Introduction
Echinococcosis, also known as hydatid disease, is a
type of zoonotic parasitic disease caused by the
Echinococcus larvae infection. This disease is severely
harmful to both human and animal health. The disease has a
worldwide distribution, being identified in Asia, Africa, Europe,
and North America (1,2). The two types of Echinococcus
that may cause echinococcosis in humans are Echinococcosis
granulosus (Eg) and Echinococcus multilocularis (Em). A
high incidence of hydatid disease has been identified in China,
with primary hydatid disease being identified in 25 regions,
including provinces, municipalities and autonomous regions
(3). The disease is predominantly
endemic in pasture areas including Xinjiang, Qinghai, Gansu and
Ningxia and semi-pasture areas. According to the incomplete
statistics obtained for a number of provinces experiencing an
epidemic of this disease, >50 million people were threatened by
Echinococcus granulosus infection and ~50–60 million people
were treated for echinococcosis (4). Current treatments for echinococcosis
including preventive measures and surgery in combination with
chemotherapy are ineffective. Thus efforts have been made to
develop more effective prevention and treatment measures.
Consequently, with the development of molecular biotechnology,
molecular vaccine prevention against echinococcosis has become an
ideal method of treating hydatid disease (5,6).
The research and development involved in identifying
an epitope vaccine is an extremely difficult but highly targeted
technology which comprehensively utilizes the technology of
molecular biology and immunology. A key step in the preparation of
the epitope vaccine involves the identification of and obtaining
data pertaining to the epitope. Traditional epitope screening
methods, including mass spectrum technology, nuclear magnetic
resonance technology and the enzymatic method, are time-consuming,
laborious and costly contributing to the low feasibility of these
methods. Advances in technology has resulted in epitope prediction
with bioinformatics technology becoming a more convenient and
viable method. Prediction using various parameters and methods has
greately improved the accuracy of epitope prediction. The epitope
peptide vaccine has also been used in immune prevention against
viruses (7,8). The epitope vaccine is a synthesized
polypeptide based on the information of B- and T-cell epitopes.
Utilization of these vaccines may protect the body against specific
pathogens by activating B cells to produce specific antibodies.
These vaccines potentially activate the cytotoxic T lymphocytes
(CD8+ T cells) to eliminate virus-infected cells
(9,10). In addition, CD4+ T cells
may be activated to mediate humoral immune response.
The wide application of immunology, genetic
engineering, protein engineering, synthetic peptide technology,
peptide library technology, biophysical techniques, immunoassay
technology and computer technology have resulted in the
identification of additional antigen epitopes. Lightowlers et
al(11,12) demonstrated that sheep (intermediate
host) immunized with the Eg95 recombinant protein vaccine were
protected against oncosphere infection. The immune protective
effect was ≤95–100%, of which 86% were fully protected. These data
suggest that Eg95 is an ideal protective antigen. In a previous
study (13), Eg95 gene was
identified as a candidate gene in Echinococcus with high
antigenicity. Additionally, the secondary structure of Eg95 had
many random coils, which indicated a high flexibility. The regions
with a high flexibility were potential epitope regions. Previously,
we also predicted the T- and B-cell epitopes of Eg95. In this
study, in order to examine the antigenicity of Eg95, we initially
predicted its tertiary structure. Based on the T/B epitope
information obtained as well as the tertiary structure, the T- and
B-combined epitopes of Eg95 were analyzed. The results of the
present study therefore provided additional evidence on the epitope
information of Eg95.
Materials and methods
Reagents and materials
TRIzol was purchased from Invitrogen (Carlsbad, CA,
USA). DL2000 DNA marker, λ HindIII, BamIII,
SacI, NotI, Taq enzyme and T4 DNA ligase were
purchased from Takara Bio, Inc. (Shiga, Japan). AMV First Strand
cDNA Synthesis kit and pUCm-T were purchased from MBI. UNIQ-10 mini
plasmid extraction kit, UNIQ-10 column DNA gel extraction kit,
X-gal, IPTG, LB medium (liquid and solid) and ampicillin were
purchased from Sangon, Shanghai, China.
E. coli DH5α was provided by the
Echinococcosis Institute of the First Affiliated Hospital of
Xinjiang Medical University.
