Introduction
Acute pancreatitis is a condition where inflammation
occurs suddenly in the pancreas. The pancreas, located behind the
stomach in the upper abdomen, produces digestive enzymes and the
sugar-processing hormones, insulin and glucagon. Although the exact
etiology of acute pancreatitis remains controversial (1), gallstones and heavy alcohol
consumption are the two most common causes (2). With symptoms including a sudden onset
of dull and steady pain in the upper abdomen, acute pancreatitis
occurs at an incidence rate of 2.9 per 10,000 persons and affects
382,014 (0.029%) individuals annually in China (3).
Acute pancreatitis is mild in 80% of cases and
severe in the remaining 20% of cases (2). Mild acute pancreatitis, also called
edematous or interstitial pancreatitis, is defined as pancreatic
inflammation and edema associated with minimal organ dysfunction,
whereas severe acute pancreatitis is defined as pancreatic necrosis
associated with secondary injury to extrapancreatic organs leading
to multiple organ dysfunction syndrome (MODS) and/or local
complications (4).
Mild acute pancreatitis usually resolves within a
few days with conservative management. However, severe acute
pancreatitis may be life-threatening and requires management in an
intensive care unit. Although extensive research and clinical
efforts have been made in the management of acute pancreatitis
during the past few decades (5),
to date no effective cure is available (6) and the mortality from severe acute
pancreatitis remains high (7).
Therefore novel therapeutic strategies are required to improve the
outcomes of patients with severe pancreatitis.
Given that MODS is the primary cause of morbidity
and mortality associated with severe acute pancreatitis, novel
therapeutic approaches aiming to prevent injury of the vital organs
have become a subject of intensive investigation. In a previous
study, we assessed the potential of sivelestat, a competitive
inhibitor of human neutrophil elastase (NE) (8), in the protection against acute
pancreatitis-associated lung injury in a rat model (9). As an extension of the analyses in our
previous study, the present study aimed to evaluate the ability of
sivelestat to protect against renal injury in acute pancreatitis in
rats.
Materials and methods
Animals, experimental design and specimen
collection
Since this study was an extension of a previous
study from our group, the animals and their allocation, as well as
the methods of pancreatitis induction and sivelestat treatment,
were the same as described in our previous study (9). In summary, adult male Sprague-Dawley
rats were randomized into the following groups: i) the experimental
acute pancreatitis (EAP) group in which rats were induced to
develop acute pancreatitis by the administration of sodium
taurocholate through laparotomy under anesthesia; ii) the EAP plus
sivelestat treatment group in which rats were injected with 2 mg/kg
sivelestat through the superficial dorsal vein of the penis
immediately following EAP induction; and iii) the control group in
which a sham laparotomy was performed. At 6, 12 and 24 h after
sivelestat or vehicle treatment, six rats from each group were
sacrificed by depletion. Immediately before and after animal
sacrifice, blood samples were collected through cardiac puncture
and serum was prepared. Tissue blocks of the kidneys and pancreas
were excised and fixed in 10% formalin or snap-frozen in liquid
nitrogen The study protocol was approved by the Institutional
Review Board of Zhejiang University (Hangzhou, China).
Histological examination
Formalin-fixed kidney tissue blocks were dehydrated,
embedded in paraffin, sectioned at 5 μm and stained with
hematoxylin and eosin (H&E). Abnormalities, including
vacuolization of the tubular epithelial lining in the subcapsular
region, patchy areas of hemorrhage in the interstitium and necrosis
in the epithelial lining of the tubules towards the medullary
region were assessed by two independent pathologists who were blind
to the study design and the specimen identities.
Renal function test
Serum levels of blood urea nitrogen (BUN) and
creatinine (CR) were determined using standard laboratory
methods.
Measurement of serum tumor necrosis
factor-α (TNF-α)
Serum levels of TNF-α were determined using an
immunoassay kit (Biosource, Grand Island, NY, USA) following the
manufacturer’s instructions.
