclera (11), auricular cartilage (12), hard palatal mucosa (13), allogeneic dura mater (14) and tarsal plate (8), among others. Repair of palpebral
conjunctival defects can be performed by sliding conjunctival
fornices or autologous lip mucosae (5).
The tarsus is an integral component of eyelid
tissue. Internal and external canthal ligaments and tarsi play
vital roles in maintaining the appearance and stability of eyelids.
Therefore, repairing the posterior laminar palpebral conjunctiva
during the repair of eyelid defects warrants further
investigation.
Either composite transplants of the nasal septal
chondromucosa or ear cartilage tissue can easily lead to many
sequelae, for instance, stiff eyelids, poor activity and thickening
eyelids, among others. Thus, our surgery was limited to repairing
only the lower eyelid defect. Most significantly with regard to the
transplant of nasal mucosa, inappropriate use of feather and
chamfer edges can cause necrosis of the nasal mucosa
postoperatively. Drawing materials from the costicartilage is more
difficult. Moreover, its curve and thickness differ from those of
the tarsus, which is why such materials have to be further
processed. The postoperative appearance associated with these
materials is not good, and palpebral conjunctivae require
transplanting. Although the thickness and curve of ear cartilage
tissue are close to those of the tarsus, palpebral conjunctivae
also require transplantation. However, inappropriate performance of
the surgery may lead to auricle atrophy and distortion. Tarsal
defects can also be repaired via transplantation of hard palatal
mucosa, which works better; however, this method requires another
wound.
Some advanced methods using the Mustarde technique
(to repair the upper eyelid using the lower eyelid full-thickness
rotation flap), Cutler-Beard technique (to repair the lower eyelid
using the lower eyelid full-thickness sliding flap) and Hughes
technique (for upper tarsus palpebral conjunctival sliding instead
of the inner part of the lower eyelid defect), among others, may be
considered. These technologies are made from the best materials,
but their use is largely restricted and can damage healthy eyelids,
rendering them unideal.
Autogenic materials outside the eyelid are not only
mainly composed of collagenous fibers but also approximate the
eyelid’s structure, making them convenient for transplants. In
fact, they are not as hard as tarsi. Owing to lack of sustenance,
they would not reach satisfactory stability with the repair of
lower eyelid defects. Furthermore, due to absorption in various
degrees after the transplantation of allogeneic sclera, palpebral
conjunctivae would require transplanting.
Tarsal plate and palpebral conjunctival
transplantation is widely reported in the literature. We have
repaired palpebral conjunctival defects by mostly performing
conjunctival metastasis repair. For the repair of larger tarsal
defects, we combined allogeneic tarsal plate and palpebral
conjunctival transplantation, instead of using sliding autologous
conjunctival fornix or autologous lip mucosa transplantation, and
obtained nearly optimal outcomes. Only one case failed in our
observation. The eyes of all the other patients were cured or
improved. With a smooth palpebral conjunctival surface,
symblepharon and tarsal collapse did not occur after the
reconstruction of the eyelid, thereby reducing damage to the
autologous conjunctival fornix. As to the repair of tarsal defects,
the combined allogeneic tarsal plate and palpebral conjunctival
transplantation could take the place of tarsal plate
transplantation, sliding autologous conjunctival fornix
transplantation and autologous lip mucosa transplantation.
The advantages of a combined tarsal plate and
palpebral conjunctival transplantation procedure are as follows: i)
the tarsal plate is similar to the autogenous tarsus in terms of
thickness, curve and toughness, and the eyelid margin closely
approximates with the physiological state. This procedure allows
the right size of tarsus to be used, even the full tarsus,
according to the exact site of the eyelid defect. It yields fairly
satisfactory results; ii) it makes it easy to fix the levator
palpebrae superioris and avoids ptosis of the eyelid and limited
movement; iii) it is associated with lower immunogenicity; iv)
under this procedure, tarsal plates and palpebral conjunctivae may
also avoid stimulation on cartilage that lacks a smooth surface,
thereby reducing the risk of corneal injury; v) it allows for
convenient selection of materials with simple solution and
preserving conditions (2).
The advantages of tarsal plate and palpebral
conjunctival transplantation in practical applications are as
follows: i) this procedure does not require complex decoration on
the tarsal plate as the combined effect of the method is extremely
effective. On account of the toughness of the tarsus and its
flexible shape, the risk for postoperative deformation is lower
under this procedure; ii) this procedure could avoid or reduce
trauma in other parts of the body.
In the animal experiments, all tarsal plate and
palpebral conjunctival transplants were preserved under three
conditions. During the first week, the three groups responded
differently to the transplantation with inflammatory infiltration,
necrosis or gland necrosis. Within the first month, inflammatory
infiltration had disappeared. Collagen fiber and connective tissue
hyperplasias then appeared. In Group 3, the alcohol-preserved
group, which was affected by physical and chemical factors,
proteins of allo-tissue structure became inactive and some surface
antigenic determinants even disappeared. However, some determinants
of antigen molecules emerged. Denatured protein represented
decreasing antigenicity, but new antigenic specificity followed.
