Introduction
The Shiitake mushroom, or Lentinus edodes (L.
edodes) has been recognized for having beneficial
bioactivities. Shiitake mushrooms are an important ingredient in
Asian foods, since they possess a desirable taste and odor.
Additionally, this fungus has an excellent nutritional value with
high levels of vitamins B and D 1). A bioactive compounds from
L. edodes, eritadenine, has been shown to exert
anti-hypercholemic effects in previous studies (2,3). In
addition to this, eritadenine may affect lipid metabolism through
the inhibition of S-adenosyl homocysteine hydrolase (SAH) (4–6).
Homocysteine is a non-protein amino acid that is
biosynthesized through the metabolism of methionine (6). Elevated levels of homocysteine have
been associated with number of diseases, including heart failure
and bone disorders. High homocysteine concentrations increase the
susceptibility to endothelial injury, which leads to inflammation
in various tissues and may result in ischemic injury and metabolic
imbalances (7–9). In humans, normal plasma levels of
total homocysteine are 5–15 mmol/l. An increase in this amino acid
to 5 μmol/l is associated with an elevated risk of coronary
heart disease by 60–80% for males and females (10). In hyperhomocysteinemic patients,
high levels of homocysteine are significantly reduced by folic acid
and vitamin B12 supplementation (11). Deficiencies in B vitamins (vitamin
B6, 9 and 12) may lead to hyperhomocysteinemia (12). In addition, folate and vitamin B12
deficiencies cause DNA damage, which may result from
hypomethylation (3,13).
DNA methylation is an important epigenetic mechanism
that selectively regulates the expression of targeted genes and is
associated with various cardiac diseases (14). Methylation at the promoter region
controls gene transcription and in turn attracts histone
deacetylases (HDACs). DNA methylation is mediated by DNA methyl
transferase (DNMT); this enzyme uses S-adenosyl methionine (SAM) as
a methyl group source. DNMTs include DNMT1, 2 and 3. Homocysteine
selectively reduces the activity of DNMT1, resulting in increased
levels of SAH in human endothelial cells (15).
The nutritional role of dietary L. edodes has
not yet been elucidated in the mouse model of hyperhomocysteinemia.
In the present study, we investigated the effect of L.
edodes in a mouse model of hyperhomocysteinemia induced by a
folate- and vitamin B12-deficient diet (DFV) during the growth
stage of the animals (between 4 and 12 weeks of age). The serum and
hepatic levels of homocysteine were measured using serum chemistry
and high performance liquid chromatography (HPLC). Expression of
DNMTs in the liver was also evaluated in order to determine the
potential anti-hyperhomocysteinemic effects of L.
edodes.
Materials and methods
Experimental animals
ICR mice (4 weeks-old) were obtained from Koatech
(Pyeongtaek, Gyeonggi, South Korea). All animals were housed in
polycarbonate cages and acclimated in an environmentally controlled
room (temperature, 23±2°C; relative humidity, 50±10%; frequent
ventilation; and a 12/12-h light-dark cycle) prior to use. The mice
(n=60) were divided into six groups (n=10 per group).
Homocysteinemia was induced in five groups by the administration of
DFV for 6 weeks.
To assess the preventative effect of L.
edodes on hyperhomocysteinemia, mice received DFV alone as a
negative control (NC), DFV + eritadenine (10 mg/kg) as a positive
control (PC) or DFV + 5% (T1; w/w), 10% (T2; w/w) or 20% (T3; w/w)
L. edodes, from 10- to 12 weeks of age. A sham group
received AIN-93M pellets. The body weights of the mice were
measured before and after the experiment. The Ethics Committee of
Chungbuk National University (Cheongju, Korea) approved all
experimental procedures.
