Introduction
Bullous keratopathy (BK) develops as a result of
corneal endothelial cell damage or a reduction in the number of
corneal endothelial cells due to lesions. Corneal endothelial cell
dysfunction is often a late clinical development. Intraocular lens
implantation has become popular and widespread due to the
application of laser technology and the increased incidence of BK,
which cannot be cured using drugs or conservative treatments
(1–5). For a number of years, penetrating
keratoplasty (PK) has been the standard treatment for BK (6). Although the success rate of this
procedure is as high as 90%, the post-operative complications are a
serious clinical problem. During the 20th century, high precision
surgical instruments and microscopes, medicines, anti-immune
rejection medicines and improvements in corneal surgery, as well as
corneal topography techniques, have been developed. Archila et
al(7) attempted the first
clinical implementation of a corneal flap following lamellar
keratoplasty. Since the publication of the study, optical corneal
transplantation has been widely performed in China (8). Clinicians have made advancements in
elements of the cornea transplant procedure and corneal
transplantation has become accurate to the corneal cell level
(9). Deep lamellar endothelial
keratoplasty (DLEK) is the type of lamellar keratoplasty (10,11)
that is most often used for the treatment of corneal endothelial
cell lesions. Lamellar corneal transplantation is often used to
specifically address these lesions.
In the current study, a 1-mm syringe needle was
inserted into the anterior chamber of the cornea and the Descemet’s
membrane (DM film) and endothelial cells were removed to create the
rabbit BK model. The results of PK and DLEK surgeries were assessed
after 1, 2 and 3 months of recovery by observing the corneal
topography and morphology and the presence or absence of an immune
rejection and corneal astigmatism. The aim of the present study was
to identify a safe and effective surgical treatment for BK and to
support the validity of this approach using experimental findings
(12–14).
Materials and methods
Experimental animals
A total of 36 healthy and eye disease-free New
Zealand white rabbits (male and female; Inner Mongolia Medical
College Hospital Animal Experimental Center, Hohhot, China),
weighing 1.5–2.0 kg, were maintained at room temperature.
The 36 rabbits were randomly divided into the DLEK,
PK and experimental control group. Each group had 12 rabbits. The
DLEK and PK groups were further divided into groups that were
observed 1, 2 or 3 months subsequent to the surgery. The right eye
of each rabbit was used as the experimental eye (for surgery). The
corneal donor group (12 rabbits, 24 eyes) provided corneas to the
DLEK and PK graft groups. This study was carried out in strict
accordance with the recommendations in the Guide for the Care and
Use of Laboratory Animals of the National Institutes of Health. The
animal use protocol has been reviewed and approved by the
Institutional Animal Care and Use Committee (IACUC) of the
Affiliated Hospital of Inner Mongolia Medical College.
Experimental models
SU-MIAN-XIN (0.2 ml/kg), made by the Veterinary
Research Institute of the Military Medical Science Academy of the
PLA in Beijing, China, was administered to the rabbits and then the
animals were anesthetized with 0.5% tetracaine. Each rabbit was
covered with a surgical drape and its eyelid was opened. Using a
microscope for visualization, a surgical knife was used to puncture
the cornea 1 mm from the limbus. The wound was closed and a 1-mm
syringe needle was inserted into the anterior chamber of the cornea
and bent at an obtuse angle. The DM film was curetted and the
anterior chamber was washed with BSS to establish the
characteristics of BK. Each animal was observed using a slit lamp
following the surgery and examined every 4 weeks to test for
corneal opacity, corneal edema, thickening of the cornea that
exceeded >3 times its normal thickness, hyphema, ocular
infections, glaucoma or other complications. The rabbit models of
BK were confirmed to be steadied prior to determining that the
animals were ready for surgical treatment.
Pre-operative preparation
Prior to surgery, the conjunctival sac of the right
eye was treated with 1% pilocarpine eye drops (to induce miosis)
and a gentamicin and saline flush. Each animal was placed in the
left lateral position while under general anesthesia. Next, the
animal’s eyelid was disinfected, the area was draped and a speculum
was used to open the eyelid.
