Introduction
Augmenter of liver regeneration (ALR) is a
non-specific hepatocyte growth-promoting factor with heat
stability, identified by Hagiya et al(1) in 1994 during a study of hepatic
stimulator substance (HSS), and is different from hepatocyte growth
factor (HGF) (2). Similar to
insulin-like growth factor (IGF) and epidermal growth factor (EGF),
ALR plays an important role in the regeneration of hepatocytes
(3). In order to further
understand the human ALR (hALR) concentration of serum and study
the association of hALR with various liver diseases, particularly
with the different stages of type-B hepatitis, it is necessary to
investigate the serum hALR concentration in various types and
extents of hepatitis and cirrhosis, and the correlation of hALR and
disease in detail. According to the classical immunology theory
(4), we aimed to establish a
reliable method for measuring hALR using a directly competitive
inhibition reaction, which is different from previous double
antibody sandwich or indirect enzyme-linked immunosorbent assays
(ELISAs). Eu3+-labeled hALR was used to compete with the
pure hALR protein as an antigen in the competitive inhibition, and
an anti-hALR monoclonal hybridoma cell line was used to produce
anti-hALR monoclonal antibody; these processes established a direct
competitive method for measuring serum hALR to aid the
understanding of serum hALR concentration and its significance in
various liver diseases.
Materials and methods
Materials and reagents
Recombinant plasmid pQE30-hALR was constructed in
the Institute for Viral Hepatitis (Chongqing Medical University,
Chongqing, China), as previously described (5). The polyhistidine protein purification
kit (Ni2+-NTA Resin) was purchased from Qiagen GmbH
(Hilden, Germany) and the capillary electrophoresis (CE) system
(PACE 5500) was purchased from Beckman (Beckman Coulter Inc., Brea,
CA, USA). BALB/c mice were provided by the Experimental Animal
Centre of Chongqing Medical University (Chongqing, China).
Eu3+ chelator, Eu3+-labeled dilution
solution, fluorescence enhancer, time-resolved fluorometer,
automicroplate washer and plate shaker were purchased from Sym-Bio
Life Science (Zhejiang, China).
Serum samples
The serum samples were collected from patients in
the outpatient or inpatient ward of the Department of Infectious
Diseases, the Second Affiliated Hospital, Chongqing Medical
University between September 2005 and April 2006. The diagnosis met
with the Viral Hepatitis Prevention & Treatment Strategy
released by the Chinese Society of Hepatology and Society of
Infectious Diseases (2005)(6). The
blood samples were collected in the morning after fasting,
incubated at 37˚C for 2 h, centrifuged at 1,200 × g for 10 min and
stored at −20°C. This study complied with the Declaration of
Helsinki, and was approved by the Ethics Committee of the Second
Affiliated Hospital of Chongqing Medical University. All
participants provided written informed consent.
Methods
Preparation of antigens and antigen
labeling
i) Pronucleus expression of hALR: following
identification, the recombinant plasmid pQE30-hALR was transformed
into E. Coli SG13009 for expression induced by IPTG. The
product was identified with 15% SDS-PAGE. ii) Affinity
chromatography purification of hALR: hALR protein was purified
using Ni-NTA and identified using 15% SDS-PAGE and capillary
electrophoresis. iii) hALR labeling: purified hALR protein was
labeled with Eu3+ via DTTA chelation (7) and the labeled product was identified
by 15% SDS-PAGE and time-resolved fluorescence (TRF) immunoassay
(TRFIA).
Antibody preparation and
identification
Anti-hALR hybridoma (AAMA) cells were established in
our laboratory (8) and were
cultured and inoculated intraperitoneally into BALB/c mice
(9) as previously described. SP2/0
myeloma cells were used as a negative control. The harvested
ascitic fluid was tested for the reactivity of hALR proteins and
human albumin using an ELISA and immunoblot assay.