Measurement of SAM, SAH and serum
homocysteine levels
Frozen mouse liver samples were homogenized with 0.4
M HClO4 buffer. The tissue homogenates were centrifuged
at 2,000 × g at 4°C for 20 min. Supernatants were filtered with a
0.45-μm filter. SAM and SAH concentrations were measured
with Shimadzu LC-10 HPLC apparatus (Tokyo, Japan) equipped with a
250×4.6-mm Ultrasphere 5-μm ODS Betasil analytical column
(Thermo Hypersil-Keystone, Runcorn, UK) according to a previously
described protocol (16). Blood
was collected from each mouse, transferred to serum separator tubes
and centrifuged at 400 × g for 20 min. The supernatant was
transferred into 1.5 ml tubes and analysis of serum homocysteine
levels was performed using a chemiluminescent immunoassay method
with an ADVIA Centaur Assay system (Siemens Medical Solution
Diagnostics, Dublin, Ireland) according to the manufacturer’s
instructions.
RNA extraction and quantitative PCR
Total RNA was extracted from mouse liver using
TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA)
according to the manufacturer’s instructions. The RNA concentration
was determined using an Epoch micro-plate spectrophotometer (BioTek
Instruments, Inc., Winooski, VT, USA) at an absorbance of 260 nm
and RNA quality was evaluated by electrophoresis in 1% agarose
gels. Total RNA (1 mg) was reverse transcribed into first-strand
complementary DNA (cDNA) using Moloney murine leukemia virus
reverse transcriptase (Invitrogen Life Technologies) and a random
primer (9-mer; Takara Bio Inc., Shiga, Japan). Each cDNA sample (1
ml) was amplified by 10 ml 2X SYBR® Premix Ex Taq™
(Takara Bio Inc.) and 10 pmol each primer. Amplification was
performed in a 7300 Real time PCR System (Applied Biosystems,
Foster City, CA, USA) using the following parameters: denaturation
at 95°C for 5 min, then 40 cycles of denaturation at 95°C for 30
sec, annealing at 60°C for 30 sec and extension at 72°C for 45 sec.
The oligonucleotide primer sequences used in this study were as
follows: 5′-AAC CAA GCA AGA AGT GAA GCC C-3′ (sense) and 5′-GCA AAA
TGA GAT GTG ATG GTG G-3′ (antisense) for DNMT1 (product size, 185
bp); 5′-GGA GGA ATG TGC CAA AAC TG-3′ (sense) and 5′-GCA GTT GTT
GTT TCC GCA C-3′ (antisense) to amplify DNMT3a (product size, 132
bp); and 5′-AAC AGC ATC GGC AGG AAC-3′ (sense) and 5′-ATC TTT CCC
CAC ACG AGG-3′ (antisense) for DNMT3b (product size, 249 bp). The
oligonucleotide primer sequences used to amplify β-actin (product
size, 131 bp) were: 5′-GGC ACC CAG CAC AAT GAA G-3′ (sense) and
5′-GCA AAA TGA GAT TGT ATG GTG G-3′ (antisense). The relative
expression levels of DNMT1 (normalized to the level of β-actin) in
each sample were determined using RQ software (Applied Biosystems).
All real-time PCR experiments were repeated twice.
Data analysis
Data are presented as the mean ± standard error of
the mean (SEM) and were analyzed with a one-way analysis of
variance (ANOVA) followed by Tukey’s multiple comparison test.
Statistical analyses were performed using Prism Graph Pad (v.4.0;
GraphPad Software Inc., San Diego, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Body weight and daily dietary intake
Differences in body-weight and daily intake between
mice who ate a normal diet and those who received DFV for 6 weeks
were evaluated. The mice from the six groups had similar body
weight gains as shown in Table I.
The body weights of mice in the sham, NC and PC (eritadenine)
groups did not change in response to the DFV. The groups treated
with L. edodes flour (10 and 20%) had lower final body
weights compared with those of the other groups at 6 weeks;
however, the differences in weight were not significant.
 | Table I.Body weight and daily intake. |
Table I.