DLEK group
Twelve eyes were obtained from the corneal donor
group to construct a donor cornea transplant sample of DLEK. The
corneal epithelium was removed by swabbing it with a sponge while
the animal’s cornea was visualized using a microscope. A micro
knife was used to make a corneal lamellar incision that was 120–160
μm thick, with a diameter of 9.0–9.5 mm and a pedicle flap.
The flap was removed; following the lamellar corneal cutting
procedure, a ring with a diameter of 7.25 mm was used to confirm
the complete separation of the grafts and the removal of the
original tissue.
The right eyes from the DLEK group were used to
construct a receptor cornea transplant bed of DLEK. After using a
swab to wipe the corneal epithelium that was visualized using a
microscope, a corneal lamellar knife was used to make an incision
120–160 μm thick with a diameter of 9.0–9.5 mm. The corneal
flap was removed. Using a 7.0-mm trephine drill to remove part of
the corneal tissue for receptor cornea planting tablet of DLEK. The
corneal tissue was removed which in addition to the recipient
rabbit’s corneal stroma, which included the nether lamellar layer,
Descemet’s layer and the corneal cortex. An incision was made in
the cornea to a depth of two-thirds of its thickness. The structure
was completely separated from the planting bed using a drill
subsequent to the clamp clip being removed.
The corneal transplant process of DLEK was conducted
as follows: The donor corneal endothelium was placed face down on
the corneal bed and the graft was fixed using interrupted sutures.
The pedicle flap of the corneal tissue was reset and closed using
combined, interrupted sutures.
PK group
Twelve eyes from corneal donor groups were used to
create transplant samples of PK. Using a microscope to visualize
the cornea, a cut was made using a drill with a diameter of 7.25
mm. Drilling was stopped when a loss of resistance was felt by the
operator. The complete separation of the grafts was then confirmed.
Care was taken to ensure that the grafts did not move and were kept
in reserve.
The right eyes of the PK group were used to create
receptor cornea transplant bed and corneal transplant process of
PK. The corneal beds were prepared for the transplant using the
same method of graft production that was described earlier. A hole
7.0 mm in diameter was drilled in the cornea and the corneal pieces
were removed to complete the preparation of the bed.
The donor corneal endothelium was placed face down
on the graft and then attached to the DM using interrupted
sutures.
Post-operative treatment
The rabbits were handled using routine
post-operative care. The sutures were removed as required after 1
month of recovery. The rabbits’ eyes were examined after 3 months
of recovery. The animals were euthanized and the eyes were removed.
For each eye, the cornea was separated from the limbus and stored
in fixative as preparation for hematoxylin and eosin (H&E)
staining.
Post-operative observation
The eyes were observed once a week using slit lamp
microscopy and assessed for the presence of new blood vessels in
the corneal bed, graft edema, opacity, melting, slab gap,
epithelial growth on the transplant, the presence of immune
rejection and any morphological changes of the cornea. The
topography was examined after 1, 2 and 3 months of recovery and any
changes in the astigmatism (Astig) values were recorded. Subsequent
to 3 months of recovery, two rabbits from the PK group and two
rabbits from the DLEK group were chosen for corneal H&E
staining to observe the cornea-receptor interface.
Statistical analysis
The data were analyzed using SPSS 13.0 software for
comparison of the groups and are expressed as mean ± standard
deviation (SD). A t-test was used for a single factor analysis to
compare the experimental and control groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
DLEK group
At day 1 post-surgery, 10 of the rabbits’ eyes
exhibited severe eyelid spasms, moderate conjunctival hyperemia,
corneal opacity (grade I) and moderate edema. After 2 weeks, the
conjunctival congestion was alleviated and the cornea became clear
in the center, although the periphery remained pale and opaque. The
corneal graft remained in place and the graft edema persisted
(grade I). The sutures were intermittently removed after 1 month
and the blepharospasms were significantly reduced at this time. The
cornea gradually became transparent, although the periphery
remained white and opaque. The graft edema disappeared and a small
amount of corneal neovascularization was observed. After 3 months
of recovery, it was possible to flip the eyelid and the rabbits
remained emotionally stable. The central section of the transparent
cornea became clear, although the peripheral cornea remained
off-white. No eye infections or other complications were observed.
