Introduction
Type 2 diabetes is preceded by the inability of
β-cells to secrete sufficient insulin to overcome insulin
resistance or reduced insulin sensitivity, combined with reduced
insulin secretion. Degeneration of the islets of Langerhans with
β-cell loss is secondary to insulin resistance and is regarded as
the pathophysiology of type 2 diabetes (1). Oral hypoglycemic agents directly
stimulate insulin release from β-cells to overcome insulin
resistance and normalize blood glucose levels. However, these drugs
may induce certain adverse effects, such as hypoglycemia (2,3). The
consumption of anthocyanins (ANCs) has been suggested to be
correlated with a reduced risk of degenerative diseases, such as
atherosclerosis (4),
cardiovascular diseases (5),
cancer (6) and diabetes (7). ANCs extracted from Calendula
officinalis fruits have been reported to enhance insulin
release from pancreatic β-cells in vitro(8). Mulberry leaves and fruits have been
used in the treatment of numerous diseases (9–12).
The mulberry fruit (Morus alba L., family Moraceae) contains
abundant ANCs, which scavenge reactive oxygen species (13), have anti-obesity effects and
inhibit low-density lipoprotein oxidation (14). The predominant ANCs in mulberry,
cyanidin 3-rutinoside and cyanidin 3-glucoside, have been
demonstrated to dose-dependently inhibit the migration and invasion
of highly metastatic A549 human lung carcinoma cells (15). Furthermore, it was recently
demonstrated that the cyanidin 3-O-β-D-glucopyranoside fraction
from mulberry fruit protected against bladder dysfunction in
streptozotocin-induced diabetic rats (16). However, it has not yet been
elucidated whether the ANCs in mulberry are able to significantly
lower blood glucose levels and whether they may be useful in the
treatment of the pathogenesis of type 2 diabetes.
The evolution of diabetes in male leptin
receptor-deficient Zucker diabetic fatty (ZDF) rats (ZDF/CrlCrlj)
has resulted in it becoming a popular model for preclinical studies
of type 2 diabetes, due to the fact that these rats exhibit
disrupted islet architecture, β-cell degranulation and increased
β-cell death (17,18). Therefore, ZDF male rats were used
as a rodent model of type 2 diabetes in the present study. It was
hypothesized that the consumption of an ANC extract from Thai
Morus alba L. fruits was likely to result in
glucose-lowering effects and enhanced insulin secretion. The
purpose of this study was to determine the ANC composition of Thai
Morus alba L. fruits, and to assess the effect of an ANC
extract on the blood glucose and insulin levels in ZDF rats. To the
best of our knowledge, the present study has demonstrated for the
first time that ANCs extracted from Thai Morus alba L. have
significant anti-diabetic activity. Furthermore, the ANC extract
appeared to prevent the development of pathogenic lesions in
diabetic islets by suppressing islet degeneration.
Material and methods
Plant material and extraction
Mulberry fruits were obtained from Kamnan Jul Farm,
Petchaboon Province, Thailand. The fruit was extracted in
ethanol-water (50/50, v/v%), prior to the extract being filtered
through a Buchner funnel and filter paper (Chmlab, Barcelona,
Spain) and transferred to a 100 ml flask. The extract was then
collected and condensed at 40°C using a Büchi B-490 rotary
evaporator (Büchi Labortechnik AG, Flawil, Switzerland) under a
vacuum and lyophilized with a freeze-dryer (Labconco Corp., Kansas
City, MO, USA).
Isolation and purification of mulberry
ANCs
A C18 Sep-Pak cartridge (Waters Corp., Milford, MO,
US) was activated for 30 min with distilled water and
high-performance liquid chromatography (HPLC)-grade methanol (Merck
KGaA, Darmstadt, Germany). The ANC extract was then loaded onto the
column. Following successive washes with five volumes of distilled
water (acidified with 0.01% HCl) and ethyl acetate (Fisher
Scientific UK Ltd., Loughborough, UK), the ANCs were eluted with
methanol containing 0.01% HCl. The ANC solution was then collected
and condensed at 40°C using a Büchi B-490 rotary evaporator under
vacuum.