Measurement of NE activity and
cytokine-induced neutrophil chemoattractant-1 (CINC-1) level in
renal tissue
Tissue homogenate was prepared from frozen renal
specimens using the method described for the homogenate preparation
of lung tissue in our previous study (9). NE activity was determined
spectrophotometrically using a chromogenic substrate. Levels of
CINC-1 were measured with a sandwich enzyme-linked immunosorbent
assay (ELISA) kit (R&D Systems, Minneapolis, MN, USA).
Statistical analysis
Data are expressed as arithmetic means ± standard
deviation (SD) and analyzed with one-way analysis of variance
(ANOVA) and Bonferroni test. SPSS software was used for statistical
analyses (SPSS Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Renal histopathology
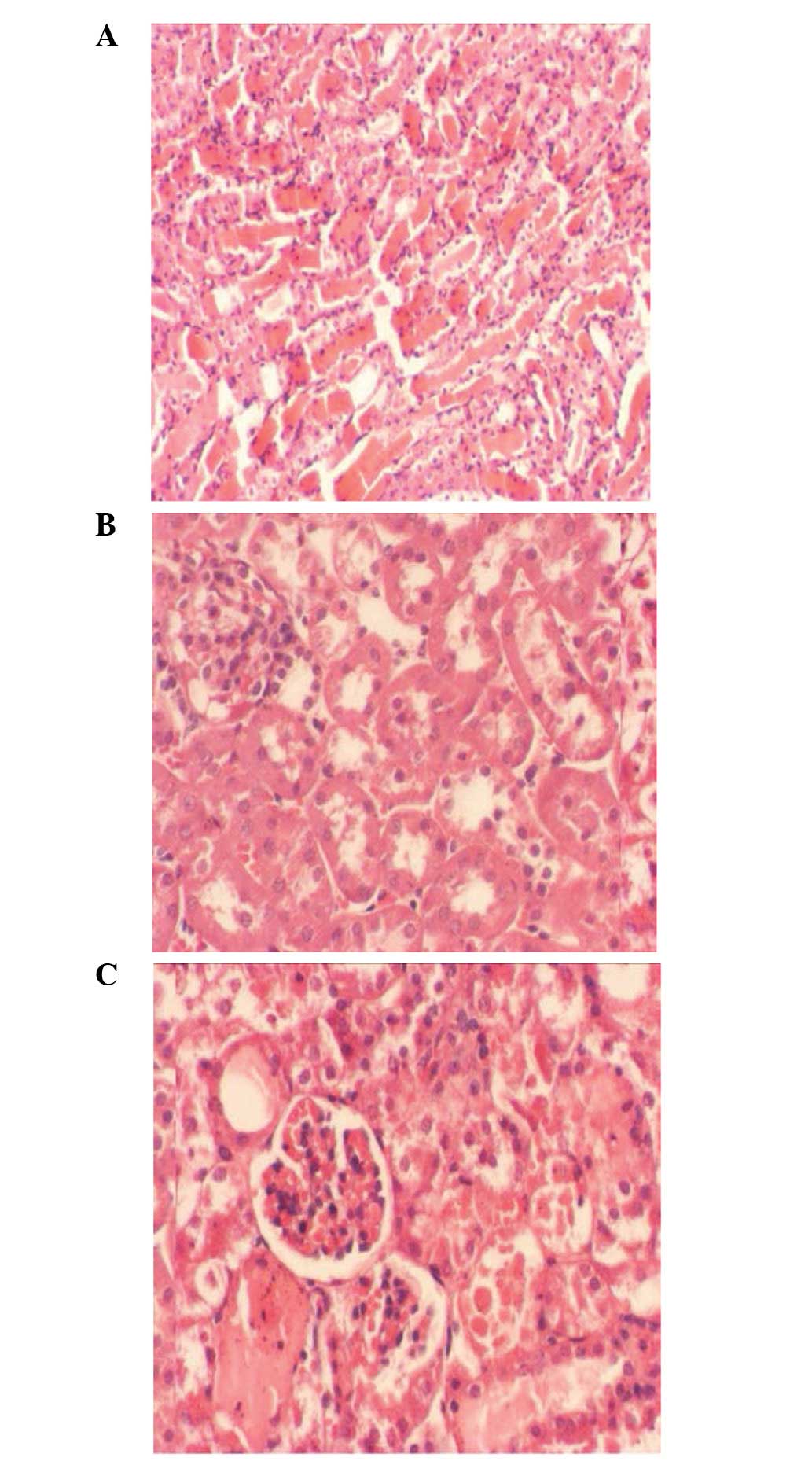
Representative images of H&E-stained renal
tissue sections 24 h after acute pancreatitis induction and
sivelestat treatment are presented in Fig. 1. Structural anomalies were
significant in the kidneys of rats treated with sodium taurocholate
as compared with the control rats. Manifestations of these
anomalies included apparent vacuolization of the tubular epithelial
lining in the subcapsular region, patchy areas of hemorrhage in the
interstitium and necrosis in the epithelial lining of the tubules
towards the medullary region. Sivelestat treatment effectively
ameliorated the sodium taurocholate-induced renal structure
anomalies.
Renal functions
As presented in Table
I, the serum levels of BUN were consistent over time in the
control rats; however, they were significantly increased at all
three time-points in the rats with sodium taurocholate-induced
acute pancreatitis (P<0.05). Sivelestat treatment effectively
attenuated the taurocholate-induced increase in the serum levels of