Establishment of the measuring method and
clinical application
Direct antigen competition was used to coat the
96-well ELISA plate with an optimal working concentration of
anti-hALR (monoclonal antibody 100 μl/well, 1:800) at 4°C
overnight. BSA/PBST was added (3%, 200 μl/well) and the well was
blocked at 37°C for 3 h. Standard purified hALR protein (50 μl, 2
μg/ml) diluted with PBS at 1:10, 1:50, 1:100, 1:500, 1:1,000 and
1:5,000 was mixed with 50 μl Eu3+-hALR in the same
reaction well for competitive inhibition reaction in the plate
shaker and incubated at 37°C for 1 h. Three parallel wells were
used for each concentration. Fluorescence enhancing solution (100
μl) was added to each well, the plates were incubated at 37°C for 5
min and the resulting samples were analyzed by TRFIA to create a
standard curve. The serum samples from the patients with various
liver diseases were used to replace the standard protein and
competitively react with Eu3+-hALR in the blank,
negative antibody control and negative quality control serum wells.
A regression analysis of the results was performed using the
standard curve to calculate the hALR concentration in the sera of
the 90 patients with various liver diseases. Calf serum was used as
a medium to prepare serial concentrations of hALR at 5, 10, 20 and
40 ng/ml to be detected with the direct competitive assay, and then
the coefficient of recovery and the variation coefficient were
calculated.
Statistical analysis
The data are expressed as mean ± SD. SPSS 13.0
(SPSS, Inc., Chicago, IL, USA) was used to compare intra-group
differences using a Student’s t-test. P<0.05 was considered to
indicate a statistically significant result.
Results
Antigens and antigen labeling
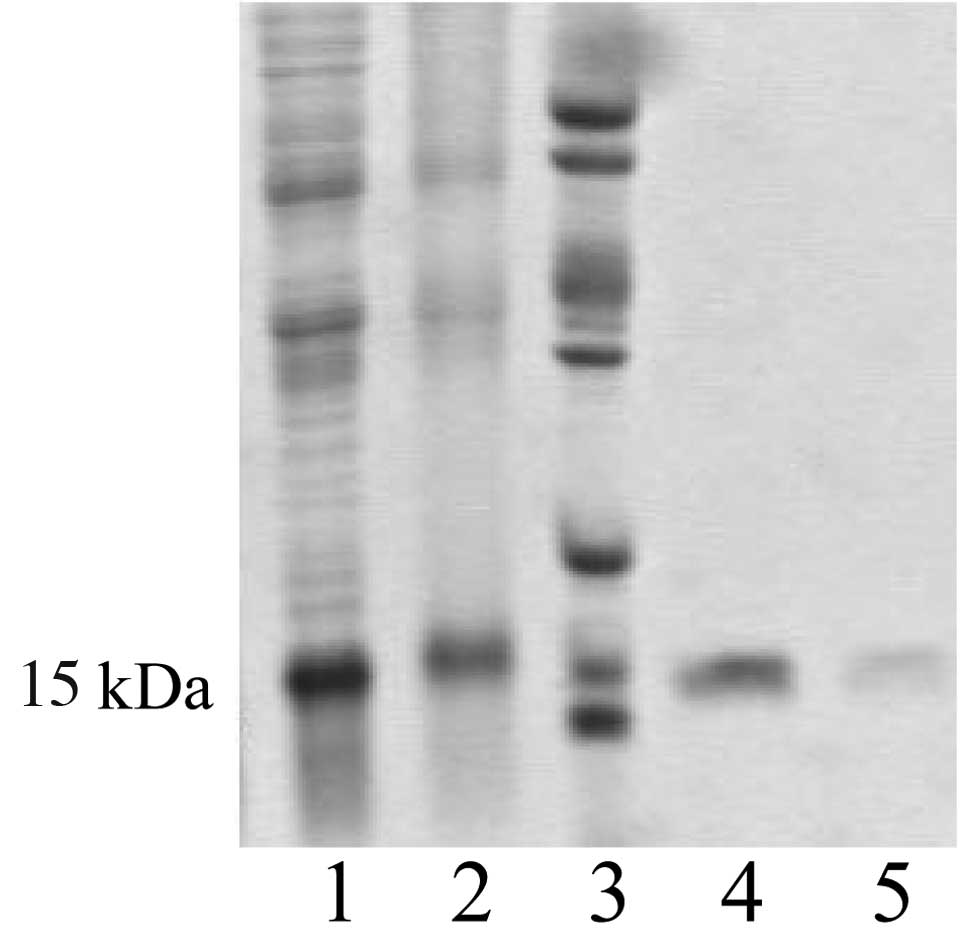
The recombinant plasmid pQE30-hALR showed high
expression in the host bacteria and the molecular weight of the
product was approximately 15 kDa (Fig.