Body weight and daily intake.
| Group | Weight (g)
| Daily intake (g) |
|---|
| Baseline | 8 weeks later |
|---|
| Sham | 37.1±0.9 | 40.1±0.3 | 4.2±0.5 |
| Negative | 37.2±0.9 | 41.3±3.4 | 4.6±0.9 |
| Positive | 37.6±0.7 | 41.0±0.5 | 4.6±0.6 |
| 5% L.
edodes | 37.5±0.9 | 41.9±0.7 | 4.2±0.5 |
| 10% L.
edodes | 37.8±0.1 | 39.7±1.3 | 4.4±0.8 |
| 20% L.
edodes | 37.6±0.8 | 39.1±2.1 | 4.6±1.0 |
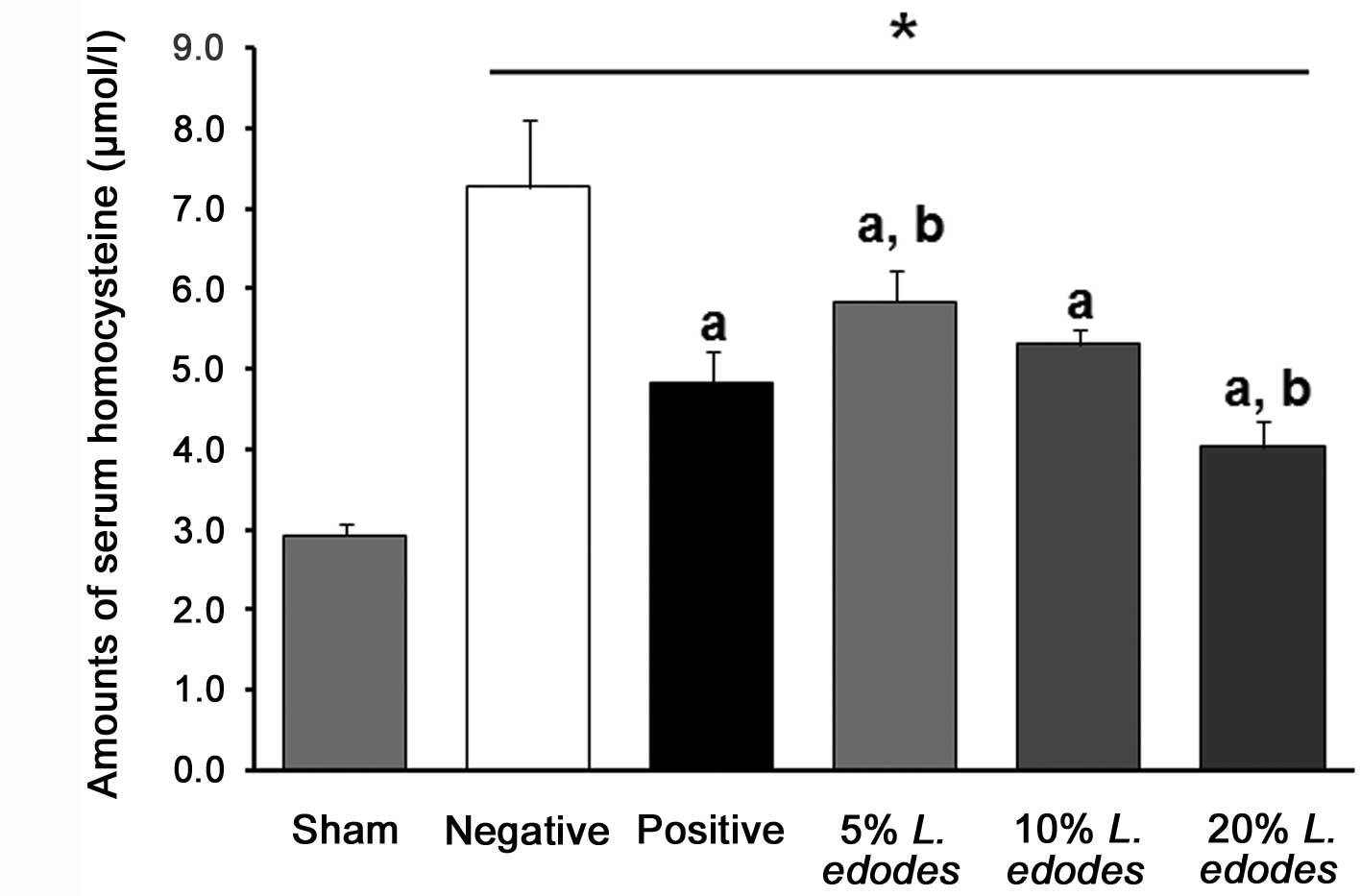
Serum levels of homocysteine in
hyperhomocysteinemic mice
Dietary supplementation with L. edodes
affected the serum levels of homocysteineine in mice with
hyperhomocysteinemia, caused by the DFV (Fig. 1). The DFV resulted in a marked
increase in homocysteine levels from 2.9±0.1 to 7.3±0.8 mmol/l
after 6 weeks. The addition of dietary eritadenine (10 mg/kg) or
L. edodes flour (5, 10 or 20%) attenuated the rise in serum
homocysteine levels caused by the DFV. In the groups receiving
L. edodes (5, 10 and 20%), the levels of homocysteine were
reduced in a dose-dependent manner compared with those in the NC
group.