HPLC-electrospray ionization (ESI)-mass
spectrometry (MS)
ANCs in the partially purified extracts were
separated and quantified by reverse-phase HPLC using a Hypersil™
Gold C18 column (inner diameter, 5 μm; 4.6×250 mm; Thermo Fisher
Scientific Inc., Salt Lake City, IL, USA). The column was eluted
with a mobile phase consisting of water, 3.75% formic acid (VWR
International, Ltd., Lutterworth, UK) and 15% methanol at a flow
rate of 1 ml/min. The separated ANCs were detected and measured at
530 nm, and were identified based on the retention times and
ultraviolet (UV)-visible (Vis) wavelength spectra of pure authentic
standards (cyanidin 3-O-glucoside, cyanidin 3-rutinoside,
pelargonidin 3-glucoside and pelargonidin 3-rutinoside; Sigma, St.
Louis, MO, USA). The identity of each peak was verified by LC-MS
(Agilent 1100; Agilent Technologies, Santa Clara, CA, USA) using
ESI and operating in a single quadrupole mode. The instrument was
scanned over the m/z range of 200–1,500 in the ESI positive
ion mode. The LC-MS was eluted with acetonitrile (Fisher Scientific
UK Ltd.) and 0.5% ammonium hydroxide (90:10, v/v%).
Quantification of ANCs by UV-Vis
spectroscopy
The ANCs were quantified by UV-Vis spectroscopy, as
previously described (19). The
model reaction solution was diluted with 0.01% HCl in distilled
water and the absorbance at 510 nm was compared with that of known
standard solutions using a Genesys 10 UV spectrophotometer (Thermo
Spectronic, Rochester, NY, USA).
Determination of total phenolic
content
The total phenolic content was determined using the
Folin-Ciocalteau reagent (FCR), as previously described (20), with minor modifications. Briefly,
2.5 ml ethanolic mulberry extract was mixed with 0.5 ml FCR (Sigma)
and 1.0 ml 20 g/100 g solution of sodium carbonate. The mixture was
then incubated for 2 h in the dark at 25°C. The absorbance of the
mixture was measured at 765 nm using a UV-Vis Genesys 10 UV
spectrophotometer (Thermo Spectronic). A standard curve was plotted
using gallic acid (0.07–10 mg/ml in methanol; Sigma) as a standard.
The total phenolic content was expressed as gallic acid equivalents
(GAEmM/Gfw). The assay was carried out in triplicate and the mean
value was recorded.
Determination of ferric-reducing
antioxidant power (FRAP)
FRAP was measured as previously described (21). Briefly, FRAP reagent, which
consisted of 0.3 M acetate buffer (pH 3.6), 10 mM
2,4,6-tris(2-pyridyl)-s-triazine (TPTZ) (Fluka, Buchs, Switzerland)
in 40 mM HCl and 20 mM FeCl3.6H2O at a ratio
of 10:1:1 (v/v/v) was freshly prepared prior to each measurement.
Following this, 200 μl mulberry extract was mixed with 1.3 ml FRAP
reagent and incubated for 30 min at 37°C. The absorption was
measured at 595 nm using an Epoch spectrophotometer (Bio-Tek
Instruments, Inc. Winooski, VT, USA) with the Gen5 Data Analysis
Software interface. Aqueous or methanol solutions containing known
Fe(II) concentrations were used to calibrate the FRAP assay. FRAP
values, expressed as mmol of Fe(II) equivalents (FeFmM/gFW), were
determined by comparing the change in the absorption of the test
mixture with that of the Fe(II) standards. The assay was carried
out in triplicate and the mean value was recorded.