Therefore, in Group 3, the substitutions of allo-tarsus had no
activity but exhibited antigenicity (11). The regular dry agent glycerine was
inactive during the preservation of tarsal plates and palpebral
conjunctivae. In fact, glycerine served as an antiseptic
preservative (15).
Compared with glycerine and alcohol preservation,
liquid nitrogen cryopreservation, in which cell energy metabolism
is completely inhibited, is a novel method. Cells subjected to
cryopreservation become so dormant that they are preserved for a
long time. After thawing under proper conditions, the cells are
restored to metabolize, exerting their biological functions. Thus,
this type of preservation is active (4). Furthermore, much research has shown
that cryopreservation reduces the immunogenicity of tissue to a
certain degree (16). In the
present study, milder inflammatory infiltration and less necrosis
were observed in Group 1, the cryopreserved group, in the early
stage compared with Groups 2 and 3, but a number of gland cells did
not survive. A milder case of connective hyperplasia was also
observed in Group 1 during the late period.
Apoptosis after organ transplantation is correlated
with a series of processes, such as graft preservation, ischemia,
reperfusion injury, immunological rejection reaction and
immunological tolerance (6,17–20).
Apoptosis and necrosis are the characteristic morphological changes
in allograft rejection. The levels of apoptosis are positively
correlated with the degree of rejection (21). By contrast, apoptosis eliminates
the cellular immunity of the host so as to induce immune tolerance
(22). Studies have confirmed that
cytotoxic T lymphocyte (CTL)-mediated cytotoxicity is the major
cause of immunological rejection. CTL-mediated apoptosis primarily
results from the Fas/FasL system, whereas the killing effects of
CTLs on target cells are exerted by apoptotic pathways. The
coexistence of apoptosis and necrosis in target cells is
attributable to CTLs (3). These
data suggest that CTLs, which are involved in immunological
rejection, lead to transplant injury.
In the present study, all specimens from the three
groups exhibited apoptotic cells under postoperative TEM and SEM
observations. However, the number of apoptotic cells in Group 1 was
lower compared with the numbers in Groups 2 and 3 within the same
period. These results showed that cryopreservation has significant
advantages over the traditional glycerine and alcohol preservation
methods in terms of preservation and postoperative rejection.
Cryopreservation was performed only in an eye bank, making it
extremely safe and convenient to obtain, preserve and assign tarsal
plates and palpebral conjunctivae, thus providing us with broader
space for our basic work.
Alloantigens, which were detected in the tarsal
plates and palpebral conjunctivae, may have caused the
postoperative rejection documented in this study. To repair
conjunctival defects, we mainly performed conjunctival metastasis
repair. If the defect was larger, we performed transplantation of
human autologous mucosa. An allogeneic tarsus is the same as a
broad tissue receptor. Its fewer blood vessels and inactivity
characterize it as a weak antigen.
Antibodies in the tissue could be tolerated
postoperatively and do not cause evident immune responses, which
makes healing satisfactory (23,24).
However, we repaired tarsal defects of experimental animals in this
group by combined allo-tarsal palpebral conjunctival
transplantation. We did not find any animal model of graft failure
caused only by local and general rejection. Allogeneic tissue
grafts inevitably cause immune responses, but research has shown
that slight immune responses should be beneficial for the healing
of implants and organisms (25).
Stimulation of implants, lymphocytes and eosinophils trigger
numerous reactions, including the formation of granulation tissue
and mother cells of fibroblasts. As a result, local nutrition is
strengthened and metabolism is improved. In the process of
transplantation, antibodies of body fluid also develop. Antibodies
are capable of facilitating transplant damage; however, they are
also able to protect transplants against cell responses (i.e.,
immunoenhancement). Non-cytotoxic antibodies may contribute to the
survival of transplants. After having been transplanted into a
receptor for a long time, a graft gradually adapts to the receptor.
When allo-transplant immunogenicity decreases, on the contrary, the
receptor would gradually increase its immunological tolerance and
no longer exhibit an immune response to the transplant.
It is in this light that we did not observe any
failure of transplantation caused by rejection among the rabbits
after tarsal plate and palpebral conjunctival transplantation in
the present study. Neovascularization increasingly developed into
the tarsal plate and palpebral conjunctival margin after
transplantation according to our postoperative clinical
observations. Within the first 2 months postsurgery, amyotrophy of
parts of the tarsal tissue appeared and the tissue became thinner.
However, the figure of the eyelid had already been reconstructed at
that time, and it had no effect on the outcomes of surgery. This
study has demonstrated that liquid nitrogen-cryopreserved tarsal
plate and palpebral conjunctival transplantation is an ideal method
for repairing tarsal defects. As this procedure remains largely
unexplored, the pathological changes and immune responses
post-transplantation warrant further investigation.
Acknowledgements
This study was supported by Nanjing
Medical Development Projects of Science and Technology (No:
YKK0241).
References
|
1.
|
Wang SZ, Xu ZL and Geng Y: Clinical
observation of eyelid reconstrucyion with a preserved foreign
tarsus. Zhonghua Zhong Liu Fang Zhi Za Zhi. 9:628–629. 2002.(In
Chinese).
|
|
2.
|
Shi JT, An YZ and Min Y: A clinical
observation of preserved donor tarsal plate transplantation for
repair of tarsus defect. Zhonghua Yan Ke Za Zhi. 37:203–206.