In the DLEK group, 2 cases of perforated corneal flaps occurred
which caused the surgery to be terminated.
PK group
In the PK group, 9 of the 12 rabbit eyes exhibited
severe eyelid spasms at day 1 post-surgery. The rabbits were
confused and moderate conjunctival hyperemia, corneal edema (grade
II) and turbidity (grade II) were observed. After day 7, the
corneal edema persisted, the periphery was a hazy gray, the corneal
sutures remained in place and numerous new blood vessels (with a
length of 1–2 mm) were observed between the corneal limbus and
cornea. At ∼2 weeks post-surgery, the conjunctival hyperemia was
reduced and the central section of the cornea became transparent.
The area surrounding the grafts was a hazy gray and the corneal
limbal neovascularization grew to between 2 and 3 mm. The corneal
sutures were removed after 1 month of recovery. The corneal
neovascularization subsided and no endophthalmitis was observed. At
∼3 months post-surgery, the rabbits remained emotionally stable
when the eyelid was flipped and the cornea was nearly transparent.
At this time, 3 eyes exhibited severe corneal opacity and numerous
new blood vessels covered the entire graft. Corneal melting (grade
I) was observed and an immune graft rejection occurred.
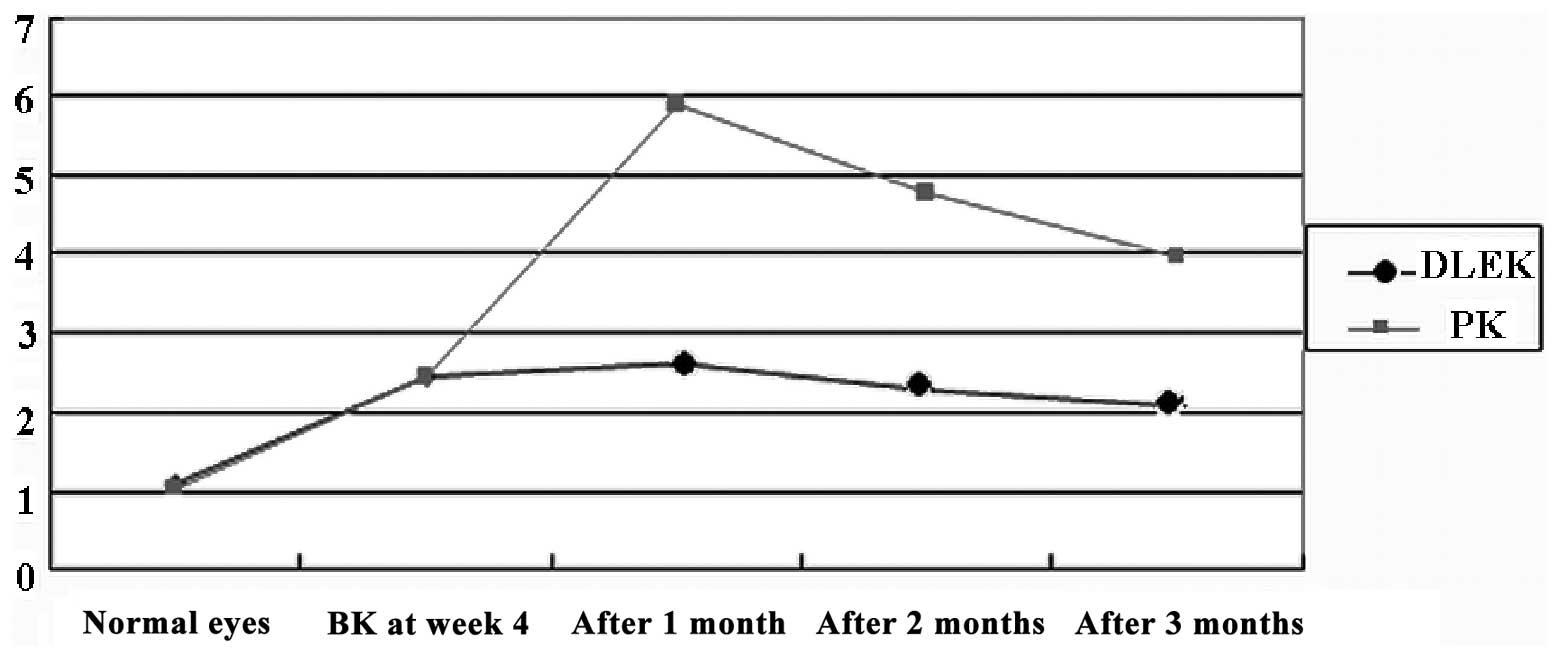
Corneal Astig value
In the DLEK group, the post-operative corneal Astig
value was significantly different from that of the normal rabbit
eyes (P<0.05) at several time points. Compared to the BK 4 week,
the mean corneal Astig value was signifcantly decreased at 3 months
after surgery (t=10.251, P<0.05, n=10). Compared to the observed
normal rabbit eyes at several time points, the mean corneal Astig
value was signifcantly increased in the PK group (P<0.05). At 3
months subsequent to the corneal surgery, the Astig value was