BUN. Similarly, serum levels of CR were significantly higher in the
rats treated with sodium taurocholate than in the control rats at
all three time-points (P<0.05) and sivelestat treatment returned
the serum level of CR to the normal value observed in the control
rats (Table II).
 | Table I.Serum levels of BUN (mmol/l) in the
different groups at various time-points. |
Table I.
Serum levels of BUN (mmol/l) in the
different groups at various time-points.
| Group | 6 h | 12 h | 24 h |
|---|
| Control | 5.06±0.75 | 5.13±1.05 | 5.22±0.78 |
| AP | 16.82±1.51a | 17.30±1.98a | 19.62±2.04a |
| AP+S | 11.57±1.92b | 11.94±2.06b | 12.43±2.15b |
 | Table II.Serum levels of creatinine
(μmol/l) in the different groups at various time-points. |
Table II.
Serum levels of creatinine
(μmol/l) in the different groups at various time-points.
| Group | 6 h | 12 h | 24 h |
|---|
| Control | 21.06±1.75 | 21.73±1.51 | 22.03±1.69 |
| AP | 35.42±1.90a | 36.81±1.84a | 38.42±2.06a |
| AP+S | 23.79±1.92b | 24.54±1.07b | 27.23±1.85b |
Serum levels of TNF-α
The results of TNF-α measurement are summarized in
Table III. The baseline level of
TNF-α in the serum of control rats was between 4.17±1.04 and
5.73±0.81 pg/ml, with little difference between the three
time-points (P<0.05). Sodium taurocholate induced a robust
increase (P<0.001) in the serum level of TNF-α; however, this
increase decreased in magnitude with time (22.8-fold at 6 h,
11.6-fold at 12 h and and 7.1-fold at 24 h). Sivelestat treatment
significantly attenuated the taurocholate-induced increase in the
serum level of TNF-α at all three time-points (P<0.01); however,
it failed to return the level to normal.
 | Table III.Serum levels of TNF-α (pg/ml) in the
different groups at various time-points. |
Table III.
