Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune
disease characterized by swelling joints, synovitis and
degeneration (1). Rat adjuvant
arthritis (AA), an experimental model, resembles RA in histological
pathology. The progression of synovitis in AA and RA is
characterized by pronounced tumor-like expansion of the synovium.
Consequently, neovascularization may play a pivotal role during
disease progression (2). The
similarities in joint pathology between AA and RA may be employed
for the screening of new drugs to treat RA (3). Non-steroidal anti-inflammatory drugs
(NSAIDs), steroidal agents and immunosuppressants are usually used
for the treatment of RA, but the side-effects indicate a
requirement for new and more effective natural drugs. Resveratrol
(3,5,4-trihydroxystilbene; Res) is a naturally occurring polyphenol
present in >70 species of plants. It has been demonstrated that
Res possesses anti-inflammatory, analgesic and immune-regulatory
actions, and has marked preventive and therapeutic effects on AA
rats (4,5). In the current study, immunology was
used to observe the effect of Res on the synoviocytes of AA rats,
the secretion of inflammatory cytokines and the possible
mechanism(s) of action of synoviocytes.
Materials and methods
Animals
A total of 60 male Sprague-Dawley (SD) rats, 220–250
g, were provided by the laboratory animal center of Anhui Medical
University (Hefei, China; Certificate No. 01). The rats were
maintained in a colony room at an ambient temperature of 25±1°C.
The lighting duration in the colony room was 12 h (from 7:00 a.m.
to 19:00 p.m.). Food and water were provided ad libitum. The
rats were acclimatized under standard laboratory conditions for 1
week prior to experimentation. The experiments were approved by the
Committee of Laboratory Animals of Anhui Medical University (Hefei,
China).
Drugs and reagents
Approximately 99% pure Res was obtained from
Sigma-Aldrich (St. Louis, MO, USA). A stock solution was prepared
in dimethyl sulfoxide (DMSO) and further diluted in a RPMI-1640 to
achieve the desired final concentration. The concentration of DMSO
was 0.05% (v/v). Bacillus Calmette-Guérin (BCG) and Tripterygium
wilfordii polyglucoside tablet (TPT; positive control compound)
were purchased from Shanghai Biochemical Institute (Shanghai,
China), and suspended in 0.5% sodium carboxymethylcellulose
(CMC-Na) to produce the necessary concentration for the experiment.
The RPMI-1640 medium was purchased from Gibco (Carlsbad, CA, USA).
125I-IL-1β and 125I-TNF-α radioimmunoassay
(RIA) kits were purchased from Beijing Northern Biomedicine Company
(Beijing, China), goat anti-rabbit monoclonal antibody to
phosphorylated ERK1/2 (p-ERK1/2) was purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA), and protein kinase C (PKC)
suppressor chelerythrine from Promega Corporation (Madison, WI,
USA). All other reagents were of analytical purity and were from
commercial sources.
Animal groupings and drug treatment
The SD rats were divided randomly into six groups
(n=10 each), and the drug treatment groups were treated with Res
intragastrically (10, 50 or 100 mg/kg/day) or the reference drug
TPT (100 mg/kg/day). AA rats were created as previously described
(6,7). Briefly, rats received immunization
(day 0) with a single intradermal injection of 0.1 ml Freund’s
complete adjuvant (FCA). FCA was prepared by mixing 10 mg
heat-inactived (80°C, 1 h) BCG with 1 ml sterile paraffin oil which
was then injected into the right hind foot pads of the rats. The
rats in the drug treatment groups were given a continuous
intragastric gavage (i.g. 10 ml/kg/day) between days 12 and 24
after immunization. For the normal and AA model rats, an equal
amount of 0.5% CMC-Na solution was administered.
AA evaluation
AA rats were evaluated as previously described
(8). Briefly, the left hind paw
(non-injection) volume was determined using a YLS-TA volume meter
(Shandong Medicine Academy, Jinan, China) prior to immunization
(basic value, day 0) and at 4-day intervals following
administration of the drugs. The paw swelling (Δ ml) was defined as
the increase in paw volume evaluated following inflammation.