significantly different from the BK 4 week value (t= −78.184,
P<0.05, n=10). Compared to the PK group at several time points
after surgery, the corneal Astig value was also signifcantly
decreased in the DLEK group (F=3833.614, P<0.05; Table I and Fig. 1).
 | Table IComparison of corneal Astig values (D)
at various time points following surgery (mean ± SD). |
Table I
Comparison of corneal Astig values (D)
at various time points following surgery (mean ± SD).
| Control group | BK 4 weeks | 1 month after
surgery | 2 months after
surgery | 3 months after
surgery | F | P-value |
|---|
| DLEK | 1.08±0.25 | 2.43±0.24 | 2.62±0.21 | 2.29±0.34 | 2.07±0.19 | 827.84 | 0.00 |
| PK | 1.03±0.28 | 2.43±0.32 | 5.92±0.65 | 4.78±0.61 | 3.99±0.36 | 6071.82 | 0.00 |
| t | - | - | 110.68 | 82.242 | 43.666 | - | - |
| P-value | - | - | 0.00 | 0.00 | 0.00 | - | - |
Histological changes
Normal corneal tissue has 4 layers; the epithelium,
stroma, DM and the endothelial cell layer membrane (Fig. 2A). In 2 rabbits, the DM appeared as
a layer of endothelial cells attached to the membrane at 4 week
post-surgery (Fig. 2B). The
continuity of the collagen fiber interface was established in the
DLEK group subsequent to 3 months of recovery (Fig. 2C), and the corneas in the PK group
were essentially normal at this time (Fig. 2D).
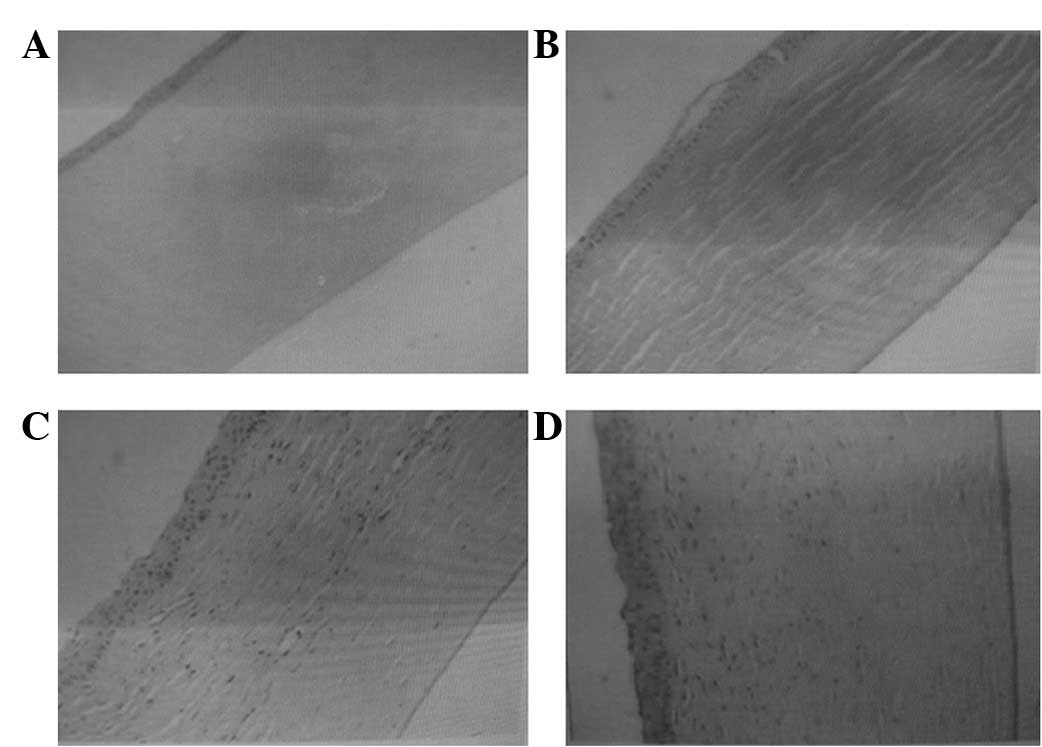 | Figure 2(A) A normal cornea (H&E,
magnification, ×200); (B) a normal corneal endothelium attached to
the DM membrane of endothelial cells following simple curettage at
4 weeks (H&E, magnification, ×100); (C) a cornea 3 months after
DLEK surgery (H&E, magnification, ×100); (D) a cornea 3 months
after PK surgery (H&E, magnification ×100). H&E,
hematoxylin and eosin; DM, Descemet’s membrane; DLEK, deep lamellar
endothelial keratoplasty; PK, penetrating keratoplasty. |
