Contents
Introduction
Homeostasis of the epidermal barrier
Adverse effects induced by UVB in the epidermal
barrier
Reduced levels of covalently bound Cer
Disrupted multilamellar structures in the
intercellular space of the SC
Increased GlcCer levels
Disrupted epidermal calcium gradient
Beneficial effects induced by UVB in the epidermal
barrier
Conclusions
Introduction
The epidermal barrier is important for maintaining
the homeostasis of the skin. The skin is constantly exposed to
ultraviolet A (UVA) and ultraviolet B (UVB) irradiation while
ultraviolet C radiation is absorbed by the ozone layer. Due to
their different wavelength ranges, UVA and UVB act at two different
levels of the skin. UVA predominantly affects the dermis and the
DNA, whereas UVB affects the epidermis (1–3). UV
irradiation of the skin is known to induce disruption of the
epidermal barrier (4–7). However, UVB irradiation has been used
for the treatment of atopic dermatitis, a skin disease involving a
defective epidermal barrier (8–10).
We reviewed the homeostasis of the epidermal barrier and the
previous studies investigating the adverse and beneficial effects
of UVB irradiation in the epidermal barrier, with the aim of
understanding the potential therapeutic strategy of using UVB
irradiation in the treatment of skin diseases with a disrupted
epidermal barrier.
Homeostasis of the epidermal barrier
The skin barrier properties are primarily localized
in the outer epidermal layer, the stratum corneum (SC) (11). The SC consists of corneocytes
surrounded by a neutral lipid-enriched extracellular matrix. The
mechanical strength of the skin is provided by the corneocytes,
which are encased by a cornified cell envelope (CE) (12). The hydrophobic extracellular lipid
matrix provides a barrier against the movement of water and
electrolytes (11). Thus, the SC
is involved in the regulation of water release from the organism
and into the atmosphere, known as transepidermal water loss (TEWL)
(13). TEWL is used as an
indicator of the functional integrity of the SC (14,15).
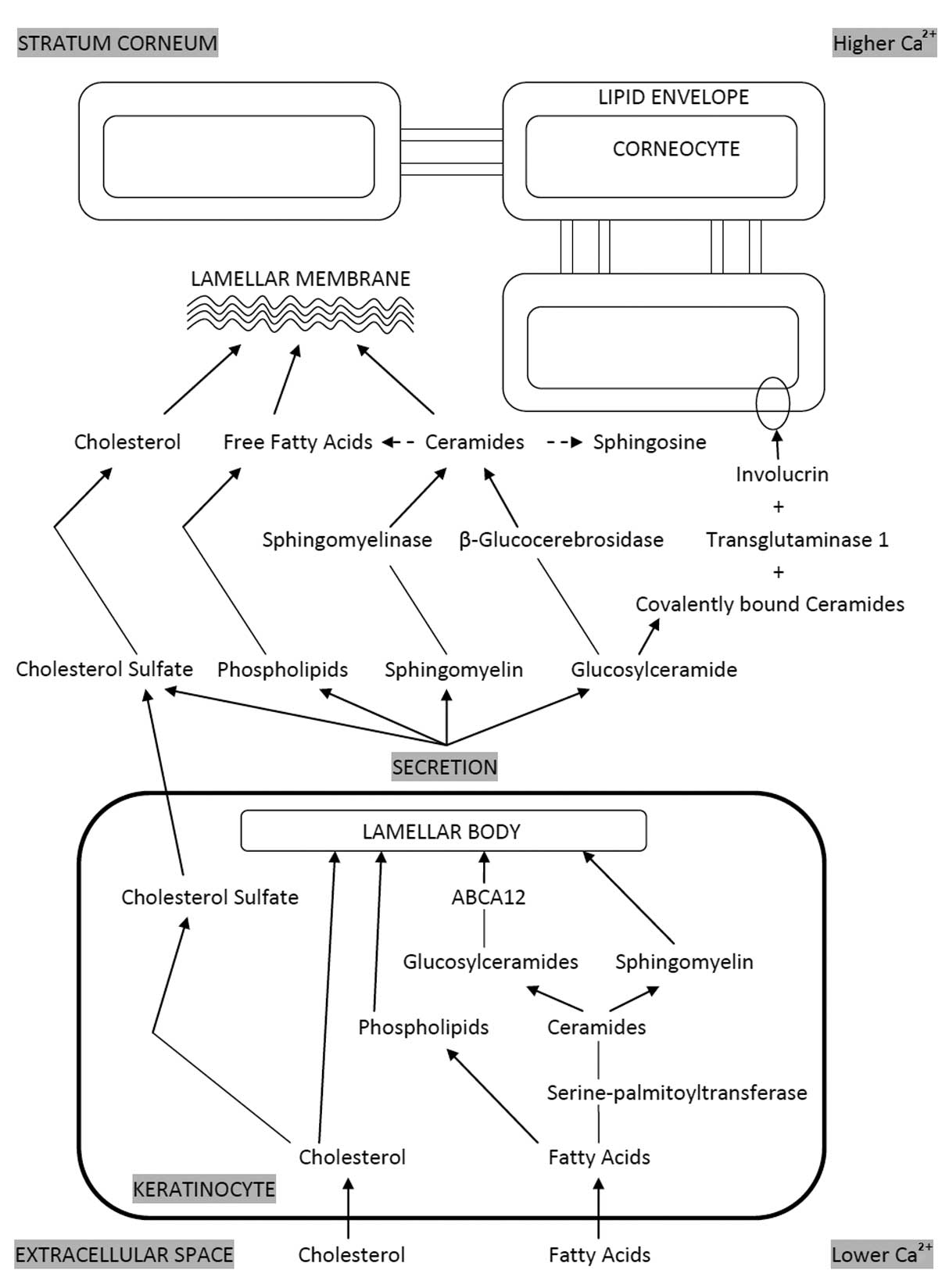
Lamellar bodies (LBs) are abundantly located in the
differentiated keratinocytes, particularly in the stratum
granulosum (16). LBs are
responsible for supplying the lipids for the lipid envelope of the
CE and the extracellular lipid matrix (Fig. 1) (16,17).
LBs contain lipid precursors and numerous enzymes, including lipid
hydrolases and proteases (16). It
is considered that the incorporation of the lipid hydrolases and
proteases into LBs requires the prior or concurrent delivery of
lipids to the LBs (18). Thus, if
lipids are deficient or lipid synthesis is disrupted, the enzymes
that are characteristically found in LBs are not transported from