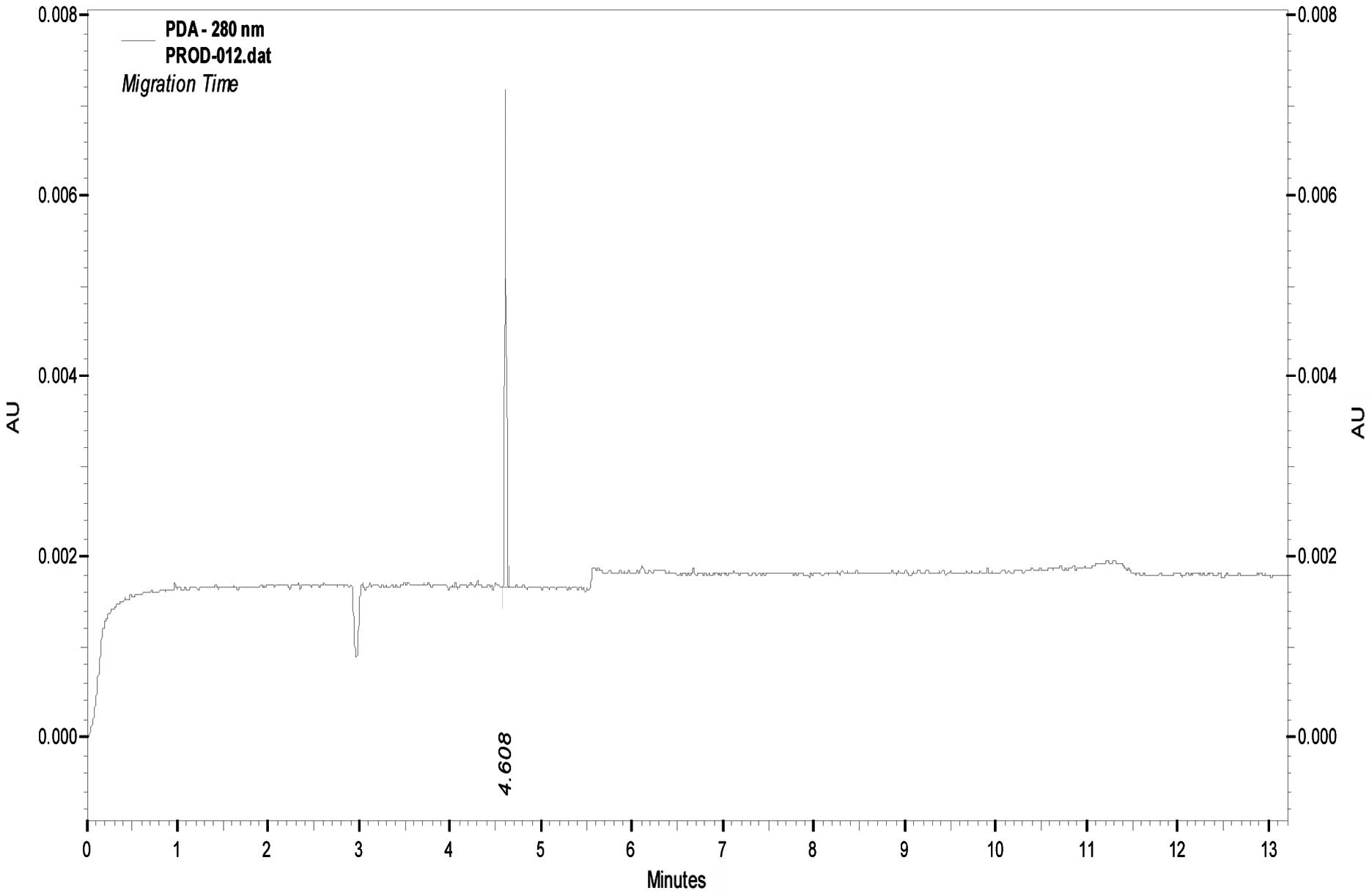
1). Following purification with affinity chromatography, the
protein was identified as one band by SDS-PAGE with a purity of 90%
by CE (Fig. 2).