Echinococcus protoscolex collection
Echinococcus protoscolex were collected from
fresh goat liver infected with Echinococcus. Briefly,
Echinococcus cyst fluid was taken with a sterile syringe and
placed in a sterile beaker. The protoscolex were then allowed to
naturally precipitate. After rinsing three times with sterile
saline the collected protoscolex were stored at −80°C prior to
analysis.
Reverse-transcribed PCR (RT-PCR)
Echinococcus protoscolex were ground in
liquid nitrogen prior to RNA extraction. Total RNAs were extracted
by TRIzol reagents according to the manufacturer’s instructions and
dissolved in 50 μl DEPC water. Then, 5 μl were run on a 1.2%
MOPS-formaldehyde denaturing gel. Eg95 cDNA was reversed
transcribed using the AMV of First Strand cDNA Synthesis kit
following the manufacturer’s instructions. Briefly, 1 μl of total
RNA was reverse transcribed into cDNA in 20 μl reaction mixtures
containing 200 units of Moloney murine leukemia virus reverse
transcriptase, 1 μl per reaction of oligo(dT)18 primers, and 0.5 mM
each of dNTPs, dATP, dCTP, dGTP, and dTTP. The reaction mixture was
then incubated at 42°C for 1 h and at 70°C for 5 min to deactivate
the reverse transcriptase.
Cloning of Eg95 gene by PCR
The Eg95 gene was cloned using the
protoscolex cDNA. The primer pairs were designed by DNAman software
and synthesized by Sangon. Primer sequences used were: upstream
(P1): 5′-ATGGCATTCCAG TTATGTCTC-3′ and downstream (P2):
5′-TCACATTACA GTGCTTTCCTTCTTGC-3′. Amplification of Eg95 was
conducted in a 20 μl mixture of 1 μl cDNA template, 2 μl 10X
buffer, 0.5 μl of each primer, 0.5 μl of 10 mM dNTP, 0.5 μl
Taq enzyme and 15.5 μl pure water. PCR reaction was carried
out according to the following procedures: initial denaturation at
95°C for 6 min, then 30 consecutive cycles of denaturation at 95°C
for 30 sec, annealing at 55°C for 30 sec, extension at 72°C for 2
min, and a final extension at 72°C for 10 min. The PCR products
were detected by electrophoresis on a 1.2% agarose gel.
Construction of recombinant plasmid
pUCm-T/Eg95
After purification, PCR products were cloned into
the pUCm-T vector. The ligation system was conducted in a 10 μl
mixture of 2 μl PCR product, 0.5 μl pUCm-T vector, 1 μl 10X
ligation buffer, 0.5 μl T4 DNA ligase and 6 μl de-ionized water.
After ligation at 16°C for 16 h, the ligation product was
transformed into E. coli DH5α competent cells. For clone
screening, ampicillin LB plates were pre-treated with IPTG and
X-Gal. After overnight incubation at 37°C, the positive white
colonies were selected and incubated for PCR identification. The
correct recombination plasmid was confirmed by sequencing and
blasting.
Tertiary structure prediction and
analysis
Predictive analysis of the Eg95 protein tertiary
structure was conducted by online server 3DLigandsite (http://www.sbg.bio.ic.ac.uk/~3dligandsite/) and the
iterative-TASSER (I-TASSER). RasMol version 2.7.5.2 software was
used to analyze different modes of the tertiary structure. The
tertiary structure was displayed in the modes of Cartoon, Structure
and Group.
T- and B-combined epitope prediction and
analysis
The B-cell epitope prediction software included
DNAStar (V5.0) (http://www.dnastar.com) and the online prediction
software IEDB (http://tools.immuneepitope.org/main/index.html). The
T-cell epitope prediction software included SYFPEITHI (http://www.syfpeithi.de) and BIMAS (http://bimas.dcrt.nihgov/molbiothe/hla_bind/).