Serum levels of TNF-α (pg/ml) in the
different groups at various time-points.
| Group | 6 h | 12 h | 24 h |
|---|
| Control | 4.17±1.04 | 5.73±0.81 | 5.34±1.20 |
| AP | 95.12±21.42a | 66.48±27.94a | 38.12±22.17a |
| AP+S | 63.77±25.92b | 44.54±23.07b | 26.23±19.85b |
NE activity and CINC-1 concentration in
renal tissue
As shown in Table
IV, the NE activity in the renal tissue homogenate was
consistent in control rats over time (P>0.05); however, it
significantly (P<0.01) increased in animals with
taurocholate-induced acute pancreatitis in a time-dependent manner
(5.3-fold at 6 h, 8.2-fold at 12 h and 11.4-fold at 24 h). Although
sivelestat treatment was not able to restore the normal baseline
level of renal NE activity, it significantly attenuated the
taurocholate-induced increase in NE activity at all time-points
(P<0.01). Similar patterns of change were observed for the renal
level of CINC-1 in the three groups; however, the magnitude of
change induced by taurocholate was significantly larger
(>100-fold) than that for the change in NE activity (Table V).
 | Table IV.Neutrophil elastase activity (pg/ml)
in the renal tissue in the different groups at various
time-points. |
Table IV.
Neutrophil elastase activity (pg/ml)
in the renal tissue in the different groups at various
time-points.
| Group | 6 h | 12 h | 24 h |
|---|
| Control | 1.35±0.37 | 1.42±0.28 | 1.34±0.25 |
| AP | 7.14±1.35a | 11.65±1.98a | 15.37±2.14a |
| AP+S | 4.36±1.92b | 6.89±2.07b | 9.23±1.85b |
 | Table V.CINC-1 concentration (pg/g) in renal
tissue in the different groups at various time-points. |
Table V.
CINC-1 concentration (pg/g) in renal
tissue in the different groups at various time-points.
| Group | 6 h | 12 h | 24 h |
|---|
| Control | 52.23±3.77 | 57.42±5.34 | 61.34±7.85 |
| AP |
4500.14±538.30a |
5374.65±577.48a |
6208.37±534.23a |
| AP+S |
3409.71±421.92b |
4518.89±378.16b |
5400.32±456.80b |
Discussion
Acute pancreatitis may affect organs near to and
distant from the pancreas, including the lungs, kidneys, liver and
the cardiovascular and central nervous systems. According to the
revised Atlanta classification of acute pancreatitis (10), organ failure is one of the major
determinants of the severity of acute pancreatitis. While no organ
failure is present in mild acute pancreatitis (the most common form
of the disease) and organ failure is only transient in moderate
acute pancreatitis, persistent (>48 h) and multiple organ
failure or dysfunction (MODS) commonly occurs in severe acute
pancreatitis (10). Moreover,
previous clinical data has demonstrated that the number of organs
experiencing function failure is positively associated with the
mortality rate in severe acute pancreatitis (11). Therefore, protection against severe
acute pancreatitis-associated organ failure improves the survival
rate of patients with severe acute pancreatitis.
In a previous study (9), with the aim of identifying novel
organ-protective agents, we evaluated the effects of the NE
inhibitor, sivelestat, on lung dysfunction in a rat model of
experimental acute pancreatitis. We observed the development of
histopathological and biochemical abnormalities in the circulation,
lungs and pancreas characteristic of acute pancreatitis in rats
following the surgical administration of sodium taurocholate, as
well as the effective attenuation of the taurocholate-induced
abnormalities by sivelestat, suggesting a potential role for
sivelestat in the protection against acute pancreatitis-associated
pulmonary injury. Utilizing saved serum samples and renal tissue
specimens from that study, in the present study we assessed the
renoprotective activity of sivelestat.
We first assessed changes in the histology of
kidneys from rats at 6, 12 and 24 h after taurocholate induction in
the presence or absence of sivelestat treatment in a parallel
comparison with sham-operated control animals. In agreement with
results from a previous study (12), we observed histological anomalies
in the renal tubules following taurocholate administration,
confirming renal injury in rats with experimental acute
pancreatitis. We also observed a significant amelioration in the
taurocholate-induced renal histological changes in rats following
sivelestat treatment, indicating that sivelestat has a beneficial
effect on renal histology.