Discussion
Corneas have been used for tissue and organ
transplants for 100 years and are the most commonly transplanted
organ. Many patients with corneal blindness are able to sense light
following this type of transplant. The aim of a corneal transplant
is to replace an opaque cornea with healthy corneal tissue and to
restore a patient’s sight. Each year, 3,000 corneal transplants are
conducted in China, 40,000 in the United States and 2,000 in Japan
(15).
Future experimental studies using DLEK for the
treatment of BK require the establishment of a reliable animal
model. Rabbits are the most commonly used animal model in
ophthalmology studies (17–19),
however, the rabbit and human corneal endothelia differ in their
biological characteristics, mainly in terms of endothelial
regeneration (16–18). The establishment of a stable and
lasting corneal endothelial injury model is worthwhile. The results
of the present study suggest that scraping the endothelium and the
basement membrane of corneal endothelial adhesions (the DM film)
may significantly delay the regeneration of rabbit corneal
endothelial cells and prolong the effects of endothelial cell
injury. These effects are in agreement with the clinical
pathological characteristics of BK and support the use of this
animal model for evaluating the effects of an experimental DLEK
surgery accurately and objectively.
Langerhans cells within the corneal epithelium
express HLA antigens, which give these cells the ability to
recognize non-self antigens. Compared with PK, in theory, DLEK is
able to decrease the risk of antigenic material production as the
graft does not contain epithelial cells. As the donor epithelial
tissue has been separated from the patient’s own corneal stroma,
the Langerhans cells are not in direct contact with donor antigens.