Results
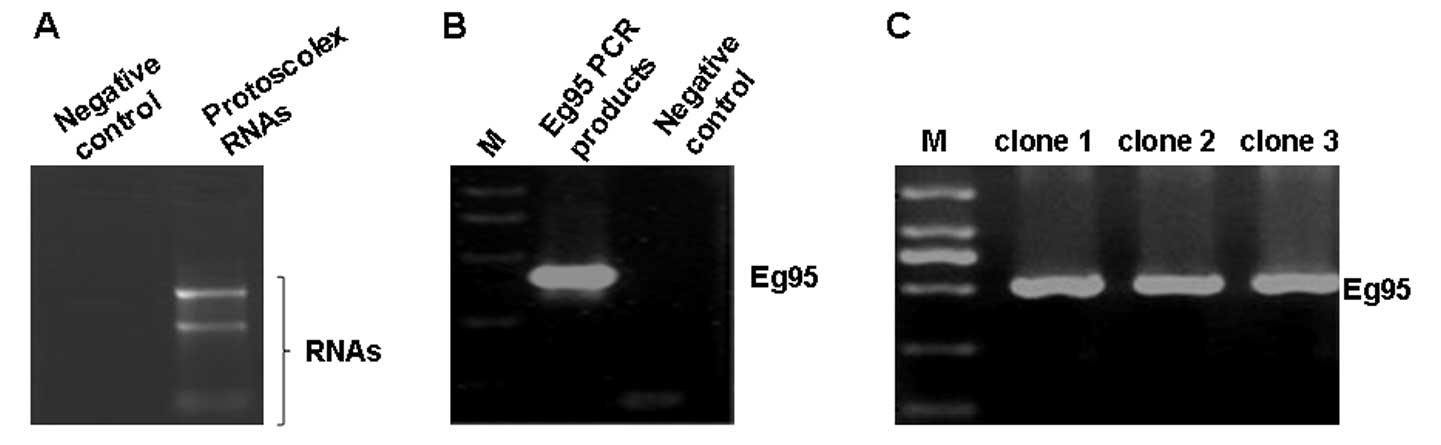
Total RNAs were successfully extracted
from Echinococcus protoscolex
In order to check the quality of the extracted RNA,
total RNAs were analyzed on 1.2% MOPS-formaldehyde denaturing gel.
The electrophoresis results are shown in Fig. 1A. On the gel, two bands were
evident: The lower band was extremely smeared, while the upper band
was much clearer. RNA absorbance of the upper band was measured at
260 and 280 nm. The value of A260/A280 was 1.81, indicating a good
purity of RNA. This result suggests that total RNAs were extracted
with good purity and could be used for subsequent analysis.
Successful amplification of the Eg95
gene
A PCR assay was performed to clone the Eg95
gene. Firstly the extracted RNAs were reversed transcribed into
cDNA by RT-PCR. cDNA was subsequently used as a template to perform
PCR. The PCR products were verified by gel electrophoresis. As
shown in Fig. 1B, in the
Eg95 PCR products, a specific band of ~500 bp was identified
while no such band was found in the negative control group. In
theory, the gene length of Eg95 was 471 bp. The length of
this PCR fragment was close to that of the Eg95 gene. This
result indicates that a specific PCR fragment was amplified from
the cDNA. However, whether the sequence of corresponds to the
sequence of Eg95 gene remains to be verified.
Successful construction of the
recombinant plasmid pUCm-T/Eg95
To evaluate the sequence of the amplified
Eg95 gene, we initially cloned the Eg95 gene into the
pUCm-T vector and the gene was screened using blue-white selection.
A PCR assay was performed with the white clones to identify
correctly constructed plasmids. Three white colonies were randomly
selected for PCR analysis. As shown in Fig. 1C, all three colonies contained the
Eg95 gene fragment, demonstrating that the plasmid was
successfully constructed.
Recombinant plasmid was sequenced and the sequence
was compared to the Eg95 gene sequence (Genebank accession
no. HM345607) in BLAST. The alignment result showed that the
sequence in the recombinant plasmid was correct.
Therefore, the Eg95 gene was correctly
amplified and the pUCm-T/Eg95 plasmid was successfully
constructed.