Antibody preparation and
identification
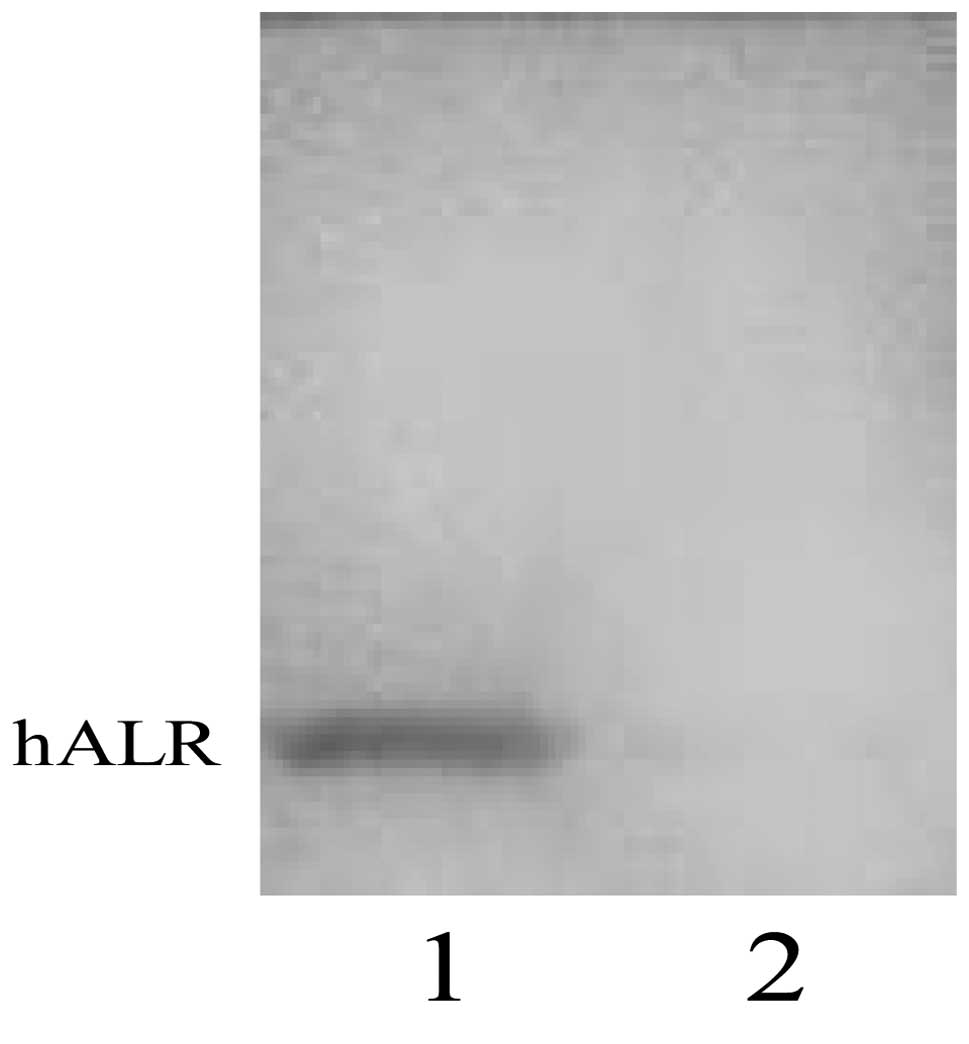
The harvested anti-hALR monoclonal antibody ascites
were measured by ELISA with an optimal working concentration of
1:800 (Table I). Western blotting
showed the monoclonal antibody of hALR protein as a single band
without interaction with the natural albumin protein in human serum
(Fig. 3).
 | Table IOD450 of hALR combined with
mouse ascites in different concentrations. |
Table I
OD450 of hALR combined with
mouse ascites in different concentrations.