Synoviocyte culture
AA rats were sacrificed on the 28th day via
subaxillary exsanguination under intraperitoneal anesthesia with 1%
0.2 ml sodium pentobarbital following immunization. The joint
tissues were prepared by first removing the skin and separating the
limb below the ankle joint. Synovium from the knee joints of the
rats was excised under sterile conditions and digested with a
sequential incubation of 0.2% (w/v) collagenase type II and 0.25%
(w/v) trypsin. Fibroblast-like cells were isolated from affected
joints by collagenase digestion, as described previously (9). Synoviocytes were resuspended with
RPMI-1640 medium at a concentration of 1×109 cells/l;
the synoviocyte suspension (500 μl) and LPS (500 μl;
10 mg/l) were added to six-well plates. Following incubation at
37°C in a 5% CO2 atmosphere for 48 h, the supernatant
containing IL-1β and TNF-α was collected and stored at −80°C.
Immunohistochemistry
Vascular endothelial growth factor (VEGF) was
visualized immunohistochemically as previously described (10). The joint specimens were initially
decalcified for 2 weeks in EDTA-containing buffer and embedded in
10% formalin solution (v/v). Prior to staining, 5-μm frozen
sections were fixed for 30 min in ice-cold acetone. Endogenous
peroxidase activity was quenched by incubating the slides for an
additional 30 min in methanol (absolute) and 3% hydrogen peroxide.
The slides were then incubated with polyclonal goat anti-mouse VEGF
antibodies (1:500 dilution). Biotinylated rabbit anti-goat IgG
(Beijing Zhongshan Golden Bridge Biotechnology Co., Beijing, China)
and peroxidase-conjugated streptavidin were used as second and
third reagents, respectively. The slides were incubated in the dark
for 10 min at room temperature with a solution of the chromogen
3,3′-diaminobenzidine tetrahydrochloride (DAB reaction kit; Beijing
Zhongshan Golden Bridge Biotechnology Co.). After rinsing with
distilled water, the slides were counter-stained with Mayer’s
hematoxylin.
RIA for IL-1β and TNF-α
IL-1β and TNF-α levels in the cultured synoviocyte
supernatant were assayed according to the manufacturer’s
instructions (11). Briefly, a
commercially available RIA kit was used. IL-1β and TNF-α were
derived from the cultured supernatant. Test samples and standards
of IL-1β and TNF-α were assayed in duplicate in polystyrene tubes,
which were incubated at room temperature for 24 h with 100
μl anti-IL-1β and anti-TNF-α antibodies. The supernatants
were decanted and pellets were counted using a γ counter.
Simultaneously, in certain assay samples, phosphate-buffered saline
was added instead of an antibody to measure the nonspecific binding
of the labeled IL-1β and TNF-α. The radioactivity of the pellets
was then measured and a linear transformation curve was constructed
for the calculations.
Measurement of mRNA expression of IL-1β
and TNF-α in synoviocytes
The total RNA of IL-1β and TNF-α in the synoviocytes
were extracted by reverse transcription (RT)-PCR assay (12). Total RNA from synoviocytes was
extracted using TRIzol, according to the manufacturer’s
instructions (Invitrogen, Carlsbad, CA, USA). For each reaction, 1
μg total RNA served as a template. For amplification, the
size of the GAPDH, IL-1β and TNF-α products were 462, 377 and 249
bp after 30 amplification cycles, respectively. The specific primer
pairs for GAPDH, IL-1β and TNF-α were GAPDH, forward: 5′-CGT GGA
AGG ACT CAT GAC CA-3′ and reverse: 5′-TCC AGG GGT CTT ACT CCT
TG-3′; IL-1β, forward: 5′-TTG TGG CTG TGG AGA AGC TG-3′ and
reverse: 5′-GCC GTC TTT CAT ACA CAG GG-3′; and TNF-α, forward:
5′-CTG GGC AGC GTT TAT TCT-3′ and reverse: 5′-TTG CTT CTT CCC TGT
TCC-3′. In all experiments, the reactions were controlled by
substituting sterile nuclease-free water for the RNA template in
the reaction. The PCR reactions were separated on 2% agarose gel
containing 0.3 μg/ml ethidium bromide and were visualized
and photographed using UV transillumination. A gel electrophoresis
(Bio-Rad, Hercules, CA, USA) was used for scanning and Lab Image
3.2 gel image analysis software was utilized to determine the grey
ratio of the object band and internal control GAPDH standard band.
The experiments were repeated at least three times for each
condition.