the Golgi to the LBs (18). The
lipids that constitute the extracellular matrix comprise 15% fatty
acids, 25% cholesterol and 50% ceramides (Cer) (16). The relative quantities of these
three key lipids are important for the formation of LBs. An excess
or deficiency of a particular lipid may disturb LB formation
(12).
In the normal human and murine epidermis, the
extracellular calcium (Ca2+) content is low in the basal
and spinous layers, with a gradual increase from the inner to the
outer layers and a maximal concentration within the outer stratum
granulosum (19–21). The change in calcium concentration
appears to be the primary signal inducing LB secretion (12). Following secretion from the
granular cells into the intercellular space, these LB-derived
lipids are further metabolized in the SC extracellular spaces by
enzymes that are cosecreted in the LBs (16,17,22–24).
Specifically, β-glucocerebrosidase (β-GlcCerase) converts
glucosylceramides (GlcCer) into Cer (25,26).
A minor but important component of the LB-derived
lipids are acylglucosylceramides, of which approximately two-thirds
are converted to ω-hydroxyceramides that become covalently attached
by ester linkages to the CE peptides (27–30).
ω-hydroxyceramides are the predominant lipid species of the
corneocyte lipid envelope in the epidermis (31). These contain linoleic esters, which
are linked by the action of transglutaminase-1 (TGase 1) to
glutamine and to glutamate residues of a number of CE structural
proteins, most notably involucrin (30,32).
These covalently bound Cer contribute a hydrophobic surface to the
corneocyte that has important consequences for water barrier
function by interactions with, and perhaps organization of,
intercellular lipids (33).
Adverse effects induced by UVB in the
epidermal barrier
Excessive exposure to UV radiation may lead to skin
cancer and premature aging, and UVB is the most effective waveband
at causing these changes (34–36).
Exposure of the skin to UVB radiation may induce changes in the
epidermal barrier (37).
Reduced levels of covalently bound Cer
Studies have demonstrated that the levels of
covalently bound Cer are significantly reduced in parallel with
significant increases in TEWL following irradiation with a single
UVB dose of 75 or 160 mJ/cm2, and following continuous
UVB irradiation [40 mJ/cm2 or 0.5 × the minimal
erythemal dose (MED)/day for 14 days] in hairless mice and rats
(38,39). This suggests a close correlation
between the alteration of covalently bound Cer and the UVB-induced
perturbation of the skin barrier.
It has been observed that the level of involucrin
did not change following a single UVB irradiation at a dose of 50
mJ/cm2; however, the expression of TGase 1 mRNA was
shown to be significantly downregulated (38,40).