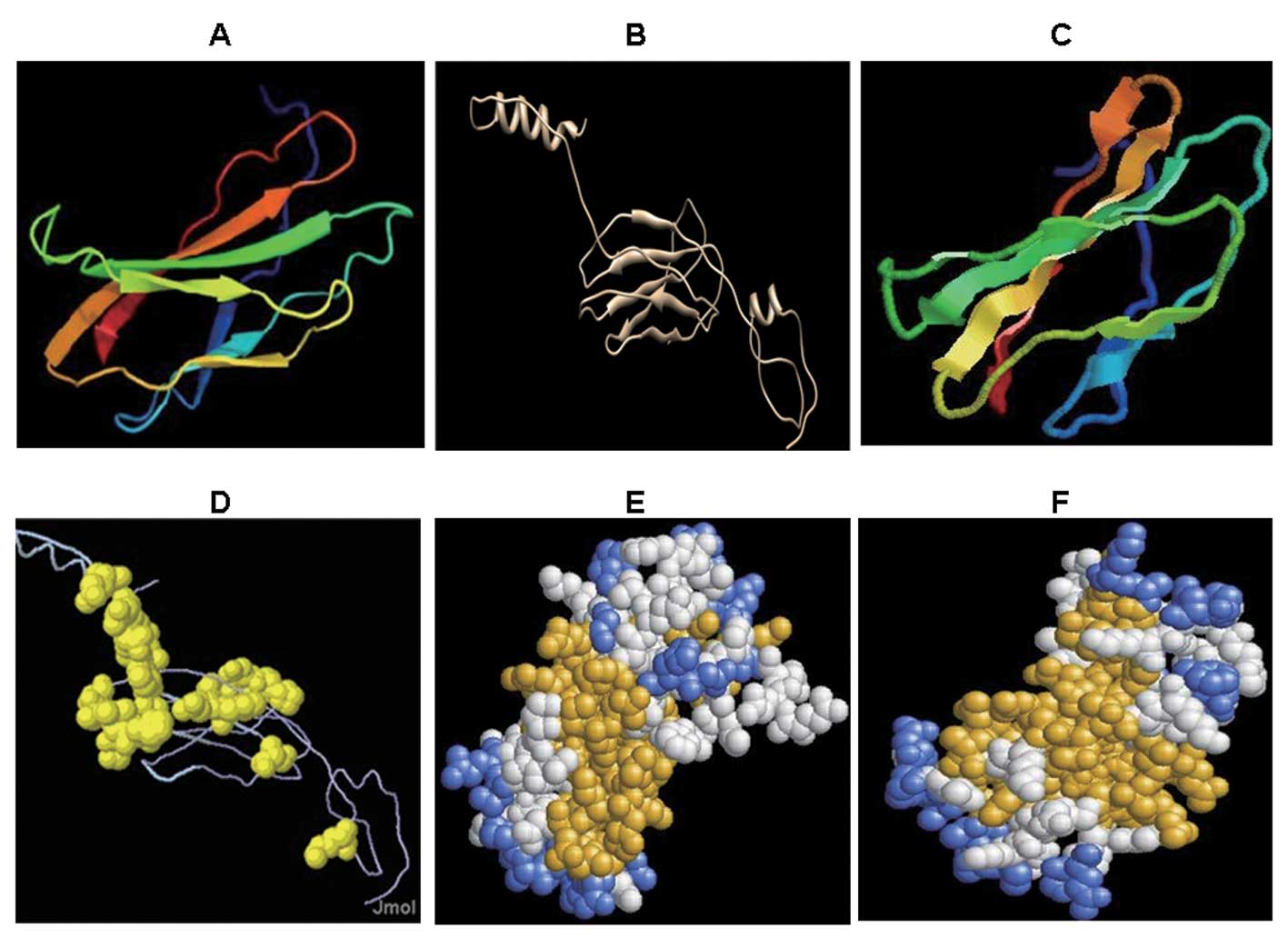
Tertiary structure prediction results of
Eg95 protein
In a previous study (13), we predicted the secondary structure
of the Eg95 protein. The secondary structure indicated high
flexibility and scalability of the Eg95 antigen. To determine the
conformational structure of the Eg95 antigen, in the present study
we predicted its tertiary structure. Fig. 2A was analyzed by the 3DLigandsite
server. The tertiary structure of Eg95 comprised α helixes (shown
by the curved lines) and β folds (shown by the laminated
structures).
To gain a better understanding of its conformational
structure, the tertiary structure of Eg95 was analyzed by I-TASSER.