Hepatic SAH and SAM levels, and SAM/SAH
ratios in hyperhomocysteinemic mice
HPLC was used to measure SAM and SAH levels, and the
SAM/SAH ratios in liver tissues were calculated. Hepatic SAH levels
were increased by the DFV as shown in Table II. The level of SAH was increased
in the NC group. The increase in SAH levels was significantly
reduced by eritadenine, as well as by L. edodes (5, 10 and
20%) in a dose-dependent manner. In addition, the SAM/SAH ratio was
reduced by the DFV and increased by dietary supplementation with
eritadenine and L. edodes flour (5, 10 and 20%).
 | Table II.Hepatic SAH and SAM levels, and
SAM/SAH ratios in hyperhomocysteinemic mice. |
Table II.
Hepatic SAH and SAM levels, and
SAM/SAH ratios in hyperhomocysteinemic mice.
| Group | SAH (nmol/g
tissue) | SAM (nmol/g
tissue) | SAM/SAH ratio |
|---|
| Sham | 5.2±0.2 | 10.8±0.6 | 2.1±0.1 |
| Negative | 13.5±0.5 | 7.7±0.4 | 0.58±0.04 |
| Positive | 6.6±0.4a | 10.3±0.9a | 1.59±0.19a |
| 5% L.
edodes | 8.7±0.4a | 7.8±0.8 | 0.90±0.08a |
| 10% L.
edodes | 7.5±0.4ab | 8.0±0.8 | 1.01±0.07ab |
| 20% L.
edodes | 6.5±0.3ac | 8.2±1.0 | 1.16±0.08ac |
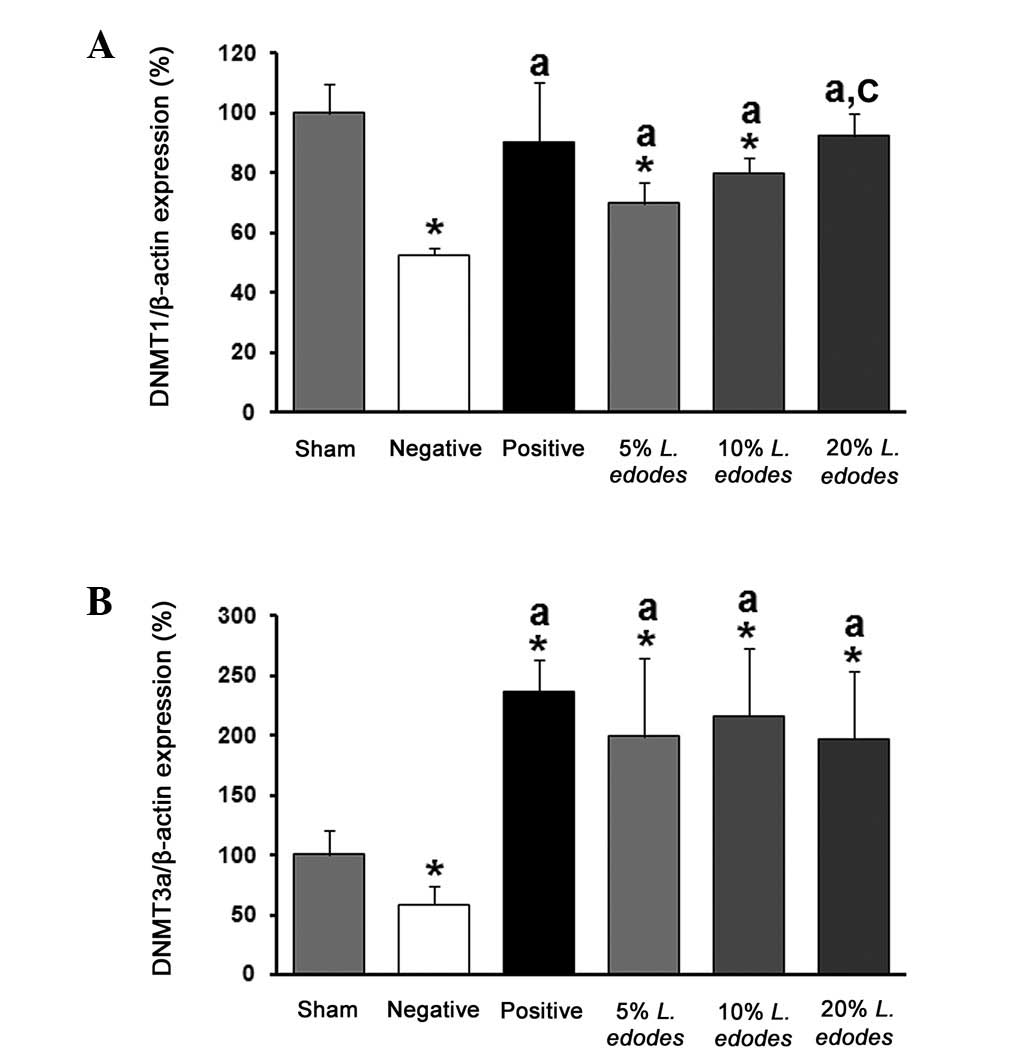
Expression of DNMT (1 and 3a) mRNA in the
livers of hyperhomocysteinemic mice
To measure the expression of hepatic DNMT1, the mice
were separated into six groups (sham, NC, PC, T1, T2, and T3). As
shown in Fig. 2, the level of
DNMT1 mRNA was reduced by the DFV in the NC group compared with
that in the sham group, and was significantly increased by
eritadenine supplementation in the PC group. Hepatic DNMT1 mRNA
expression was also dose-dependently increased by supplementation
with L. edodes (5, 10 and 20%). Hepatic DNMT3b mRNA was not
abundant in the liver of this mouse model while DNMT3a expression