Evaluation of the anti-diabetic effects
of ANCs in ZDF rats
Five-week-old male ZDF (Leprfa/CrlCrlj)
and age-matched lean rats (Leprfa/±) were used in this
study. All rats were ordered as bred from Charles River
Laboratories International (Wilmington, MA, USA). All animal
studies were conducted according to the National Institutes of
Health Guidelines for the Care and Use of Animals, and were
reviewed and approved by the Committee on Animal Experimentation of
Kagoshima University (Kagoshima, Japan). The rats were kept under
pathogen-free conditions with a 12-h light-dark cycle (lights on at
07:00) at 22±1°C.
The ZDF and lean rats were treated with 125 or 250
mg ANCs/body weight dissolved in 1% CMC (Sigma) in distilled water
by gavage, twice daily. The control groups received 1%
carboxymethylcellulose (CMC) in distilled water alone.
Following the allocation of the rats to each
experimental group, the rats were left to acclimatize and were fed
a control diet for 1 week. Food was then withheld for 24 h and tail
vein blood samples were collected subsequent to cutting the tip of
the tail with a scalpel. The blood samples were centrifuged, and
the plasma was stored at −20°C until assay. Blood glucose levels
were monitored every week using a glucose meter
(Accu-Chek® Active; Roche Diagnostics). Following 5
weeks of treatment with ANCs or CMC, the rats were sacrificed by
heart puncture using sterile needles and syringes under anesthesia
with diethyl ether, and blood was collected. Plasma insulin levels
were measured using an enzyme immunoassay (Cayman Chemical Co., Ann
Arbor, MI, USA). All experiments were performed using conscious
unrestrained rats.
Following the sacrifice of the rats, the pancreas
was perfused with physiological saline and rapidly excised. The
tissue samples were maintained in 10% neutral-buffered formalin,
dehydrated in a graded ethanol series, cleared in xylene and
embedded in paraffin wax. Sections (4 μm thick) were stained with
hematoxylin and eosin (H&E). For histological analysis, the
tissue sections were photographed using a high-resolution color
digital camera mounted on an Olympus BX51 microscope (Olympus,
Tokyo, Japan), and the images were transferred to a computer. Four
sections were examined from each animal in each treatment
group.
Cell culture and treatment
Murine macrophage-like cells (RAW 264.7) and rat
renal tubular epithelial cells (NRK-52E) were obtained from the
American Type Culture Collection (Manassas, VA, USA). RAW 264.7
cells were maintained in RPMI-1640 medium (Gibco BRL, Grand Island,
NY, USA) supplemented with 10% fetal bovine serum and 2 mmol/l
glutamine (Hyclone, Logan, UT, USA). NRK-52E cells were grown in
Dulbecco’s modified Eagle’s medium (DMEM; Gibco BRL) containing 7%
(v/v%) fetal bovine serum and 2 mmol/l glutamine (Hyclone). RAW
264.7 cells (3.5×104 cells/well) and NRK-52E cells
(4×104 cells/well) were cultured in serum-free
Opti-MEM® I medium (Gibco BRL) and serum-free DMEM,
respectively, prior to stimulation with various concentrations (0,
2, 10, 30, 50 or 100 μg/ml) of mulberry extract for 24 h.
Methylthiazolyl-diphenyl-tetrazolium
bromide (MTT) assay
Cell viability was assessed using a modified MTT
assay. Briefly, following the exposure of the cells to the
specified concentration of mulberry extract for 48 h, MTT solution
was added to each well of the six-well plate. Three hours
subsequently, dimethyl sulfoxide (DMSO) was added and the plate was
incubated for 24 h at 37°C. Absorbance was measured at 570 nm using
an automatic microplate reader (ImmunoMini NJ-2300; InterMed,
Tokyo, Japan).
Statistical analysis
Data were analyzed using SPSS statistical software
version 3.0 (SPSS, Inc., Chicago, IL, USA). Data are shown as the
mean ± standard deviation. The significance of the differences
between two groups was assessed using the Student’s t-test, and
differences between multiple groups were assessed by one-way
analysis of variance (ANOVA) followed by the Scheffé’s multiple
range test. Values of P<0.05 were considered to indicate a
statistically significant difference.