In I-TASSER, the threading method and homology modeling method were
used. Subsequent to minor adjustments by the Swiss PDB viewer
(SPDBV) software, the tertiary structure predicted by I-TASSER were
identified (Fig. 2B). In the N-
and C-terminal of the protein structure, a number of α helixes were
identified. These structures showed that the Eg95 protein had good
spatial flexibility and scalability. β folds similar to FN3 domain
were predominantly evident in the middle. These β folds were
connected by random coils and randomly folded into the
conformational structure. Furthermore, the majority of these random
coils contained >5 amino acid residues. Therefore, the β fold
regions were the potential positions of the epitope.
Mode display of Eg95 tertiary
structure
Different modes were utilized to present the 3D
structure of the protein. The tertiary structure predicted by
3DLigandsite was then analyzed by RasMol version 2.7.5.2. The
tertiary structure was presented in Cartoon, Structure and Group
modes, respectively (Fig. 2C–F).
The Cartoon mode (Fig. 2C) was
similar to that in Fig. 2A, with
flexible regions in the laminated structures of the protein. In the
Structure mode (Fig. 2D), the
amino acids in the yellow area corresponded to the flexible region
(flexible areas are easy to fold and to form antigen epitopes) in
the secondary structure. The front and back view of the Group mode
(aggregation mode) showed that the yellow region was gathered and
distributed substantially at the surface of the structure (Fig. 2E and F). When an antibody binds an
antigen, the antigen epitope needs to be fully exposed to
facilitate better binding. Thus we speculate that the yellow area
in this mode is the antigen epitope that potentially binds to the
antibody.
T- and B-combined epitope prediction
results of Eg95 antigen
Based on the tertiary structure of Eg95 protein, we
predicted the potential T- and B-combined epitopes of Eg95 antigen.
The T-cell epitope was predicted by the SYFPEITHI and BIMAS
software, while the B-cell epitope was predicted by software
included DNAStar (V5.0) (http://www.dnastar.com) and the online prediction
software IEDB. The prediction results are shown in Table I, with the T epitopes in bold. The
overlapping regions of T- and B-cell epitopes formed the T- and
B-combined epitopes. Four and six T- and B-combined epitopes were
of human and mouse origin, respectively. Additionally, four T- and
B-combined epitopes were of both human and mouse origin. Taken
together, these results clearly demonstrate the specific regions of
these T- and B-combined epitopes in the Eg95 antigen, further
providing experimental data for epitope vaccine development.
 | Table IThe T-B cell epitope prediction of
human and mouse. |
Table I
The T-B cell epitope prediction of
human and mouse.
| Epitope orign | Epitope regions | Epitope
sequences |
|---|
| Human | 14–38 |
VLAQEYKGVGKGQGQQETPLRNHFN |
| 48–67 |
RLSWEVQHLSDLKGTDISLR |
| 92–113 |
GELKPSTLYKMTVEAVKAKKTI |
| 120–135 |
IETPRAGKKESTVMTS |
| Mouse | 16–38 |
AQEYKGVGKGQGQQETPLRNHFN |
| 34–48 |
RNHFNLTPVGSQGIR |
| 50–72 |
SWEVQHLSDLKGTDISLRAVNPS |
| 67–90 |
RAVNPSDPLVCKRQTAKFSDGQLA |
| 103–113 |
LGFTVDIETPR |
| 120–132 |
TVMTSGSALTSAI |
| Human and mouse | 14–38 |
VLAQEYKGVGKGQGQQETPLRNHFN |
| 48–72 |
RLSWEVQHLSDLKGTDISLRAVNPS |
| 92–113 |
GELKPSTLYKMTVEAVKAKKTI |
| 120–135 |
IETPRAGKKESTVMTS |
Discussion
In our previous study (13), we predicted the secondary structure
of Eg95. In order to gain a better understanding of the spatial
structure of the Eg95 protein, we predicted its tertiary structure
in the present study. Based on the T/B epitope information
obtained, as well as the tertiary structure, we analyzed the T- and
B-combined epitopes of Eg95. The results demonstrate the
information of the potential epitopes for Eg95 in detail.
Methods employed for tertiary structure prediction
include the homology modeling, the threading and the ab initio
prediction methods. The first two are also referred to as
template-based methods. Based on the Swiss model, we conducted
homology modeling with Eg95 protein. The similarity between these
two methods was 16.39%. The Qmean Z-score was only −5.72, while the
model similarity was <30%. Of note, the predicted Qmean Z-score
was extremely low. Subsequently, the threading method was employed.