Western blotting examination of p-ERK1/2
in Res-treated synoviocytes
Untreated AA model synoviocytes were used as the
control group. In the Res-stimulated groups, Res was added to
synoviocytes from the AA model rats at concentrations of 0.5, 5 and
50 mg/l. In addition, the model synoviocytes were pretreated with
chelerythrine for 2 h prior to treatment with 5 mg/l Res. The
synoviocytes were maintained at 37°C with 5% CO2 for 24
h and then the cells were collected. Precooled cell lysate (100
μl) was added per 1×107 cells and the solution
was mixed. The mixture was put on ice and centrifuged at 12,000 × g
for 20 min. The concentration of protein, adjusted to 3 g/l, was
detected by Bradford assay. Approximately 15 μl solution
from each sample was detected by 15% SDS-PAGE, which was blocked
with 5% skimmed milk powder following overnight incubation.
Anti-p-ERK1/2 and mouse monoclonal anti-β-actin (1:2,000 dilution;
Sigma) were then added, the sample was maintained at 37°C for 1 h
and then developed with enhanced chemiluminescence (ECL) (13). The experiments were repeated at
least three times for each condition.
Statistical analysis
The data are expressed as mean ± SD. The analysis of
variance (ANOVA) was used to determine significant differences
between groups. P<0.05 was considered to indicate a
statistically significant result.
Results
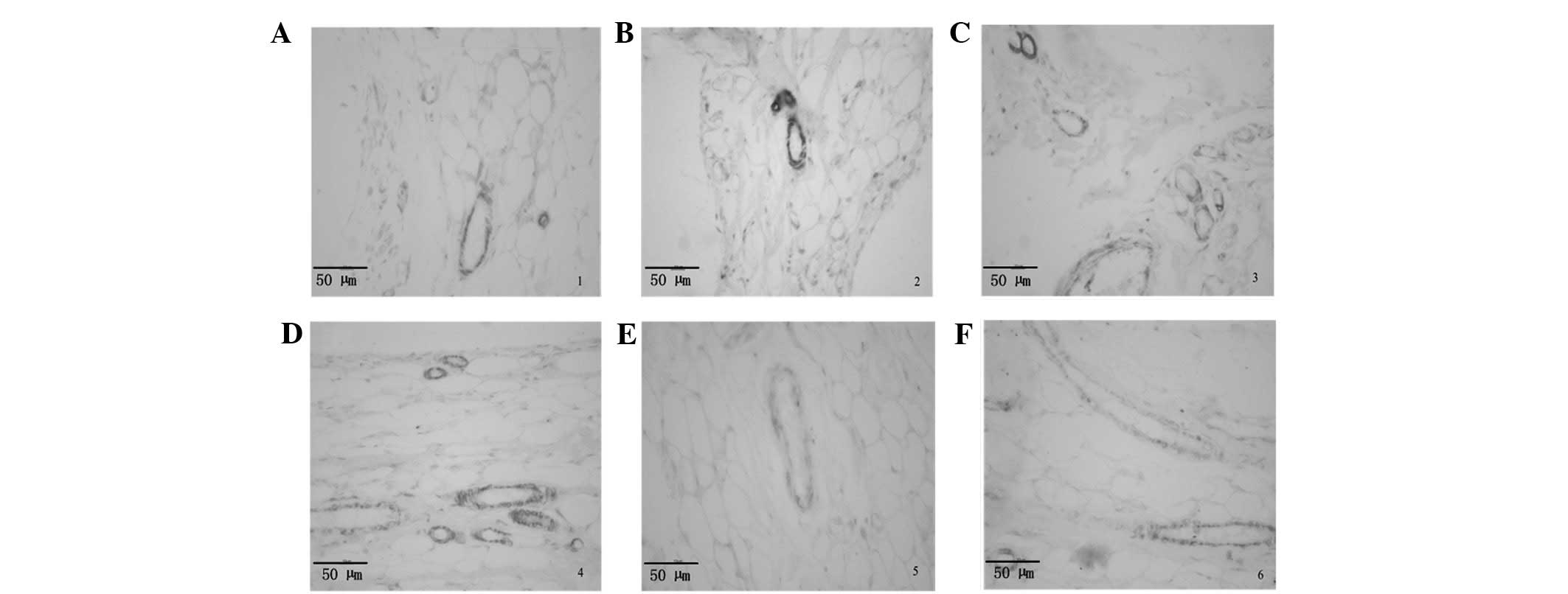
Effects of Res on the histopathology of
AA rat hind paws and immunolocalization of VEGF
Compared with normal rats, AA rats showed
hyperplastic synovium, inflammatory cell infiltration and pannus
formation. These symptoms were significantly alleviated in AA rats
following the administration of Res. Immunolocalization indicated
that VEGF was expressed by vascular endothelial cells, but only to
a small degree in normal rats (Fig.