| Concentration of
mouse ascites diluted with PBS (v/v) |
|---|
|
|
|---|
| Mouse ascites | 1:200 | 1:400 | 1:800 | 1:1000 | 1:2000 |
|---|
| SP2/0 ascites | 0.214±0.08 | 0.184±0.05 | 0.155±0.05 | 0.164±0.08 | 0.138±0.06 |
| AAMA ascites | 0.985±0.15a | 0.939±0.07a | 0.896±0.04ab | 0.785±0.01a | 0.671±0.06a |
Establishment and application of serum
measurement
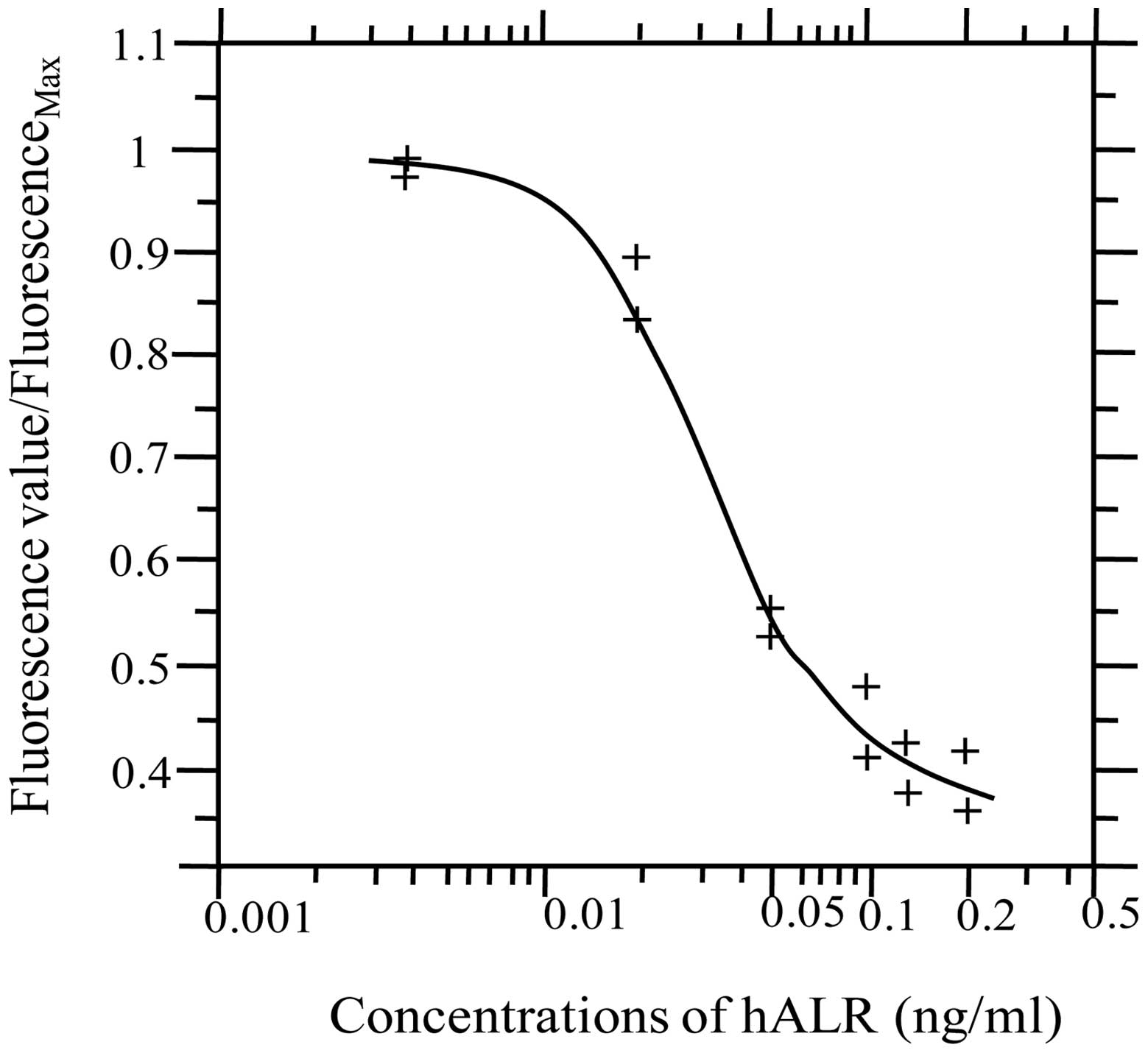
A standard curve for competitive inhibition of
Eu3+-hALR and hALR was constructed. The fluorescence
values of standard hALR and Eu3+-ALR at various
concentrations were measured by TRF. A standard curve was
constructed using matched software (Sym-Bio Life Science, Zhejiang,
China), with the concentration of standard hALR on the x-axis and
the ratio of fluorescence value in reaction wells/measured maximal
fluorescence value on the y-axis, as shown in Fig. 4.