Kidney function tests are common laboratory tests
used to evaluate how well the kidneys are working. To assess
changes in renal function in the different groups, levels of BUN
and CR were measured in the saved aliquots of serum samples
collected in our previous study. Significant elevations were
detected for BUN and CR in rats following surgery, compared with
the corresponding baseline level in control animals. Sivelestat
treatment significantly improved these renal function parameters.
In the literature, to the best of our knowledge, there are no
reports concerning the beneficial effects of sivelestat on BUN and
CR, the major parameters of renal function. Kumasaka et al
observed a beneficial effect of sivelestat on proteinuria in
nephritis rats (13). Kumasaka’s
observations and our own suggest a beneficial effect for sivelestat
on renal function.
We also assessed changes in other renal function
variables, including serum levels of TNF-α, NE activity and CINC-1
concentration in renal tissue. For the first time, we observed that
sivelestat is able to significantly improve these variables.
Acknowledgements
The authors would like to thank Dr
Ziming Yu for constructive and thoughtful input to the
manuscript.
References
|
1.
|
Khan AS and Latif SU: Controversies in the
etiologies of acute pancreatitis. JOP. 11:545–552. 2010.PubMed/NCBI
|
|
2.
|
Harper SJ and Cheslyn-Curtis S: Acute
pancreatitis. Ann Clin Biochem. 48:23–37. 2011. View Article : Google Scholar
|
|
3.
|
http://www.rightdiagnosis.com/a/acute_pancreatitis/stats-country.htmuri.
|
|
4.
|
Bradley EL: A clinically based
classification system for acute pancreatitis. Summary of the
International Symposium on Acute Pancreatitis, Atlanta, Ga,
September 11 through 13, 1992. Arch Surg. 128:586–590. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Talukdar R and Vege SS: Recent
developments in acute pancreatitis. Clin Gastroenterol Hepatol.
7:S3–S9. 2009. View Article : Google Scholar
|
|
6.
|
Waldthaler A and Schütte K: Causes and
mechanisms in acute pancreatitis. Dig Dis. 28:364–372. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Greer SE and Burchard MW: Acute
pancreatitis and critical illness: a pancreatic tale of
hypoperfusion and inflammation. Chest. 136:1413–1419. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Kawabata K and Suzuki M: ONO-5046, a novel
inhibitor of human neutrophil elastase. Biochem Biophys Res Commun.
177:814–820. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Wang HH, Tang AM, Chen L and Zhou MT:
Potential of sivelestat in protection against severe acute
pancreatitis-associated lung injury in rats. Exp Lung Res.
38:445–452. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Banks PA and Vege SS: Classification of
acute pancreatitis - 2012: revision of the Atlanta classification
and definitions by international consensus. Gut. 62:102–111. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Vege SS, Gardner TB, Chari ST, Munukuti P,
Pearson RK, Clain JE, Petersen BT, Baron TH, Farnell MB and Sarr
MG: Low mortality and high morbidity in severe acute pancreatitis
without organ failure: a case for revising the Atlanta
classification to include “moderately severe acute pancreatitis”.
Am J Gastroenterol. 104:710–715. 2009.PubMed/NCBI
|
|
12.
|
Kudari A, Wig JD, Kim V, Kochhar R,
Majumdar S, Gupta R, Yadav DT and Doley RP: Histopathological
sequential changes in sodium taurocholate-induced acute
pancreatitis. J Pancreas. 8:564–572. 2007.PubMed/NCBI
|
|
13.
|
Kumasaka R, Nakamura N, Fujita T, Murakami
R, Shimada M, Osawa H, Yamabe H and Okumura K: Beneficial effect of
neutrophil elastase inhibitor on anti-Thy1.1 nephritis in rats.
Nephrology. 13:27–32. 2008.PubMed/NCBI
|