1A). Notably, VEGF expression was markedly higher in the
vascular endothelial cells of the AA model rats than in those of
the normal control group, and the chondrocytes in chronically
inflamed joint tissue sections showed little nonspecific staining
(Fig. 1B). Compared with the AA
model rats, the level of VEGF expression in the blood vessel walls
of the synovium of the Res-treated and TPT-treated groups was
significantly lower (Fig.
1C-F).
Effects of Res on secondary
inflammation
Inflammatory polyarthritis was induced in all
immunized rats. The peak incidence occurred on the 20th day after
immunization. Treatment with Res and TPT diminished the left hind
paw swelling and polyarthritic symptoms on the 16th day after
immunization. The suppressive effect of Res 100 mg/kg was the most
marked. Res 100 mg/kg was as effective as TPT on the 24th day
(Table I).
 | Table I.Effect of Res on secondary
inflammatory reaction in AA rats (mean ± SD, n=10). |
Table I.
Effect of Res on secondary
inflammatory reaction in AA rats (mean ± SD, n=10).
| Group | Dose (mg/kg) | Hind paw swelling
(ml)
|
|---|
| Day 12 | Day 16 | Day 20 | Day 24 | Day 28 |
|---|
| Normal | - | 0.12±0.07 | 0.13±0.05 | 0.14±0.06 | 0.17±0.08 | 0.18±0.04 |
| Model | - | 0.33±0.07 | 0.37±0.08 | 0.46±0.06 | 0.41±0.07 | 0.38±0.07 |
| Res | 10 | 0.33±0.05 | 0.36±0.09 | 0.36±0.06a | 0.33±0.08b | 0.32±0.05b |
| Res | 50 | 0.32±0.06 | 0.34±0.07 | 0.35±0.05a | 0.31±0.06b | 0.29±0.04b |
| Res | 100 | 0.31±0.08 | 0.33±0.06a | 0.29±0.06b | 0.25±0.06b | 0.23±0.05b |
| TPT | 100 | 0.28±0.04 | 0.29±0.07a | 0.28±0.08b | 0.26±0.05b | 0.22±0.04b |
Synoviocyte culture
The rats were sacrificed on the 28th day by
subaxillary exsanguination under intraperitoneal anesthesia with 1%
0.2 ml sodium pentobarbital after immunization. The synovial
tissues of the bilateral knee joints of rats were obtained under
sterile conditions and the synoviocytes were cultured as previously
described (8). The synoviocytes
were resuspended in RPMI-1640 medium at a concentration of
1×109 cells/l. After smearing the synovial membrane
fibroblasts and using Giemsa stain, 300 cells were examined under
the microscope. The cell purity was >95%.