Serum sample measurement
As shown in Table
II, the concentrations of hALR in the sera of patients with
various liver diseases (acute hepatitis, chronic hepatitis,
cirrhosis and severe hepatitis) were significantly higher compared
with that in the normal control group (P<0.01).
 | Table IISerum hALR levels in 90 patients with
various liver diseases. |
Table II
Serum hALR levels in 90 patients with
various liver diseases.
| Clinical group | No. of cases | hALR concentration
(ng/ml) |
|---|
| Normal control | 10 | 3.77±1.55 |
| Acute hepatitis | 5 | 10.14±3.26a |
| Chronic
hepatitis | 30 | 8.44±2.78a |
| Cirrhosis | 30 | 10.11±4.32a |
| Severe hepatitis | 15 | 57.34±18.96a |
Recovery percentage and variation
coefficient
Using calf serum as a medium, the coefficient of
recovery was detected. Table III
shows the coefficient of recovery at four concentration levels and
the variation coefficient obtained from the repeats.
 | Table IIIRecovery percentages of the direct
competitive reaction. |
Table III
Recovery percentages of the direct
competitive reaction.
| Addition hALR
(ng/ml) | Repeated wells
(n) | Detected
concentration hALR (ng/ml) | Recovery percentage
(%) | Variation coefficient
(%) |
|---|
| 5.0 | 3 | 4.16 | 83.2 | 7.12 |
| 10.0 | 3 | 8.52 | 85.2 | 4.84 |
| 20.0 | 3 | 18.73 | 93.7 | 7.35 |
| 40.0 | 3 | 33.54 | 83.9 | 9.86 |
Discussion
ALR is a recently discovered growth factor that
promotes liver regeneration and, similar to other cellular factors
such as IGF and EGF, plays an important role in the regeneration of
hepatocytes. We aim to understand the hALR concentration in the
sera of patients with hepatitis and cirrhosis, and further
investigate its interaction with these diseases.
In previous studies (10,11),
serum hALR has been measured using a double antibody sandwich or
indirect ELISA method. However, classical immunology (4) clearly suggests that a double antibody
sandwich ELISA is not able to provide simultaneously two binding
sites for two antibodies on small molecular antigens. In addition,
large proteins in the serum may affect the binding of small
molecular antigens to antibodies in an indirect ELISA. Therefore,
the previously reported methods are not appropriate. In the present
study, we established a method for measuring serum hALR in liver
diseases of varying severity using competitive inhibition against
small molecular antigens. Direct competition is simple and fast,
and requires only one rinse and two additions of samples.
Therefore, we selected direct antigen competitive inhibition as the
response model. Through the competitive combination between
Eu3+-hALR and hALR in serum with the coated monoclonal
Abs, we established a measurement method for serum hALR.
The results indicated that the serum level of hALR
in patients with viral hepatitis was higher than that in normal
serum. In particular, severe hepatitis had the highest hALR level,
which was 15-fold that of normal serum and 6 to 7-fold that of
common hepatitis; the hALR level in acute hepatitis was 3-fold that
of normal serum, and cirrhosis and chronic hepatitis had 3-fold
higher serum hALR levels than normal serum. The hALR levels
determined in the present study were higher than those reported in
a previous study (10,11). This may be due to the different
methodologies used. The double antibody sandwich or indirect ELISA
used previously has insufficient sensitivity for small molecular
antigens, is not able to provide two binding sites for two
antibodies and is affected by temporal resistance which results in
antigens being partially undetected and so is not able to reflect
the actual levels of hALR in samples. We used a competitive
inhibition model to avoid these challenges and provide a higher
detection rate.
We noted that the serum hALR levels increased
quickly in severe hepatitis caused by hepatitis B virus (HBV)
infection. The reason for this increase is due to hepatocytes
undergoing marked necrosis and releasing large amounts of hALR into
the blood accompanied by increased secretion from other organs,
including the kidney (12) and
pancreas (13), and extensive
damage to hepatocytes decreasing the binding and metabolism of
hALR, resulting in a higher detection rate. The compensatory
increase of secretion is closely correlated with liver regeneration
and may be considered as an acute reaction of the body to liver
damage. The cause of the increase of serum hALR in acute B-type
hepatitis is similar to that in severe hepatitis, i.e., increased
release, reduced binding and reduced metabolism of hepatocytes due
to damage. In the present study, the chronic B-type hepatitic and
B-type cirrhosis patients were patients in the inpatient ward, who
had clear symptoms of chronic active hepatitis or decompensated
cirrhosis, damaged liver function and hepatocyte damage of various
extents. Therefore, the serum detection rate was high. In summary,
the serum hALR concentration in various severities of liver disease
is closely correlated with the extent of hepatocyte damage while
whether hALR has a pro-regenerative effect on the hepatocytes
depends upon the condition of the hepatocytes.