Hirao et al (41) revealed
that a reduction in the binding of ω-hydroxyceramides to involucrin
was elicited by UVB irradiation (41). Takagi et al (38) suggested that the UVB-induced
downregulation of TGase 1 may have been responsible for the
observed reduction in the level of covalently bound Cer.
A study in which hairless mice were irradiated with
a single 75 mJ/cm2 dose of UVB radiation demonstrated
that a reduction in the level of covalently bound Cer occurred in
parallel with the time when the thickness of the epidermis was
significantly increased (38).
Treatments known to induce epidermal hyperplasia, such as tape
stripping or sodium dodecylsulfate (SDS) treatment, led to
significant reductions in the levels of covalently bound Cer,
whereas the levels of non-bound Cer remained unchanged (38). This suggests that the decreased
level of bound Cer is highly correlated with epidermal hyperplasia
(38).
Disrupted multilamellar structures in the
intercellular space of the SC
LBs are secreted from the outer stratum granulosum
cells (12). Acute disruption of
the permeability barrier initiates a homeostatic repair response,
including the rapid secretion (within minutes) of the contents of
the LBs (50–80% of pre-existing LBs are secreted) (42–44).
A number of studies have demonstrated the suppression of LB release
to the space between the SC and granulosa, and the retention of the
LBs in the SC, following continuous UVB irradiation daily at a dose
of 40 mJ/cm2 or 0.5 × MED/day for 14–15 days on hairless
rats (39,45). This may have contributed to the
resulting disrupted multilamellar structures in the SC (folding,
defects or absent). Bound Cer are considered to be formed by the
fusion of LBs with the cell membrane of granulosum cells, followed
by release into the space between the SC and granulosa (17). Meguro et al (39) suggested that the suppression of the
release of the LBs into the space between the SC and the granulosa
may have resulted in the reduction in covalently bound Cer in the
SC following UVB irradiation (39).
A number of studies have shown that bound lipids may
act as templates in the formation process of multilamellar
structures by LBs released into the space between the SC and
granulosa, and may be important in the maintenance of the
multilamellar structures by connecting corneocytes to the
multilamellar layer, acting as connectors between corneocytes or
conferring resistance to proteinases derived from bacteria
(17,46,47).
Several studies have demonstrated the importance of bound lipid for
the formation of multilamellar structures between corneocytes
(39,48). In addition, certain studies have
demonstrated the disruption of multilamellar structures together
with decreased levels of covalently bound Cer following UVB
irradiation (38,39). Thus, the reduced levels of
covalently bound Cer may contribute to the disruption of
multilamellar structures. Covalently bound Cer and the formation of
multilamellar structures by LB release may contribute reciprocally
to enable normal function, while alterations induced by UVB
irradiation affect the normal regulation of the two factors.
Increased GlcCer levels
Tagaki et al (49) observed markedly suppressed
epidermal β-GlcCerase activity with a significantly increased
expression of β-GlcCerase mRNA, followed immediately by the
accumulation of GlcCer in the SC of hairless mice, one day
subsequent to a single UVB irradiation dose of 70
mJ/cm2(49). Under
normal conditions, β-GlcCerase exists at levels sufficient to
convert all GlcCer secreted from lamellar granules into Cer,
leading to complete loss of GlcCer in the SC. The accumulation of
GlcCer in the SC resulting from the inhibition of β-GlcCerase
activity leads, in turn, to barrier perturbation, concomitant with
the abnormal lamellar integrity (26,49,50).
It has been demonstrated that epidermal hyperplasia
may be induced by elevated GlcCer levels (51); thus, the accumulation of GlcCer in
the epidermis, as a result of the downregulation of β-GlcCerase,
may be the cause of the hyperproliferation induced by UVB
irradiation (49).
Decreased β-GlcCerase activity may occur due to
damage to the enzyme itself. As β-GlcCerase is predominantly
localized in the outer epidermis (52), its activity may be susceptible to
UVB irradiation, resulting in an attenuated protein-generating
capacity at specific cellular levels of the epidermis (49).
Between 70 and 80% of Cer are derived from GlcCer
through the action of β-GlcCerase (53,54).
Decreased β-GlcCerase activity, induced by UVB, gives rise to the
significant accumulation of GlcCer in the SC; however, it has been
observed that this is not accompanied by a significant reduction in
the Cer level in the SC (49).
Tagaki et al (49)
demonstrated that the increasing level of GlcCer in the SC
following UVB irradiation corresponded to <10% of the total Cer
in the SC. It was suggested that β-GlcCerase activity exists below
the SC at a level sufficient to convert more than the control level
of GLcCer (49).