was reduced by DFV compared with the level in the sham group. This
inhibitory effect was eliminated by eritadenine and all of doses of
L. edodes flour (5, 10 and 20%).
Discussion
L. edodes contains considerable
concentrations of vitamins (D, B6, B9 and B12) and other beneficial
compounds, including eritadenine and dietary fiber (1). This fungus is considered to be useful
for treating hypercholesterolemia, inflammation, hypertension and
osteoporosis (17–19). In a previous study, we examined the
anti-osteoporotic effects of L. edodes and the ability of
this fungus to induce the expression of duodenal and renal calcium
transport channels in mice with osteoporosis-like symptoms
(19). In the present study, we
investigated the anti-hyperhomocysteinemic effects of L.
edodes and determined whether it prevents increases in
homocysteine levels in mice with dietary folate and vitamin B12
deficiencies. After the mice had received specialized diets for 6
weeks, starting when the animals were 6 weeks old, we observed that
dietary L. edodes supplementation reduced the serum and
hepatic levels of homocysteine in the hyperhomocysteinemic mice.
Eritadenine was also shown to have a similar effect.
Dietary supplementation with eritadenine has been
reported to effectively suppress guanidine acetic acid
(GAA)-induced hyperhomocystenemia in rats (6). However, the mechanism underlying this
effect has not been fully elucidated. Analyses of eritadenine
content in L. edodes identified that the concentration of
this compound ranged between 3.2 and 6.3 mg/g dried mushroom
(20). The total amount of
aministered eritadenine in the PC group (10 mg/kg) was lower
compared with the amount contained in the dietary L. edodes
(>25 mg/kg); however, the anti-hyperhomocysteinemic effects were
similar.