Consequently, the incidence of graft rejection is reduced following
this technique compared with that in the matrix model. However,
immune-mediated corneal graft rejection is mainly caused by an
endothelial rejection, as the endothelial cells may express
allogeneic antigens which may cause an endothelial rejection. In
the present study, during observations of the two groups, several
rabbits from each group developed an immune rejection. In the DLEK
group, a small amount of corneal neovascularization appeared after
1 month and had increased by 3 months post-surgery. When the
corneal sutures were removed, the corneal neovascularization
gradually subsided and the central part of the cornea became
transparent. The majority of the corneal neovascularization grew
∼1–2 mm into the limbus area in the PK group and by the second week
following surgery, the corneal limbal neovascularization had
increased to 2–3 mm into the limbus area. At ∼3 months after this
increase, three corneas were still cloudy with numerous new blood
vessels and they appeared to be undergoing a severe immune graft
rejection. In the present study, the immune rejections were more
severe and occurred more frequently in the PK group than in the
DLEK group. Corneal allograft rejection is a trigger mechanism that
may be a complex process affected by numerous factors. The
following factors were identified using a multi-factor analysis of
this process: i) conducting thick graft surgery (which results in
excessive antigen release from the allogeneic tissue); ii)
stimulation by the corneal sutures causes neovascularization, which
may lead to an immune graft rejection (a large diameter graft is
more susceptible to corneal neovascularization than smaller
grafts); iii) a larger number of Langerhans cells, which are
distributed in the peripheral cornea, possess antigen-presenting
functions and are stimulated by various physical and chemical
factors. These factors may all increase the number of Langerhans
cells, which gradually migrate to the central corneal graft
position or deviate from the central cornea to the periphery. All
of these factors may lead to rejection.
PK completely replaces the diseased cornea with
allogeneic tissue. In an ocular surface reconstruction, the corneal
thickness and curvature of the recipient’s original cornea are not
identical to those of the donor cornea. In addition, suturing in
several directions and with several suture tensions may distort the
corneal surface, leading to a highly irregular post-operative
astigmatism. DLEK retains the anterior corneal surface, the cortex,
the first elastic layer and a portion of the matrix layer of the
recipient, therefore, the anterior corneal surface remains smooth
and the astigmatic curvature of the cornea that is caused by
inconsistencies and sutures is reduced. Consequently, DLEK causes
less astigmatism than PK. An analysis of the causes of astigmatism
may prevent the formation of highly irregular astigmatism. In the
present study, the corneal topography astigmatism values in the
pre-operative PK group, the BK group at 4 weeks and at several
post-operative time points were significantly different when
compared with those of the DLEK group (P<0.05). The following
possible explanations for this difference were identified: i) the
original donor may have exhibited corneal astigmatism, if the
conditions mapping the pre-operative donor cornea lens have been
selected; ii) a donor cornea may exhibit diseases that lead to
corneal curvature and abnormal thickness (if the original cornea
exhibited keratoconus angiogenesis, the rate of wound healing would
be inconsistent); iii) inconsistencies may be present in the cut of
the cornea, which may affect the the pre-operative preparations of
the recipient, lead to a low intraocular pressure and result in the
requirement of a speculum to open a sagging eye (leading to the
cornea receptors being drilled into conical tissue); iv) the ring
may deviate from the optical axis center (donor and/or recipient);
v) the donor cornea may not match the recipient’s in shape and
size; vi) the impact of the suture (the positioning of the sutures
in the 4th, 2nd and 1st pin must be a straight line) or twisted
sutures may result in needle spacing that is not uniform between
the sutures; and vii) the surgeon may not have used the surgical
keratometer to adjust the tightness of the sutures.
In addition to these points which aid in the
prevention and treatment of astigmatism following surgery, the
cornea and corneal lens measurements may be corrected using
selective suture removal in the early post-operative period.
Several authors have argued that wounds should be sutured with
interrupted 12 needles and 10-0 nylon and that 11-0 nylon
continuous sutures should be used with a 12-pin (19). Subsequent to recovery for 1 month,
surgeons should remove 1 or 2 sutures every two weeks when the
astigmatism is large, which will cause a more compact topography.
Further study is required to determine an effective method to treat
extreme astigmatism following suturing.
In summary, the results of the present study
indicated that corneal graft rejections occur less often and are
less severe following DLEK than following PK. In addition, the
results indicate that post-operative corneal astigmatism following
DLEK or PK differed in several ways. However, as visualized using
H&E staining, the corneal shape appeared to be normal 3 months
subsequent to the DLEK and PK surgeries. It is proposed that the
topography of the cornea does not guarantee an improvement in
visual acuity and stability. The present study did not examine the
process of wound healing following DLEK surgery. The effect of the
corneal topography on long-term vision should be studied further.