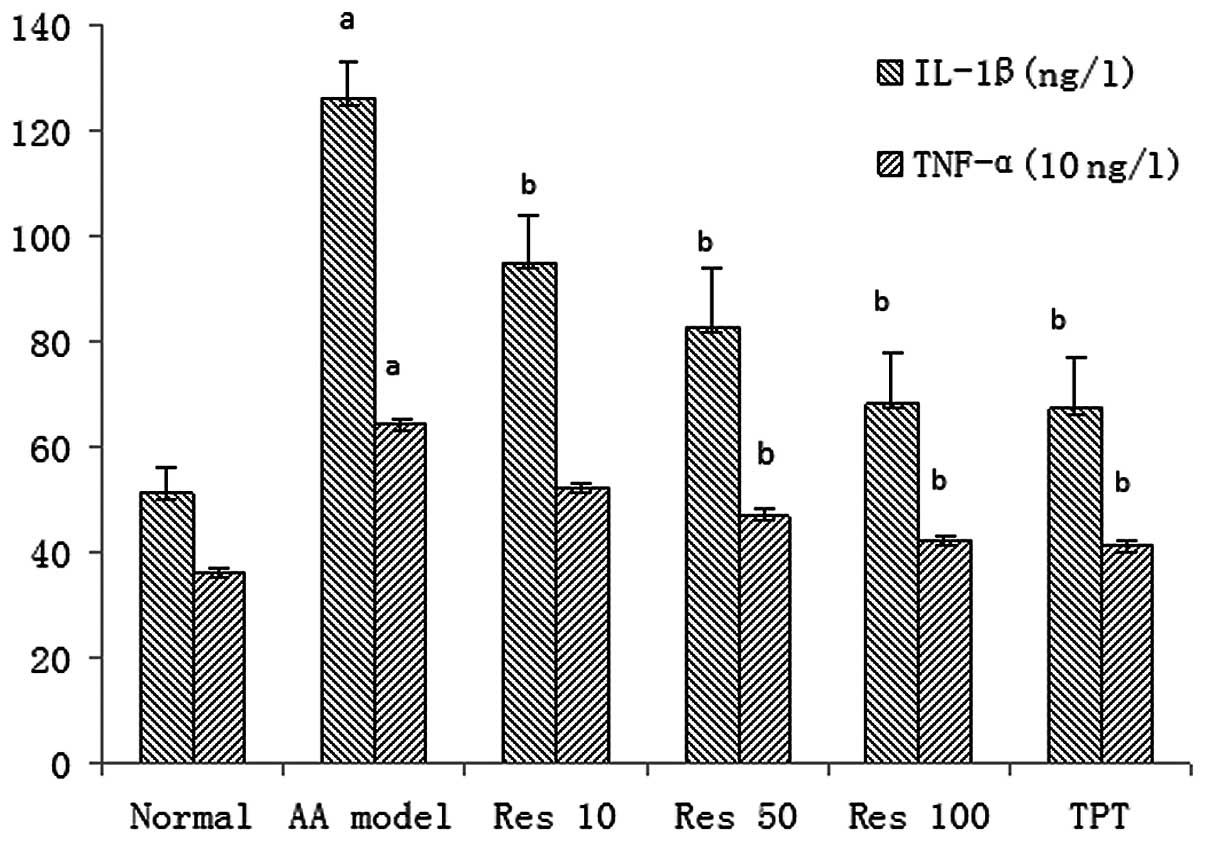
RIA assay of IL-1β and TNF-α levels in
synoviocyte suspensions
Synoviocyte suspensions from the AA model rats were
revealed by RIA to release higher levels of IL-1β and TNF-α than
those of normal rats. Res and TPT significantly inhibited the
production of IL-1β and TNF-α in the synoviocyte suspension
(Fig. 2).
Effect of different concentrations of Res
on mRNA expression of IL-1β and TNF-α in synoviocytes
In the normal group, there was weak mRNA expression
of IL-1β and TNF-α, while the expression of cytokines was
significantly increased in the AA group (P<0.05). As the dose of
Res gradually increased from 10 mg/kg to 100 mg/kg, mRNA expression
of IL-1β and TNF-α was reduced in a concentration-dependent manner.
Steady-state expression of GAPDH was used to control equal loading
of the PCR product onto gels (Fig.
3).
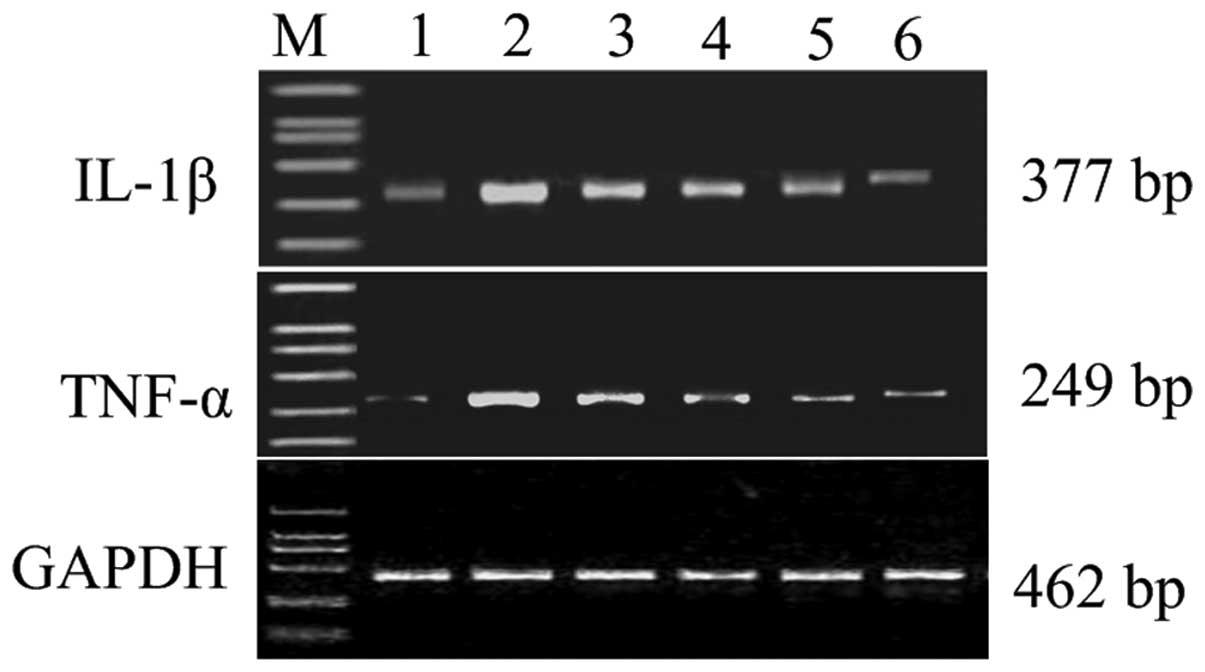 | Figure 3.Representative gels stained for RT-PCR
products of IL-1β and TNF-α mRNA expression of synoviocytes in AA
model rats treated with Res. Lane M, marker; lane 1, normal; lane
2, AA model group; lane 3, Res 10 mg/kg group; lane 4, Res 50 mg/kg
group; lane 5, Res 100 mg/kg group; lane 6, TPT 100 mg/kg group.
RT-PCR, reverse transcription-PCR; AA, adjuvant arthritis; Res,
resveratrol; TPT, Tripterygium wilfordii polyglucoside
tablet. |
Effect of Res on p-ERK1/2 in rat
synoviocytes
To explore the effect of Res (0.5, 5 and 50 mg/l) on
p-ERK in the synoviocytes of AA model rats in vitro, the
synoviocytes were stimulated with Res. In addition, the
synoviocytes were pretreated with the PKC inhibitor chelerythrine
prior to stimulation with Res. The expression levels of p-ERK1/2 in
the synoviocytes were detected by western blotting. The p-ERK1/2
activity was low in the AA model synoviocytes. Following the
stimulation of the synoviocytes with Res, the protein expression
level of p-ERK1/2 was notably higher than that of the AA model
group. Following pretreatment of the synoviocytes with
chelerythrine for 2 h prior to treatment with Res, the protein