Results
Analysis of mulberry ANCs
The ANC composition of mulberry fruit was determined
by HPLC-ESI-MS. The ANC extract was purified using a C-18 Sep-Pak
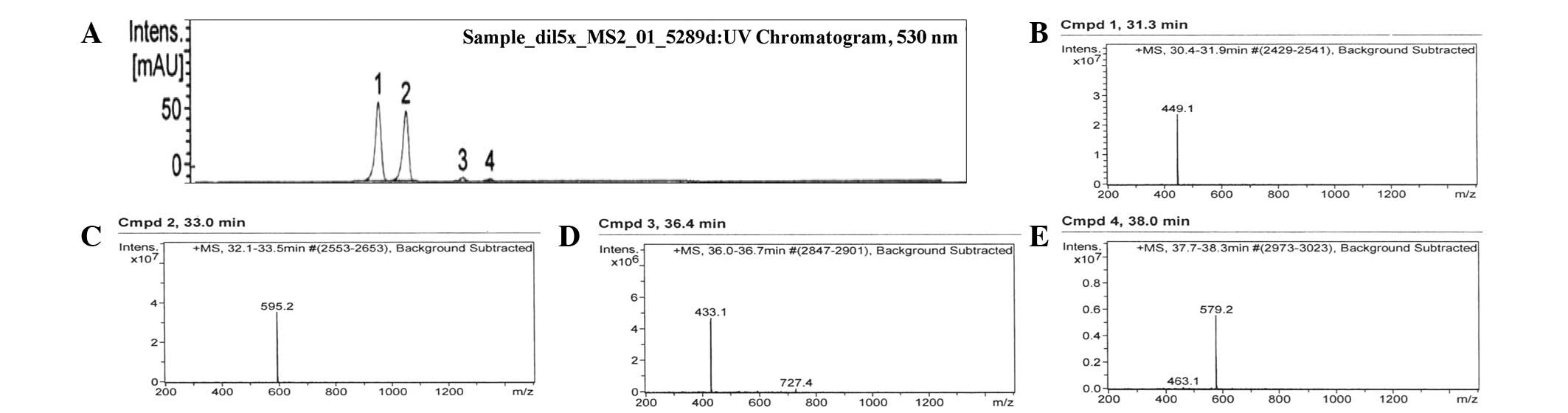
cartridge, and the resulting chromatogram, at 520 nm, is shown in
Fig. 1. The chromatogram contained
four peaks within the retention time of 31–38 min, indicating the
presence of four different ANCs in mulberry fruit (Table I). Peak 1, with a retention time of
31.3 min, M+ at m/z 449.1 and a fragment ion at m/z
287.0, was identified as cyanidin 3-O-glucoside (51.4%). Peak 2,
with a retention time of 33.0 min, M+ at m/z 595.2 and
fragment ions at m/z 449.1 and 287.0, was identified as
cyanidin 3-rutinoside (45.3%). Peak 3, with a retention time of
36.4 min, M+ at m/z 433.1 and a fragment ion at m/z
271.0, was identified as pelargonidin 3-glucoside (2.1%). Peak 4,
with a retention time of 38.0 min, M+ at m/z 579.1 and
fragment ions at m/z 433.1 and 271.0, was identified as
pelargonidin 3-rutinoside (1.2%). The results of the UV-Vis
quantification of the total ANC content showed that the
phenolic-rich extract contained 28 mg/g of total ANCs (calculated
as cyanidin-3-O-glucoside equivalents). The total phenolic content
of ANC extracts, expressed as mmol of Fe(II) equivalents and gallic
acid equivalents, was 67.28 GAEmM/Gfw and 22.67 FeFmM/gFW,
respectively (data not shown).
 | Table IIdentification of anthocyanins (ANCs)
in mulberry fruit. |
Table I
Identification of anthocyanins (ANCs)
in mulberry fruit.