The expression of DNMT1 mRNA, which the DFV
down-regulated, was increased by eritadenine and dose-dependently
increased by L. edodes (5, 10 and 20%) in the mice with
hyperhomocysteinemia compared with the level in the NC group.
Similarly, the level of DNMT3a mRNA was decreased by the DFV; this
inhibitory effect was abolished and the level of DNMT3a was
elevated by eritadenine and all doses L. edodes (5, 10 and
20%) compared with the sham group. A previous study demonstrated
that homocysteine selectively reduces the activity of DNMT1 and
increases SAH levels in human endothelial cells, but does not
affect DNMT3 (15). In another
study, DNMT1 and DNMT3 expression was reported to be elevated by
homocysteine in human monocytes (21). The levels of DNMT1 and DNMT3 have
been demonstrated to be mediated or selectively regulated by
homocysteine (15,21). DNMT3 levels have been found to
differ between mouse liver, HUVECs and human monocytes, and may
vary according to tissue type and species (15,21,22).
These results indicate that homocysteine is affected by
upregulation of DNA methylation through the suppression of HDAC
activity and increased DNMT1 and DNMT3 activities.
In conclusion, the present study demonstrated that
supplementation with eritadenine and L. edodes (5, 10 and
20%) significantly inhibited the effects of DFV-induced
hyperhomocysteinemia in mice. Reduced serum and hepatic
homocysteine levels illustrated the beneficial effects of L.
edodes on hyperhomocysteinemia-like symptoms. Serum and hepatic
homocysteine levels were significantly reduced by the
administration of eritadenine and L. edodes to
hyperhomocysteinemic mice with dietary folate and vitamin B12
deficiencies compared with their levels in the NC group. In
addition, we examined the expression of DNMT1 and DNMT3a in the
livers of mice and demonstrated that hepatic DNMT1 and DNMT3a
levels were increased to the levels observed in sham animals by
eritadenine and L. edodes. Based on our findings, we propose
that L. edodes may improve hyperhomocysteinemic symptoms due
to the beneficial compounds it contains.
Acknowledgements
This study was supported by a National
Research Foundation of Korea (NRF) grant funded by the Korean
Ministry of Education, Science and Technology (MEST; No.
2010-0011433).
References
|
1.
|
Wasser SP: Shiitake (Lentinus
edodes). Encyclopedia of Dietary Supplements. Coates PM,
Blackman MR, Cragg GM, et al: 1st edition. Marcel Dekker; New York,
NY: pp. 653–664. 2005
|
|
2.
|
Ngai PH and Ng TB: Lentin, a novel and
potent antifungal protein from shitake mushroom with inhibitory
effects on activity of human immunodeficiency virus-1 reverse
transcriptase and proliferation of leukemia cells. Life Sci.
73:3363–3374. 2003. View Article : Google Scholar
|
|
3.
|
Shimada Y, Morita T and Sugiyama K:
Eritadenine-induced alterations of plasma lipoprotein lipid
concentrations and phosphatidylcholine molecular species profile in
rats fed cholesterol-free and cholesterol-enriched diets. Biosci
Biotechnol Biochem. 67:996–1006. 2003. View Article : Google Scholar
|
|
4.
|
Sugiyama K, Akachi T and Yamakawa A:
Hypocholesterolemic action of eritadenine is mediated by a
modification of hepatic phospholipid metabolism in rats. J Nutr.
125:2134–2144. 1995.PubMed/NCBI
|
|
5.
|
Shimada Y, Morita T and Sugiyama K:
Dietary eritadenine and ethanolamine depress fatty acid desaturase
activities by increasing liver microsomal phosphatidylethanolamine
in rats. J Nutr. 133:758–765. 2003.PubMed/NCBI
|
|
6.
|
Fukada S, Setoue M, Morita T and Sugiyama
K: Dietary eritadenine suppresses guanidinoacetic acid-induced
hyperhomocysteinemia in rats. J Nutr. 136:2797–2802.