If the patient maintains a relatively stable long-term corneal
topography following surgery, the corneal interface in the
recipient maintains optical transparency. If standardized surgical
procedures, a proficiency in the surgical techniques and
appropriate surgical instruments are available, it is feasible that
DLEK may be an effective method to replace PK as a standard corneal
transplantation method for treating BK.
Acknowledgements
This study was supported by the
Ministry of Health of Inner Mongolia autonomous region (No.
2006061) and the Ministry of Education of Inner Mongolia autonomous
region.
References
|
1.
|
Wu ZQ, Yang YN and Xing YQ: Current
progress on etiology and clinical treatment bullous keratopathy.
Recent Advances In Ophthalmology. 27:625–629. 2007.
|
|
2.
|
Mamalis N, Anderson CW, Kreisler KR, et
al: Changing trends in the indications for penetrating
keratoplasty. Arch Ophthalmol. 110:1409–1411. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Lindquist TD, McGlothan JS, Rotkis WM and
Chandler JW: Indications for penetrating keratoplasty: 1980–1988.
Cornea. 10:210–216. 1991.
|
|
4.
|
Beekhuis WH: Current clinician’s opinions
on risk factors in corneal grafting. Results of a survey among
surgeons in the eurotransplant area. Cornea. 14:39–42. 1995.
|
|
5.
|
Li L and He SK: Lamellar keratoplasty.
Ophthalmology research. 20:370–372. 2002.
|
|
6.
|
Maeno A, Naor J, Lee HM, Hunter WS and
Rootman DS: Three decades of corneal transplantation: indications
and patient characteristics. Cornea. 19:7–11. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Archila EA: Deep lamellar keratoplasty
dissection of host tissue with intrastromol air injection. Cornea.
3:217–218. 1985.PubMed/NCBI
|
|
8.
|
Chen JQ: Of corneal review and outlook.
Chinese Journal of Ophthalmology. 3:182–186. 2005.
|
|
9.
|
Terry MA: A new approach for endothelial
transplantation: deep lamellar endothelial keratoplasty. Int
Ophthalmol Clin. 43:183–193. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Terry MA: Endothelial replacement: the
limbal pocket approach. Ophthalmol Clin North Am. 16:103–112. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Xie LX: Chapter 17 section 1. Corneal
Transplantation. People’s Health Press; Beijing: pp. 2412000
|
|
12.
|
Melles GR, Lander F, van Dooren BT, Pels E
and Beekhuis WH: Preliminary clinical results of posterior lamellar
keratoplasty through a sclerocorneal pocket incision.
Ophthalmology. 107:1850–1857. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Busin M, Arffa RC and Sebastiani A:
Endokeratoplasty as an alternative to penetrating keratoplasty for
the surgical treatment of diseased endothelium: initial results.
Ophthalmology. 107:2077–2082. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Culbertson WW: Endothelial replacement:
flap approach. Ophthalmol Clin North Am. 16:113–118. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Yang CZ and Liu L: Chapter 1. Modern
Corneal Transplantation. People’s Military Medical Press; Beijing:
pp. 81998
|
|
16.
|
Chung JH and Fagerholm P: Endothelial
healing in rabbit corneal alkali wounds. Acta Ophthalmol (Copenh).
65:648–656. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Minkowski JS, Bartels SP, Delori FC, et
al: Corneal endothelial function and structure following
cryo-injury in the rabbit. Invest Ophthalmol Vis Sci. 25:1416–1425.
1984.PubMed/NCBI
|
|
18.
|
Hirsch M, Renard G, Faure JP and Pouliquen
Y: Formation of intercellular spaces and junctions in regenerating
rabbit corneal endothelium. Exp Eye Res. 23:385–397. 1976.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Li N and Zou L: Selective removal of
interrupted sutures for the controls of post keratoplasty
astigmatism. Chinese Journal of Practical Ophthalmology.
20:532–533. 2002.(In Chinese).
|