Disrupted epidermal calcium gradient
Jiang et al (55) revealed the appearance of large
clumps of calcium precipitates in the extracellular spaces of the
lower nucleated layers of the epidermis of hairless mice 48 h
subsequent to UVB irradiation at a dose of 0.15 J/cm2
(equivalent to 7.5 × MED) (55).
The normal calcium gradient was altered, with higher extracellular
calcium levels within the lower layers of the epidermis (55). The most notable feature was
observed 96 h subsequent to UVB irradiation, when the TEWL reached
the highest level. Immediately following barrier disruption, the
increased water movement through the compromised SC carried calcium
outward toward the skin surface, resulting in a reduction in the
calcium concentration surrounding the stratum granulosum cells
(20,56,57).
A gradual return to a normal calcium distribution level was
observed in parallel with the gradual return to a normal TEWL level
(55), indicating that barrier
recovery occurs in parallel with the restoration of the calcium
gradient in the epidermis (20).
This shows that a marked correlation exists between the altered
calcium gradient and the disrupted epidermal barrier.
If the reduction in calcium levels is prevented by
the provision of exogenous calcium, LB secretion does not occur and
permeability barrier repair is not initiated (20,56–58).
Conversely, if the calcium levels surrounding the stratum
granulosum cells are decreased without disrupting the permeability
barrier by either iontophoresis or sonophoresis, LB secretion is
stimulated (58,59). This shows that the calcium gradient
in the epidermis is closely linked to the presence of a normal
permeability barrier.
Several studies have investigated the UVB-induced
abnormal lamellar membrane structures in the SC interstices
(38,39,60).
Defective lamellar multilayers have also been observed in the
presence of an altered calcium gradient following UVB irradiation
(55). The altered calcium
gradient following UVB irradiation may affect the secretion of LBs
(20,56,57).
This may account for the suppression of LB release to the space
between the SC and granulosa, and the retention of LBs in the SC,
following the continuous UVB irradiation of hairless rats daily at
a dose of 40 mJ/cm2 or 0.5 × MED/day for 14–15 days
(39,45). This may contribute to the observed
abnormal lamellar membrane structures in the SC following UVB
irradiation.
Abundant evidence supports the hypothesis that
extra-cellular calcium regulates the progression of mammalian
epidermal differentiation. In human and murine keratinocytes, low
calcium concentrations stimulate proliferation, while high calcium
concentrations inhibit proliferation and enhance differentiation
(61). Moreover, it has been
demonstrated that UVB irradiation induces a variety of cutaneous
responses, including the induction of epidermal hyperplasia
(55). Thus, it is conceivable
that a critical level of cytosolic calcium is required for the
initiation of the differentiation events. Therefore, the increases
in the extracellular and cytosolic calcium levels are associated
with the changes in the epidermal proliferation and/or
differentiation process (55).
A study observed that following an increase in the
calcium concentration, the TGase 1 enzyme activated glutamine
residues (Gln107, Gln118, Gln122,
Gln133 and Gln496) of involucrin, with high
specificity (32). Since cornified
envelopes are present in the outer epidermis where the calcium
concentration is normally higher, TGase 1 functions well in such a
high calcium concentration. Therefore, the disruption of the
epidermal calcium gradient caused by UVB irradiation may further
downregulate TGase 1 enzyme activity, subsequently leading to the
disruption of the formation of covalently bound Cer.
Beneficial effects induced by UVB in the
epidermal barrier
Although narrowband UVB or UVA phototherapy is a
mainstay of treatment, natural and artificial UVB irradiation is
frequently employed in the treatment of atopic dermatitis (9,10).
Different skin types are affected differently by the same level of
UVB exposure. The MED of a fair-skinned (Fitzpatrick type I) person
is 10–25 mJ/cm2(62,63),
while individuals with darker skin have a higher MED (64). Therefore, in UVB treatment, the
skin type of the patient also determines the treatment dose. The
exposure dose is an important factor in determining the effects of
UVB exposure. UV-induced DNA damage and barrier disruption increase
linearly with increasing dosage (7).
High doses of UVB, such as a dose 4-fold higher than
the MED, are known to exert detrimental effects on permeability
barrier function (37,38,60).
By contrast, low-dose UV phototherapy has been demonstrated to be
useful for the treatment of a variety of skin disorders, including
psoriasis and atopic dermatitis (9,10,65).
An erythemal dose (≥1 × MED) may impair DNA repair mechanisms and
lead to cell elimination via apoptosis (66,67).