2006.PubMed/NCBI
|
|
7.
|
Akalin A, Alatas O and Colak O: Relation
of plasma homocysteine levels to atherosclerotic vascular disease
and inflammation markers in type 2 diabetic patients. Eur J
Endocrinol. 158:47–52. 2008. View Article : Google Scholar
|
|
8.
|
Goldstein LB: Novel risk factors for
stroke: homocysteine, inflammation, and infection. Curr Atheroscler
Rep. 2:110–114. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Kelly PJ, Kistler JP, Shih VE, et al:
Inflammation, homocysteine, and vitamin B6 status after ischemic
stroke. Stroke. 35:12–15. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Selhub J: Homocysteine metabolism. Annu
Rev Nutr. 19:217–246. 1999. View Article : Google Scholar
|
|
11.
|
McCaddon A, Hudson P, Ellis D, Hill D and
Lloyd A: Effect of supplementation with folic-acid on relation
between plasma homocysteine, folate, and vitamin B12. Lancet.
360:1732002. View Article : Google Scholar
|
|
12.
|
Miller JW, Nadeau MR, Smith D and Selhub
J: Vitamin B-6 deficiency vs folate deficiency: comparison of
responses to methionine loading in rats. Am J Clin Nutr.
59:1033–1039. 1994.PubMed/NCBI
|
|
13.
|
Kruman II, Culmsee C, Chan SL, et al:
Homocysteine elicits a DNA damage response in neurons that promotes
apoptosis and hypersensitivity to excitotoxicity. J Neurosci.
20:6920–6926. 2000.PubMed/NCBI
|
|
14.
|
Hiltunen MO and Ylä-Herttuala S: DNA
methylation, smooth muscle cells, and atherogenesis. Arterioscler
Thromb Vasc Biol. 23:1750–1753. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Jamaluddin MD, Chen I, Yang F, et al:
Homocysteine inhibits endothelial cell growth via DNA
hypomethylation of the cyclin A gene. Blood. 110:3648–3655. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Wagner J, Claverie N and Danzin C: A rapid
high-performance liquid chromatographic procedure for the
simultaneous determination of methionine, ethionine,
S-adenosylmethionine, S-adenosylethionine, and the natural
polyamines in rat tissues. Anal Biochem. 140:108–116. 1984.
View Article : Google Scholar
|
|
17.
|
Kabir Y, Yamaguchi M and Kimura S: Effect
of shiitake (Lentinus edodes) and maitake (Grifola
frondosa) mushrooms on blood pressure and plasma lipids of
spontaneously hypertensive rats. J Nutr Sci Vitaminol (Tokyo).
33:341–346. 1987.
|
|
18.
|
Carbonero ER, Gracher AHP, Komura DL,
Marcon R, Freitas CS, Baggio CH, Santos ARS, Torri G, Gorin PAJ and
Iacomini M: Lentinus edodes heterogalactan: antinociceptive
and anti-inflammatory effects. Food Chem. 111:531–537. 2008.
View Article : Google Scholar
|
|
19.
|
Lee GS, Byun HS, Yoon KH, Lee JS, Choi KC
and Jeung EB: Dietary calcium and vitamin D2 supplementation with
enhanced Lentinula edodes improves osteoporosis-like
symptoms and induces duodenal and renal active calcium transport
gene expression in mice. Eur J Nutr. 48:75–83. 2009.PubMed/NCBI
|
|
20.
|
Enman J, Rova U and Berglund KA:
Quantification of the bioactive compound eritadenine in selected
strains of shiitake mushroom (Lentinus edodes). J Agric Food
Chem. 55:1177–1180. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Jiang Y, Jiang J, Xiong J, et al:
Homocysteine-induced extra-cellular superoxide dismutase and its
epigenetic mechanisms in monocytes. J Exp Biol. 211:911–920. 2008.
View Article : Google Scholar
|
|
22.
|
Vizzardi E, Bonadei I, Zanini G, et al:
Homocysteine and heart failure: an overview. Recent Pat Cardiovasc
Drug Discov. 4:15–21. 2009. View Article : Google Scholar
|