Based on the present results, the competitive
inhibition method has sufficient accuracy, specificity and
sensitivity to meet the requirements of clinical application.
Through continuous optimization and widespread practice, this
method may be used as a clinical diagnostic index for liver
diseases and other diseases associated with hALR (such as urinary
system diseases) (14), and to
provide new theoretical evidence and a measurement method for the
diagnosis and differential diagnosis of associated diseases.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (No.s 30271178 and 30570826).
References
|
1
|
Hagiya M, Francavilla A, Polimeno L, et
al: Cloning and sequence analysis of the rat augmenter of liver
regeneration (ALR) gene: expression of biologically active
recombinant ALR and demonstration of tissue distribution. Proc Natl
Acad Sci USA. 91:8142–8146. 1994. View Article : Google Scholar
|
|
2
|
Burr AW, Toole K, Chapman C, Hines JE and
Burt AD: Anti-hepatocyte growth factor antibody inhibits hepatocyte
proliferation during liver regeneration. J Pathol. 185:298–302.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pawlowski R and Jura J: ALR and liver
regeneration. Mol Cell Biochem. 288:159–169. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Crowther JR: The ELISA Guidebook. Humana
Press; Totowa, NJ: pp. 24–33. 2001
|
|
5
|
Liu Q, Shi XF, Lou Y and Zhang DF:
Constraction of prokaryotic expression vector of hALR and its
expression in E.coll. Zhonghua Gan Zang Bing Za Zhi. 8:9–11.
2000.(In Chinese).
|
|
6
|
Chinese Society of Hepatology, Chinese
Medical Association; Chinese Society of Infectious Diseases,
Chinese Medical Association. The guidelines of prevention and
treatment for chronic hepatitis B. Zhonghua Gan Zang Bing Za Zhi.
13:881–891. 2005.(In Chinese).
|
|
7
|
Ferguson RA, Yu H, Kalyvas M, Zammit S and
Diamandis EP: Ultrasensitive detection of prostate-specific antigen
by a time-resolved immunofluorometric assay and the Immulite
immunochemiluminescent third-generation assay: potential
applications in prostate and breast cancers. Clin Chem. 42:675–684.
1996.
|
|
8
|
Ma HF and Liu Q: Preparation of monoclonal
antibody to human augmenter of liver regeneration: screening of
hybridomas with unpurifed antigen expressed by E.coli. Zhongguo
Mian Yi Xue Za Zhi. 18:671–673. 2002.(In Chinese).
|
|
9
|
Kontermann R and Dübel S: Antibody
engineering. Springer; 1. pp. 319–321. 2010
|
|
10
|
Zhou P, Yang XM, Li QF, He H, He FC and
Zhang MS: Detection of augmenter of liver regeneration in sera of
patients with various liver diseases. World Chinese Journal of
Digestology. 6:768–770. 1998.
|
|
11
|
Yu HY, Xiang DR, Huang HJ, Li J and Sheng
JF: Expression level of augmenter of liver regeneration in patients
with hepatic failure and hepatocellular carcinoma. Hepatobiliary
Pancreat Dis Int. 9:492–498. 2010.PubMed/NCBI
|
|
12
|
Liao XH, Zhang L, Liu Q, Sun H, Peng CM
and Guo H: Augmenter of liver regeneration protects kidneys from
ischaemia/reperfusion injury in rats. Nephrol Dial Transplant.
25:2921–2929. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Adams GA, Maestri M, Squiers EC, Alfrey
EJ, Starzl TE and Dafoe DC: Augmenter of liver regeneration
enhances the success rate of fetal pancreas transplantation in
rodents. Transplantation. 65:32–36. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liao XH, Zhang L, Tang XP, Liu Q and Sun
H: Expression of augmenter of liver regeneration in rats with
gentamicin-induced acute renal failure and its protective effect on
kidney. Ren Fail. 31:946–955. 2009. View Article : Google Scholar : PubMed/NCBI
|