2001.(In Chinese).
|
|
3.
|
Li YL, Jiang QX, Liu GS and Zhao JM:
Tarsal plate transplantation for repair of tarsus defect. Yan Ke
Xin Jin Zhan. 21:44–45. 2001.(In Chinese).
|
|
4.
|
Xu J, Chen JQ and Zheng JL: Study on fluid
nitrogen cryopreserved structure of limbus tissue and proliferative
activity. Ophthal Res. 21:225–228. 2003.(In Chinese).
|
|
5.
|
Min Y, You DB and Zhao SY: Outer canthal
extension of Z-shape flap for repair of eyelid defect. Ophthalmol.
15:215–217. 2006.(In Chinese).
|
|
6.
|
Zhao TL, Cheng XD and Li GZ: Repair of
eyelid full defect with composite flap pedicled with arterial arch
of palpebral margin. Zhonghua Yi Xue Mei Rong Za Zhi. 9:72–74.
2003.(In Chinese).
|
|
7.
|
O’Donnell BA and Candemir OO: Maximal
eyelid donor skin harvesting in eyelid repair after tumor excision.
Ophthal Plas Reconstr Surg. 18:436–40. 2002.PubMed/NCBI
|
|
8.
|
Gou W and Wu CY: Free tarsal conjunctival
flap repair of lower eyelid defect in 8 cases. People’s Military
Surgeon. 45:6052002.(In Chinese).
|
|
9.
|
Song YP, Zhang ZD and Lin XS: Sliding
tarsal conjunctival flap to reconstruction of lower eyelid.
Zhonghua Yan Wai Shang Zhi Ye Yan Bing Za Zhi. 6242005.(In
Chinese).
|
|
10.
|
Zhao ZM, Li SZ, Yang MY, et al: The nasal
septal chondromucosal island flap to repair of palpebral
conjunctiva and tarsus defect. Zhonghua Zheng Xing Wai Ke Za Zhi.
17:69–71. 2001.(In Chinese).
|
|
11.
|
Min Y and Li FM: Clinical and experimental
study on allogenic scleral transplantation for repair of tarsus
defect. Zhonghua Yan Ke Za Zhi. 26:3461990.(In Chinese).
|
|
12.
|
Xu J: Targus cartilage transplantation for
the treatment of eyelid defect. Yan Ke Xin Jin Zhan. 25:5072005.(In
Chinese).
|
|
13.
|
Patel MP, Shapiro MD and Spinelli HM:
Combined hard palate spacer graft, midface suspension, and lateral
canthoplasty for lower eyelid retraction: a tripartite approach.
Plast Reconstr Surg. 115:2105–2117. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Yang B and Chen JQ: Preliminary report on
repair of eyelid defects with self-made dry and frozen dura mater.
Shi Yong Yan Ke Za Zhi. 45–53. 1994.(In Chinese).
|
|
15.
|
Hu QY and Qiu XZ: Progress. in
preservation of cornea. Int J Ophthalmol. 3:65–67. 2003.(In
Chinese).
|
|
16.
|
Bradley MB and Cairo MS: Cord blood
immunology and stem cell transplantation. Hum Immunol. 66:431–446.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Ketheesan N, Whiteman C, Malczewski AB,
Hirst RG and La Brooy JT: Effect of cryopreservation on the
immunogenicity of umbilical cord blood cells. Transfus Apher Sci.
30:47–54. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Zavazava N and Kabelitz D: Alloreactivity
and apoptosis in graft rejection and transplantation tolerance. J
Leukoc Biol. 68:167–174. 2000.PubMed/NCBI
|
|
19.
|
Borderie VM, Scheer S, Bourcier T, Touzeau
O and Laroche L: Tissue crossmatch before corneal transplantation.
Br J Ophthalmol. 88:84–87. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Busch A, Marasco WA, Doebis C, Volk HD and
Seifert M: MHC class I manipulation on cell surfaces by gene
transfer of anti-MHC class I intrabodies - a tool for decreased
immunogenicity of allogeneic tissue and cell transplants. Method.
34:240–249. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Fayyazi A, Schlemminger R, Gieseler R,
Peters JH and Radzun HJ: Apoptosis in the small intestinal
allograft of the rat. Transplantation. 63:947–951. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Thomson AW and Lu L: Are dendritic cells
the key to liver transplant tolerance? Immunol Today. 20:27–32.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
He QZ, Wu HS and Cao XT: Cellular and
molecular immunology, Shanghai. Shanghai Science and Technology
Document Press; pp. 2241997, (In Chinese).
|
|
24.
|
Meng XY and Zou LH: Tarsal eyelid tumor
resection and allograft transplantation. J Practical Ophthalmol.
1:305–306. 1994.(In Chinese).
|
|
25.
|
Ruan YB and Wu ZB: Immunopathology. Wuhan:
Hubei Science and Technology Press; pp. 133–135. 1998, (In
Chinese).
|