The modeling accuracy via combination of the homology modeling and
threading method was much higher than that of the Swiss Model and
phyre. The three-dimensional structure is modeled based on the
multiple-threading alignments of LOMETS and I-TASSER. I-TASSER
usually provides the first and most reliable template parameters,
with the C-score being the confidence assessment for the quality of
prediction model. The calculation method is based on the parameters
of thread form alignment and analog assembly parameters. The C
scores usually range from −5 to 2, the higher the score a model
yields, the higher the credibility. The TM score and
root-mean-square deviation (RMSD) value are used to measure the
similarity between the two structures. When the natural structure
of a protein is unknown, they became crucial indicators of the
quality of the modeling. The similarity between the two structures
is measured by the TM value (14).
Usually, a TM value of >0.5 shows a correct topological model,
while that of <0.17 induces a random similarity model. Following
submission of the Eg95 protein sequence (GenBank Accession no.
ADJ94888.1) to the I-TASSER, a tertiary structure of Eg95 protein
was predicted. In the present study the C-score was −2.17, TM-score
was 0.46±0.15 and Exp.RMSD was 9.4±4.6. In general, the TM-score
>0.5 is considered meaningful. The protein tertiary structure
prediction results were based on the crystal diffraction model in
the protein data bank library (?). Protein crystal diffraction
models of multiple proteins are being explored and the database is
constantly updated. Through bioinformatics prediction, only the
tertiary structure that is closest to its natural conformation can
be accessed. Subsequently, the conformational structure may be
comprehensively analyzed via the phage display peptide library in
combination with spatial epitope prediction.
T cells are able to recognize linear epitopes
presented by antigen-presenting cells, while B cells are capable of
identifying linear and/or conformational epitopes. Accordingly
epitopes can be divided into T- and B-cell epitopes. The T- and
B-combined epitopes are epitopes that can be co-recognized by both
T and B cells. This co-recognization is of great significance for
the effective removal of pathogens. Combined analysis and
calculation of the antigenic T- and B-cell epitopes are able to
screen out the overlapping areas in the two epitopes. In theory,
epitopes that contain both T- and B-cell epitopes may have an
advantage in immune response. The epitopes (antigenic determinants)
are usually located on the surface of the molecule and have good
hydrophilicity and ductility. If the specific regions of these
epitopes were to be identified, the epitope fragments could be
cloned into vectors to construct plasmids. Thus these plasmids
potentially carry epitope fragments of Echinococcus that may
induce a strong immune response (15–18).
Yang et al(19) chemically
synthesized T-cell epitope genes (sequences, 21–40 peptides) of
protein VP1 of O-type foot-and-mouth disease virus (FMDV). The
T-cell epitope, B-cell epitope (sequences, 141–160 peptides) and
the β2-galactosidase genes were combined together to construct a
recombinant plasmid. This recombinant plasmid was expressed in
E. coli to generate the fusion protein vaccine. This vaccine
was subsequently used to immunize animals. The results showed that
the immune response induced by the peptide vaccine containing T-B
joint epitopes was 7-fold higher than that by the peptide vaccine
containing the B-cell epitope only. Additionally, the T-B joint
epitope vaccine has been used in immune prevention against virus
attacks. These data indicated that the T-B joint epitope vaccine
simultaneously stimulated the humoral and cellular immune response
and thus may have a strong protective effect. Therefore, the
epitope vaccine designed based on the T- and B-combined epitope was
an effective method in genetically engineered vaccine design, with
potentially beneficial applications (20,21).
In this study, we analyzed the human and mouse T- and B-combined
epitopes of Echinococcus. In conclusion, future studies
should focus on investigating the potential immune protective
effects of these epitopes to develop epitope vaccines for mice
immunization.
Acknowledgements
This study was supported by National Natural Science
Foundation (no. 31000411, 30901374, 81160378, 81060135, 31160194
and 30860263) and University Scientific Research Project of
Education Department of Xinjiang Autonomous Region (XJEDU2010S25
and XYDXK50780328).