expression level of p-ERK1/2 was markedly lower than that in the 5
mg/l Res-stimulated group (Fig.
4). Therefore, Res may activate p-ERK1/2 in synoviocytes via
the PKC pathway.
Discussion
RA is a chronic, systemic inflammatory disease
affecting ∼1% of people worldwide. RA is a disorder characterized
by persistent inflammatory synovitis, predominantly affecting the
peripheral joints. The chronic nature of this disease results in
progressive joint destruction, which leads to severe locomotive
disability and deterioration in quality of life (1). The present study examined the
therapeutic effects of Res and its mechanisms of action on AA in
rats. The administration of Res during the secondary inflammation
response (from days 12 to 24 after AA induction) markedly inhibited
the swelling of the non-immunized hind paw. Histological
examination of ankle arthritis showed that Res significantly
reduced hyperplastic synovium, inflammatory cell infiltration and
pannus formation. Due to the similarities in pathological features
between AA and RA, these results indicate that Res may be effective
in treating clinical RA (14).
Neovascularization is a complex process, involving
endothelial cell division, selective degradation of vascular
basement membranes and surrounding extracellular matrix, and
endothelial cell migration. Several polypeptide growth factors have
been identified based on their ability to stimulate the
proliferation of endothelial cells in the RA joint, which include
TNF-α, and acidic and basic fibroblast growth factors (12,13,15–17).
Another significant mediator of neovascularization is VEGF. VEGF is
an endothelial cell-specific mitogen in vitro and an
angiogenic growth factor in vivo, which is known to play an
important role in pathological conditions, including certain tumors
and RA (13,15). However, the location and time
course of VEGF expression during the development of RA remain
unclear; nor has it been determined whether VEGF is directly
involved in the induction of the synovitis observed in RA (16,17).