| Compound
numbera | Retention time
(min) | MS, M+
(m/z) | MS/MS
(m/z) | Assignmentb |
|---|
| 1 | 31.3 | 449.1 | 287.0 | Cyanidin
3-O-glucoside |
| 2 | 33.0 | 595.2 | 449.1/287.0 | Cyanidin
3-rutinoside |
| 3 | 36.4 | 433.1 | 271.0 | Pelargonidin
3-glucoside |
| 4 | 38.0 | 579.1 | 433.1/271.0 | Pelargonidin
3-rutinoside |
Hypoglycemic effects of ANCs and
histology of pancreatic islets in ZDF rats
Table II shows the
changes in body weight observed in the six groups of rats. The ZDF
rats had significantly higher body weights than their lean
littermates from 8 weeks of age, and the body weight progressively
increased with age (P<0.05). ANC treatment did not affect body
weight in either genotype. Moreover, following 4 weeks of
treatment, ZDF rats treated with 250 mg/kg ANCs tended to gain more
weight than those treated with CMC alone or with 125 mg/kg ANCs,
although this was not statistically significant (P=0.3 versus CMC;
P=0.11 versus 125 mg/kg ANCs).
 | Table IIChanges in body weight in each
experimental group. |
Table II
Changes in body weight in each
experimental group.
| Body weight
(g) |
|---|
|
|
|---|
| Group | 0 weeks | 2 weeks | 4 weeks | 5 weeks |
|---|
| Lean rats |
| +1% CMC | 124±6 | 152±2 | 226±6 | 277±10 |
| +125 ANCs | 121±13 | 153±6 | 226±4 | 288±15 |
| +250 ANCs | 118±5 | 149±7 | 211±11 | 270±13 |
| ZDF rats |
| +1% CMC | 143±2 | 182±4 | 256±30 | 324±24 |
| +125 ANCs | 139±3 | 187±6 | 257±9 | 317±34 |