References
|
1
|
Eckert J, Conraths FJ and Tackmann K:
Echinococcosis: an emerging or re-emerging zoonosis? Int J
Parasitol. 30:1283–1294. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nunnari G, Pinzone MR, Gruttadauria S, et
al: Hepatic echinococcosis: clinical and therapeutic aspects. World
J Gastroenterol. 18:1448–1458. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jia HY, Ding JB and Fu YC: Molecular
characteristics of Echinococcus EgA31 vaccine. Chin J
Parasit Dis. 3:552–555. 2008.(In Chinese).
|
|
4
|
Ye EJ, Su LT and Jiang L: Research
progress of hydatid disease prevention and control. Chin J
Parasitol and Parasitic Dis. 18:179–181. 2000.(In Chinese).
|
|
5
|
Dalton JP and Mulcahy G: Parasite vaccines
- a reality? Vet Parasitol. 98:149–167. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lightowlers MW, Flisser A, Gauci CG, Heath
DD, Jensen O and Rolfe R: Vaccination against cysticercosis and
hydatid disease. Parasitol Today. 16:191–196. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kaba SA, McCoy ME, Doll TA, et al:
Protective antibody and CD8(+) T-cell responses to the
Plasmodium falciparum circumsporozoite protein induced by a
nanoparticle vaccine. PLoS One. 7:e483042012.
|
|
8
|
de Sousa EM, da Costa AC, Trentini MM, de
Araújo Filho JA, Kipnis A and Junqueira-Kipnis AP: Immunogenicity
of a fusion protein containing immunodominant epitopes of Ag85C,
MPT51, and HspX from Mycobacterium tuberculosis in mice and
active TB infection. PLoS One. 7:e477812012.PubMed/NCBI
|
|
9
|
Zhang W, Li X, Lin Y and Tian D:
Identification of three H-2K(d) restricted CTL epitopes of NS4A and
NS4B protein from Yellow fever 17D vaccine. J Virol Methods.
187:304–313. 2012.PubMed/NCBI
|
|
10
|
Benlahrech A, Meiser A, Herath S, et al:
Fragmentation of SIV-gag vaccine induces broader T cell responses.
PLoS One. 7:e480382012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lightowlers MW, Lawrence SB, Gauci CG,
Young J, Ralston MJ, Maas D and Health DD: Vaccination against
hydatidosis using a defined recombinant antigen. Parasite Immunol.
18:457–462. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lightowlers MW, Jensen O, Fernandez E, et
al: Vaccination trials in Australia and Argentina confirm the
effectiveness of the Eg95 hydatid vaccine in sheep. Int J
Parasitol. 29:531–534. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li YJ, Wang J and Zhao H: Bioinformatics
prediction of the Echinococcus granulosus Eg95 antigenic
epitope. Chin J Zoonoses. 27:892–896. 2011.(In Chinese).
|
|
14
|
Zhang Y and Skolnick J: Scoring function
for automated assessment of protein structure template quality.
Proteins. 57:702–710. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mustafa AS: Development of new vaccines
and diagnostic reagents against tuberculosis. Mol Immunol.
39:113–119. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gulati S, Ngampasutadol J, Yamasaki R,
McQuillen DP and Rice PA: Strategies for mimicking Neisserial
saccharide epitopes as vaccines. Int Rev Immunol. 20:229–250. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Berzofsky JA, Ahlers JD and Belyakov IM:
Strategies for designing and optimizing new generation vaccines.
Nat Rev Immunol. 1:209–219. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sette A and Fikes J: Epitope-based
vaccines: an update on epitope identification, vaccine design and
delivery. Curr Opin Immunol. 15:461–470. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang ZJ, Lin Y and Li GJ: Immune reactions
in a guinea pig model induced by the anti-type O FMDV gene
engineering vaccine that contains T-cell and B-cell epitopes. Fudan
Univ J. 39:564–567. 2000.(In Chinese).
|
|
20
|
Lin L, Tan B, Pantapalangkoor P, et al:
Acinetobacter baumannii rOmpA vaccine dose alters immune
polarization and immunodominant epitopes. Vaccine. 31:313–318.
2013. View Article : Google Scholar
|
|
21
|
Testa JS, Shetty V, Hafner J, Nickens Z,
Kamal S, Sinnathamby G and Philip R: MHC class I-presented T cell
epitopes identified by immunoproteomics analysis are targets for a
cross reactive influenza-specific T cell response. PLoS One.
7:e484842012. View Article : Google Scholar : PubMed/NCBI
|