A study of hairless mice irradiated with a single dose of ∼1 × MED
UVB (75 mJ/cm2) showed significant disruption of the
barrier (38). A study of human
subjects irradiated with 0.7 × MED UVB for 10 consecutive days
revealed an unaltered expression of p53, a protein which is able to
activate DNA repair proteins when DNA has sustained damage. This
indicated that the repair systems were activated (67).
A study on hairless mice exposed to 0.5 × MED UVB
irradiation (40 mJ/cm2) daily for 14 days demonstrated a
significant reduction in the levels of covalently bound Cer and
disrupted multilamellar structures (39). By contrast, a separate study
concerning hairless mice irradiated with the same dose of UVB daily
for 3 days demonstrated no clinically evident inflammation or
barrier disruption (68). A dose
of 0.5 × MED UVB irradiation for 3 days, prior to tape-stripping,
resulted in significantly accelerated barrier recovery rates,
implying that repeated, short-term exposure to low-dose UVB
significantly accelerates the kinetics of barrier recovery
following acute insults (68).
The irradiation of hairless mice with a 0.5 × MED
dose of UVB for 3 days demonstrated the positive effects of UVB on
the epidermis, which, at least in part, were mediated by cutaneous
vitamin D3 activation (68). In parallel with the upregulation of
the cutaneous vitamin D3 system, there was an increase
in the mRNA levels for the epidermal lipid synthetic enzymes,
HMG-CoA, fatty acid synthase (FAS) and serine palmitoyl transferase
(SPT) (68). There was also an
upregulation of barrier-linked antimicrobial peptides (AMPs; LL-37
and hBD2) in the outer epidermis, which is considered to be
mediated by the cutaneous production of
1,25(OH)2D3, the most active form of vitamin
D3(111). Increases in the expression of involucrin and
filaggrin were also observed, without the concurrent development of
epidermal hyperplasia, implying that UVB may also regulate
epidermal differentiation (68).
1,25(OH)2D3 has been
demonstrated to increase the expression of a number of major
epidermal differentiation proteins, including involucrin, loricrin,
filaggrin and transglutaminase, as well as to stimulate cornified
envelope formation (68). A
suberythemal dose of UVB exposure is normally enough to generate
the synthesis of sufficient vitamin D3 to impact
downstream events in the epidermis (68). Another study on cultured human
keratinocytes showed that irradiation with a single 23
mJ/cm2 dose of UVB upregulated SPT activity, leading to
increased sphingolipid synthesis (69).
Conclusions
In addition to TEWL acting as an indicator of the
functional integrity of the SC, alterations in covalently bound Cer
and the epidermal calcium gradient are closely associated with the
disruption of the epidermal barrier.
A single high dose of UVB irradiation and
suberythemal doses of UVB irradiation for 14 days have been
demonstrated to exert negative effects on the epidermal barrier,
leading to barrier disruption. By contrast, a low dose of UVB
irradiation and suberythemal doses of UVB irradiation for 3 days
have been demonstrated to exert positive effects on the epidermal
barrier, without clinically evident inflammation or barrier
disruption.
The present review therefore shows that, despite the
known harmful effects, UVB irradiation may exert positive effects
in the epidermal barrier when administered in low doses and over a
relatively short period. This may be a useful therapeutic strategy
for the use of UVB irradiation in the treatment of skin diseases
with a disrupted epidermal barrier, such as atopic dermatitis,
while reducing or avoiding the possible side effects. Further
studies are required to determine the efficacy of low doses of UVB
irradiation on the skin of patients with atopic dermatitis.
Acknowledgements
This study was supported by grants
from the China National Natural Science Foundation (grant nos.
81000700 and 81171518).
References
|
1.
|
Herrling T, Jung K and Fuchs J:
Measurements of UV-generated free radicals/reactive oxygen species
(ROS) in skin. Spectrochim Acta A Mol Biomol Spectrosc. 63:840–845.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Haywood R, Rogge F and Lee M: Protein,
lipid, and DNA radicals to measure skin UVA damage and modulation
by melanin. Free Radic Biol Med. 44:990–1000. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Bak H, Hong SP, Jeong SK, et al: Altered
epidermal lipid layers induced by long-term exposure to
suberythemal-dose ultraviolet. Int J Dermatol. 50:832–837. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
McAuliffe DJ and Blank IH: Effects of UVA
(320–400 nm) on the barrier characteristics of the skin. J Invest
Dermatol. 96:758–762. 1991.
|
|
5.
|
Abe T and Mayuzumi J: The change and
recovery of human skin barrier functions after ultraviolet light
irradiation. Chem Pharm Bull (Tokyo). 27:458–462. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Solomon AE and Lowe NJ: Percutaneous
absorption in experimental epidermal disease. Br J Dermatol.