Our experiments showed that Res participated in VEGF expression in
the joints of AA rats and the VEGF expression levels reduced as the
Res dose was increased.
Synoviocytes are the final effector cells of RA
joint damage in the synovial membrane and articular cavity.
Analysis shows that the mitochondrion is the energy provider for
cell metabolism in synoviocytes (18–20).
Our previous results showed that the secretion and metabolism of
the synoviocytes of AA model rats became hyperfunctional at day 24
after immunization. The effects of Res on synoviocyte secretory
function may provide an explanation for its mechanism of action in
AA rats. These pharmacological effects of Res strongly suggests its
potential in the treatment of autoimmune disease, particularly in
RA (6).
Under normal circumstances, the expression and
secretion of IL-1β and TNF-α are strictly controlled by the
organism. In RA, the higher secretion of synoviocytes activated by
proinflammatory factors, including IL-1β and TNF-α, is suggested to
be a crucial process in the destruction of cartilaginous and bony
tissues in joints affected by RA (21,22).
IL-1β and TNF-α overproduction plays potential pathogenic roles in
the establishment of rheumatoid synovitis, the formation of pannus
tissue and the process of joint destruction. A study of
experimental models revealed that IL-1β and TNF-α are key cytokines
in joint swelling and cartilage destruction (23). In our study, the synoviocytes of AA
rats released higher levels of IL-1β and TNF-α than those of normal
rats. Res inhibited the production of IL-1β and TNF-α. RT-PCR also
showed that the mRNA expression levels of IL-1β and TNF-α in AA
rats were significantly increased compared with those of the normal
group, and Res downregulated the mRNA expression levels of the
inflammatory factors IL-1β and TNF-α.
The modulation of signal transduction by protein
phosphorylation and dephosphorylation is an important regulatory
mechanism. Our observations suggest that PKC-dependent p-ERK1/2
signal transduction plays an important role in AA synoviocytes, and
two PKC phosphorylation sites have been identified in the present
study (Fig. 4). The activity of
p-ERK1/2 was low in model synoviocytes. After the synoviocytes were
stimulated by Res, the protein expression levels of p-ERK1/2 were
markedly higher compared with those of the model control group.
When the synoviocytes were pretreated with chelerythrine, the
protein expression level of p-ERK1/2 was markedly lower than those
of the Res-stimulated groups.
The present study revealed the therapeutic action of
Res in AA rats was associated with its ability to balance the
secretion of cytokines by synoviocytes and modulate the abnormal
cellular immune function, and the possible mechanism may be
associated with activating p-ERK1/2 in the synoviocytes via PKC.
The pharmacological effects of Res strongly suggest its potential
therapeutic role for chronic RA.
Acknowledgements
This article is distributed under the
terms of the Natural Science Foundation of Higher Education
Institutions of Anhui Province, China (KJ2010A183), which permits
any non-commercial use.
References
|
1.
|
Trentham DE, McCune WJ, Susman P and David
JR: Autoimmunity to collagen in adjuvant arthritis of rats. J Clin
Invest. 66:1109–1117. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Marrelli A, Cipriani P, Liakouli V,
Carubbi F, Perricone C, Perricone R and Giacomelli R: Angiogenesis
in rheumatoid arthritis: a disease specific process or a common
response to chronic inflammation? Autoimmun Rev. 10:595–598. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Cimmino MA, Parodi M, Innocenti S, Succio
G, Banderali S, Silvestri E and Garlaschi G: Dynamic magnetic
resonance of the wrist in psoriatic arthritis reveals imaging
patterns similar to those of rheumatoid arthritis. Arthritis Res
Ther. 7:R725–R731. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Tang LL, Gao JS, Chen XR and Xie X:
Inhibitory effect of resveratrol on the proliferation of
synoviocytes in rheumatoid arthritis and its mechanism in vitro.
Zhong Nan Da Xue Xue Bao Yi Xue Ban. 31:528–533. 2006.(In
Chinese).
|
|
5.
|
Tian J, Gao J, Chen J, et al: Effects of
resveratrol on proliferation and apoptosis of TNF-α induced
rheumatoid arthritis fibroblast-like synoviocytes. Zhongguo Zhong
Yao Za Zhi. 35:1878–1882. 2010.(In Chinese).
|
|
6.
|
Chen XY, Li J, Cheng WM, Jiang H, Xie XF
and Hu R: Effect of total flavonoids from Chrysanthemum
indicum on ultrastructure and secretory function of
synoviocytes in adjuvant arthritis rats. Am J Chin Med. 36:695–704.