100:717–722. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Lamaud E and Schalla W: Influence of UV
irradiation on penetration of hydrocortisone. In vivo study in
hairless rat skin. Br J Dermatol. 111(Suppl 27): S152–S157. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Valkova S and Velkova A: UVA/UVB
phototherapy for atopic dermatitis revisited. J Dermatolog Treat.
15:239–244. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Jekler J and Larkö O: UVB phototherapy of
atopic dermatitis. Br J Dermatol. 119:697–705. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Wulf HC and Bech-Thomsen N: A UVB
phototherapy protocol with very low dose increments as a treatment
of atopic dermatitis. Photodermatol Photoimmunol Photomed. 14:1–6.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Elias PM: The stratum corneum as an organ
of protection: old and new concepts. Curr Probl Dermatol. 18:10–21.
1989.PubMed/NCBI
|
|
12.
|
Feingold KR: Thematic review series: skin
lipids. The role of epidermal lipids in cutaneous permeability
barrier homeostasis. J Lipid Res. 48:2531–2546. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Proksch E, Brandner JM and Jensen JM: The
skin: an indispensable barrier. Exp Dermatol. 17:1063–1072. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Nilsson GE: Measurement of water exchange
through skin. Med Biol Eng Comput. 15:209–218. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Pinnagoda J, Tupker RA, Agner T and Serup
J: Guidelines for transepidermal water loss (TEWL) measurement. A
report from the Standardization Group of the European Society of
Contact Dermatitis. Contact Dermatitis. 22:164–178. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Elias PM and Feingold KR: Skin Barrier.
Taylor & Francis; New York: 2006
|
|
17.
|
Wertz PW: Epidermal lipids. Semin
Dermatol. 11:106–113. 1992.
|
|
18.
|
Rassner U, Feingold KR, Crumrine DA and
Elias PM: Coordinate assembly of lipids and enzyme proteins into
epidermal lamellar bodies. Tissue Cell. 31:489–498. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Menon GK, Grayson S and Elias PM: Ionic
calcium reservoirs in mammalian epidermis: ultrastructural
localization by ion-capture cytochemistry. J Invest Dermatol.
84:508–512. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Menon GK, Elias PM, Lee SH and Feingold
KR: Localization of calcium in murine epidermis following
disruption and repair of the permeability barrier. Cell Tissue Res.
270:503–512. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Menon GK and Elias PM: Ultrastructural
localization of calcium in psoriatic and normal human epidermis.
Arch Dermatol. 127:57–63. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Freinkel RK and Traczyk TN: Lipid
composition and acid hydrolase content of lamellar granules of
fetal rat epidermis. J Invest Dermatol. 85:295–298. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Grayson S, Johnson-Winegar AG, Wintroub
BU, Isseroff RR, Epstein EH Jr and Elias PM: Lamellar body-enriched
fractions from neonatal mice: preparative techniques and partial
characterization. J Invest Dermatol. 85:289–294. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Wertz PW, Downing DT, Freinkel RK and
Traczyk TN: Sphingolipids of the stratum corneum and lamellar
granules of fetal rat epidermis. J Invest Dermatol. 83:193–195.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Holleran WM, Ginns EI, Menon GK, et al:
Consequences of beta-glucocerebrosidase deficiency in epidermis.
Ultrastructure and permeability barrier alterations in Gaucher
disease. J Clin Invest. 93:1756–1764. 1994. View Article : Google Scholar
|
|
26.
|
Holleran WM, Takagi Y, Menon GK, Legler G,
Feingold KR and Elias PM: Processing of epidermal glucosylceramides
is required for optimal mammalian cutaneous permeability barrier
function. J Clin Invest. 91:1656–1664. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Hedberg CL, Wertz PW and Downing DT: The
time course of lipid biosynthesis in pig epidermis. J Invest
Dermatol. 91:169–174. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Swartzendruber DC, Wertz PW, Madison KC
and Downing DT: Evidence that the corneocyte has a chemically bound
lipid envelope. J Invest Dermatol. 88:709–713. 1987. View Article : Google Scholar
|
|
29.
|
Wertz PW, Madison KC and Downing DT:
Covalently bound lipids of human stratum corneum. J Invest
Dermatol. 92:109–111. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Marekov LN and Steinert PM: Ceramides are
bound to structural proteins of the human foreskin epidermal
cornified cell envelope. J Biol Chem. 273:17763–17770. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Behne M, Uchida Y, Seki T, de Montellano
PO, Elias PM and Holleran WM: Omega-hydroxyceramides are required
for corneocyte lipid envelope (CLE) formation and normal epidermal
permeability barrier function. J Invest Dermatol. 114:185–192.
2000. View Article : Google Scholar
|
|
32.
|
Nemes Z, Marekov LN, Fésüs L and Steinert
PM: A novel function for transglutaminase 1: attachment of
long-chain omega-hydroxyceramides to involucrin by ester bond
formation. Proc Natl Acad Sci USA. 96:8402–8407. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Fitzpatrick TB, Eisen AZ, Wolff K,
Freedburg IM and Austen KF: Dermatology in General Medicine. 4th
edition. McGraw-Hill, Health Professions Division; New York:
1993
|
|
34.
|
Cole CA, Forbes PD and Davies RE: An
action spectrum for UV photocarcinogenesis. Photochem Photobiol.
43:275–284. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Sterenborg HJ, de Gruijl FR, Kelfkens G
and van der Leun JC: Evaluation of skin cancer risk resulting from
long term occupational exposure to radiation from ultraviolet
lasers in the range from 190 to 400 nm. Photochem Photobiol.
54:775–780. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Bissett DL, Hannon DP and Orr TV:
Wavelength dependence of histological, physical, and visible
changes in chronically UV-irradiated hairless mouse skin. Photochem
Photobiol. 50:763–769. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Haratake A, Uchida Y, Schmuth M, et al:
UVB-induced alterations in permeability barrier function: roles for
epidermal hyperproliferation and thymocyte-mediated response. J
Invest Dermatol. 108:769–775. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Takagi Y, Nakagawa H, Kondo H, Takema Y
and Imokawa G: Decreased levels of covalently bound ceramide are
associated with ultraviolet B-induced perturbation of the skin
barrier. J Invest Dermatol. 123:1102–1109. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Meguro S, Arai Y, Masukawa Y, Uie K and
Tokimitsu I: Relationship between covalently bound ceramides and
transepidermal water loss (TEWL). Arch Dermatol Res. 292:463–468.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Bernerd F and Asselineau D: Successive
alteration and recovery of epidermal differentiation and
morphogenesis after specific UVB-damages in skin reconstructed in
vitro. Dev Biol. 183:123–138. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Hirao T, Denda M and Takahashi M:
Identification of immature cornified envelopes in the
barrier-impaired epidermis by characterization of their
hydrophobicity and antigenicities of the components. Exp Dermatol.
10:35–44. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Proksch E, Holleran WM, Menon GK, Elias PM
and Feingold KR: Barrier function regulates epidermal lipid and DNA
synthesis. Br J Dermatol. 128:473–482. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Grubauer G, Elias PM and Feingold KR:
Transepidermal water loss: the signal for recovery of barrier
structure and function. J Lipid Res. 30:323–333. 1989.
|
|
44.
|
Menon GK, Feingold KR, Mao-Qiang M,
Schaude M and Elias PM: Structural basis for the barrier
abnormality following inhibition of HMG CoA reductase in murine
epidermis. J Invest Dermatol. 98:209–219. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Meguro S, Arai Y, Masukawa K, Uie K and
Tokimitsu I: Stratum corneum lipid abnormalities in UVB-irradiated
skin. Photochem Photobiol. 69:317–321. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Wertz PW and Downing DT: Covalently bound
omega-hydroxyacylsphingosine in the stratum corneum. Biochim
Biophys Acta. 917:108–111. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Abraham W and Downing DT: Interaction
between corneocytes and stratum corneum lipid liposomes in vitro.
Biochim Biophys Acta. 1021:119–125. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
48.
|
Chang F, Swartzendruber DC, Wertz PW and
Squier CA: Covalently bound lipids in keratinizing epithelia.
Biochim Biophys Acta. 1150:98–102. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
49.
|
Takagi Y, Nakagawa H, Yaginuma T, Takema Y
and Imokawa G: An accumulation of glucosylceramide in the stratum
corneum due to attenuated activity of beta-glucocerebrosidase is
associated with the early phase of UVB-induced alteration in
cutaneous barrier function. Arch Dermatol Res. 297:18–25. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
50.
|
Haratake A, Uchida Y, Mimura K, Elias PM
and Holleran WM: Intrinsically aged epidermis displays diminished
UVB-induced alterations in barrier function associated with
decreased proliferation. J Invest Dermatol. 108:319–323. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
51.
|
Marsh NL, Elias PM and Holleran WM:
Glucosylceramides stimulate murine epidermal hyperproliferation. J
Clin Invest. 95:2903–2909. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
52.
|
Takagi Y, Kriehuber E, Imokawa G, Elias PM
and Holleran WM: Beta-glucocerebrosidase activity in mammalian
stratum corneum. J Lipid Res. 40:861–869. 1999.PubMed/NCBI
|
|
53.
|
Hamanaka S, Hara M, Nishio H, Otsuka F,
Suzuki A and Uchida Y: Human epidermal glucosylceramides are major
precursors of stratum corneum ceramides. J Invest Dermatol.