2008.
|
|
7.
|
Wei W, Chen MZ and Xu SY: Pharmacological
effects of isoxicam. Chin Pharmacol Bull. 2:29–34. 1986.(In
Chinese).
|
|
8.
|
Gu WZ and Brandwein SR: Inhibition of type
II collagen-induced arthritis in rats by triptolide. Int J
Immunopharmacol. 20:389–400. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Wang B, Chen M and Xu SY: Separation and
cultivation of synoviocytes in rats. Chin Pharmacol Bull. 10:73–74.
1994.(In Chinese).
|
|
10.
|
Yamairi F, Utsumi H, Ono Y, Komorita N,
Tanaka M and Fukunari A: Expression of vascular endothelial growth
factor (VEGF) associated with histopathological changes in rodent
models of osteoarthritis. J Toxicol Pathol. 24:137–142. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Dulos J, Kaptein A, Kavelaars A, Heijnen C
and Boots A: Tumour necrosis factor-α stimulates
dehydroepiandrosterone metabolism in human fibroblast-like
synoviocytes: a role for nuclear factor-κB and activator protein-1
in the regulation of expression of cytochrome p450 enzyme 7b.
Arthritis Res Ther. 7:R1271–R1280. 2005.
|
|
12.
|
Martin-Armas M, Simon-Santamaria J,
Pettersen I, Moens U, Smedsrød B and Sveinbjørnsson B: Toll-like
receptor 9 (TLR9) is present in murine liver sinusoidal endothelial
cells (LSECs) and mediates the effect of CpG-oligonucleotides. J
Hepatol. 44:939–946. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Hamerlik P, Lathia JD, Rasmussen R, et al:
Autocrine VEGF-VEGFR2-Neuropilin-1 signaling promotes glioma
stem-like cell viability and tumor growth. J Exp Med. 209:507–520.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Athanasou NA: Synovial macrophages. Ann
Rheum Dis. 54:392–394. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Enciso JM, Konecny CM, Karpen HE and
Hirschi KK: Endothelial cell migration during murine yolk sac
vascular remodeling occurs by means of a Rac1 and FAK activation
pathway in vivo. Dev Dyn. 239:2570–2583. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Maffei CM, Mirels LF, Sobel RA, Clemons KV
and Stevens DA: Cytokine and inducible nitric oxide synthase
expression during experimental murine cryptococcal
meningoencephalitis. Infect Immun. 72:2338–2349. 2004. View Article : Google Scholar
|
|
17.
|
Kim WU, Yoo SA, Min SY, Park SH, Koh HS,
Song SW and Cho CS: Hydroxychloroquine potentiates Fas-mediated
apoptosis of rheumatoid synoviocytes. Clin Exp Immunol.
144:503–511. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Price FM, Levick JR and Mason RM: Changes
in glycosaminoglycan concentration and synovial permeability at
raised intra-articular pressure in rabbit knees. J Physiol.
495:821–833. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Yamanishi Y, Boyle DL, Green DR, Keystone
EC, Connor A, Zollman S and Firestein GS: p53 tumor suppressor gene
mutations in fibroblast-like synoviocytes from erosion synovium and
non-erosion synovium in rheumatoid arthritis. Arthritis Res Ther.
7:R12–R18. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Dai M, Wei W, Shen YX and Zheng YQ:
Glucosides of Chaenomeles speciosa remit rat adjuvant
arthritis by inhibiting synoviocyte activities. Acta Pharmacol Sin.
24:1161–1166. 2003.
|
|
21.
|
Eskandari F, Webster JI and Sternberg EM:
Neural immune pathways and their connection to inflammatory
diseases. Arthritis Res Ther. 5:251–265. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Udagawa N, Kotake S, Kamatani N, Takahashi
N and Suda T: The molecular mechanism of osteoclastogenesis in
rheumatoid arthritis. Arthritis Res. 4:281–289. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Braun J and Sieper J: Therapy of
ankylosing spondylitis and other spondyloarthritides: established
medical treatment, anti-TNF-α therapy and other novel approaches.
Arthritis Res. 4:307–321. 2002.PubMed/NCBI
|