119:416–423. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
54.
|
Uchida Y, Hara M, Nishio H, et al:
Epidermal sphingomyelins are precursors for selected stratum
corneum ceramides. J Lipid Res. 41:2071–2082. 2000.PubMed/NCBI
|
|
55.
|
Jiang SJ, Chu AW, Lu ZF, Pan MH, Che DF
and Zhou XJ: Ultraviolet B-induced alterations of the skin barrier
and epidermal calcium gradient. Exp Dermatol. 16:985–992. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
56.
|
Lee SH, Elias PM, Proksch E, Menon GK,
Mao-Quiang M and Feingold KR: Calcium and potassium are important
regulators of barrier homeostasis in murine epidermis. J Clin
Invest. 89:530–538. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
57.
|
Menon GK, Elias PM and Feingold KR:
Integrity of the permeability barrier is crucial for maintenance of
the epidermal calcium gradient. Br J Dermatol. 130:139–147. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
58.
|
Lee SH, Choi EH, Feingold KR, Jiang S and
Ahn SK: Iontophoresis itself on hairless mouse skin induces the
loss of the epidermal calcium gradient without skin barrier
impairment. J Invest Dermatol. 111:39–43. 1998. View Article : Google Scholar
|
|
59.
|
Menon GK, Price LF, Bommannan B, Elias PM
and Feingold KR: Selective obliteration of the epidermal calcium
gradient leads to enhanced lamellar body secretion. J Invest
Dermatol. 102:789–795. 1994. View Article : Google Scholar
|
|
60.
|
Holleran WM, Uchida Y, Halkier-Sorensen L,
et al: Structural and biochemical basis for the UVB-induced
alterations in epidermal barrier function. Photodermatol
Photoimmunol Photomed. 13:117–128. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
61.
|
Hennings H, Michael D, Cheng C, Steinert
P, Holbrook K and Yuspa SH: Calcium regulation of growth and
differentiation of mouse epidermal cells in culture. Cell.
19:245–254. 1980. View Article : Google Scholar
|
|
62.
|
Taylor SC: Skin of color: biology,
structure, function, and implications for dermatologic disease. J
Am Acad Dermatol. 46(Suppl): S41–S62. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
63.
|
Pathak MA, Nghiem P and Fitzpatrick TB:
Acute and chronic efects of the sun. Fitzpatrick’s Dermatology in
General Medicine. Freedberg IM, Eisen AZ, Wolff K, Austen LA,
Goldsmith K, Katz SI and Fitzpatrick TB: 1. 5th edition.
McGraw-Hill; New York: pp. 1598–1607. 1999
|
|
64.
|
Rigel EG, Lebwohl M, Rigel AC and Rigel
DS: Daily UVB exposure levels in high-school students measured with
digital dosimeters. J Am Acad Dermatol. 49:1112–1114. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
65.
|
Leenutaphong V, Nimkulrat P and Sudtim S:
Comparison of phototherapy two times and four times a week with low
doses of narrow-band ultraviolet B in Asian patients with
psoriasis. Photodermatol Photoimmunol Photomed. 16:202–206. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
66.
|
Murphy M, Mabruk MJ, Lenane P, et al:
Comparison of the expression of p53, p21, Bax and the induction of
apoptosis between patients with basal cell carcinoma and normal
controls in response to ultraviolet irradiation. J Clin Pathol.
55:829–833. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
67.
|
Narbutt J, Norval M, Slowik-Rylska M, et
al: Suberythemal ultraviolet B radiation alters the expression of
cell cycle-related proteins in the epidermis of human subjects
without leading to photoprotection. Br J Dermatol. 161:890–896.
2009. View Article : Google Scholar
|
|
68.
|
Hong SP, Kim MJ, Jung MY, et al:
Biopositive effects of low-dose UVB on epidermis: coordinate
upregulation of antimicrobial peptides and permeability barrier
reinforcement. J Invest Dermatol. 128:2880–2887. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
69.
|
Farrell AM, Uchida Y, Nagiec MM, et al:
UVB irradiation up-regulates serine palmitoyltransferase in
cultured human keratinocytes. J Lipid Res. 39:2031–2038. 1998.
|