| +250 ANCs | 140±6 | 188±6 | 273±15 | 331±7 |
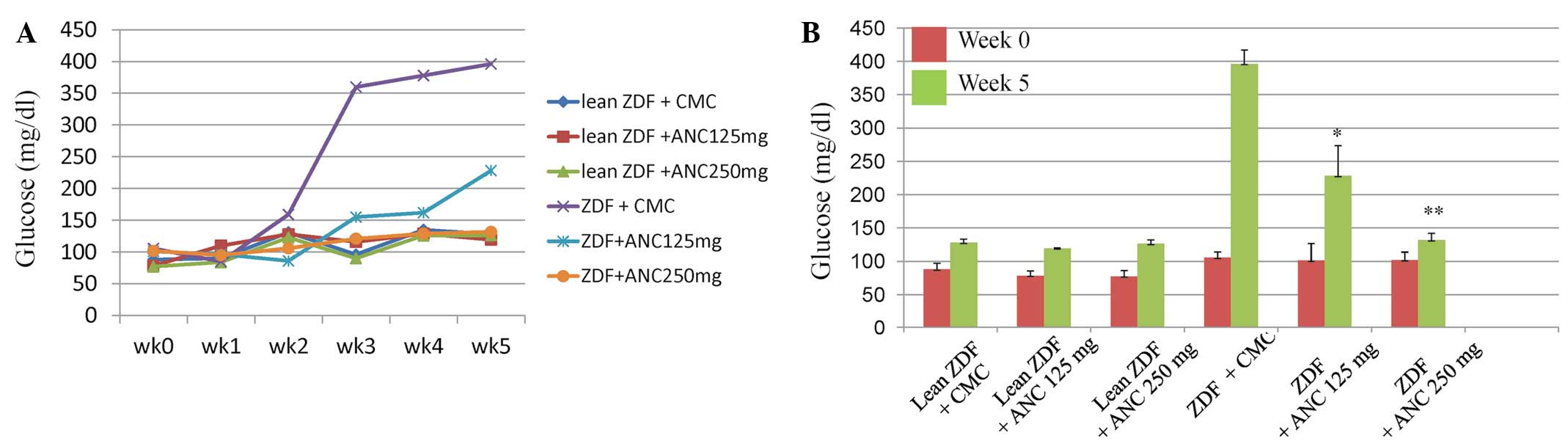
Blood glucose levels were measured in all of the
rats for 5 weeks prior to the commencement of the study and
throughout the experimental period (Fig. 2A). At 7 weeks of age, the ZDF rats
treated with the vehicle showed mild hyperglycemia (~159 mg/dl)
that rapidly progressed, reaching levels of ~396 mg/dl after 3
weeks. The administration of ANCs did not affect the glucose levels
in the lean rats. Glucose levels increased significantly from
105.5±8.7 mg/dl at 0 weeks to 396.25±21 mg/dl (P<0.0001) at 5
weeks in the ZDF rats treated with CMC; however, the glucose levels
were significantly lower in the rats treated with 125 and 250 mg/kg
ANCs (228.25±45 and 131.75±10 mg/dl, respectively; P<0.001 for
each; Fig. 2B). Treatment with 250
mg/kg ANCs reduced glucose levels in the ZDF rats to values similar
to those in their lean littermates (Fig. 2).
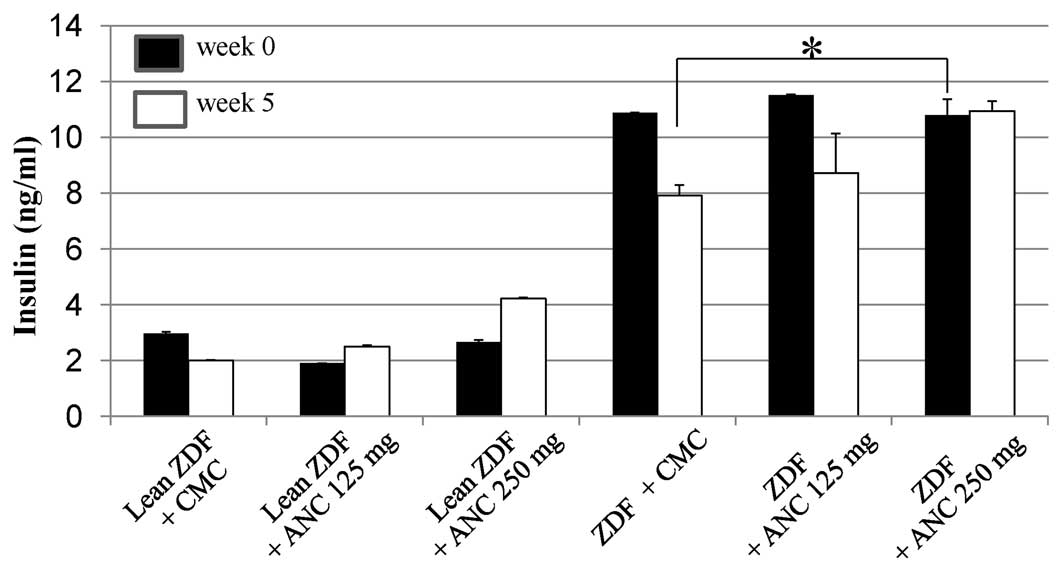
At the start of treatment, when the rats were 5
weeks of age, plasma insulin levels were significantly higher in
the ZDF rats than in the lean rats (11±0.2 versus 4.2±0.0 pg/ml;
P<0.001; Fig. 3). Between 0 and
5 weeks, the insulin levels decreased from 10.88±0.0 to 7.9±0.4
ng/ml (P<0.05) in the CMC-treated ZDF rats, and from 11.51±0.0
to 8.72±1.4 ng/ml (P<0.05) in the ZDF rats treated with 125
mg/kg ANCs. Notably, plasma insulin levels did not decrease in the
ZDF rats treated with 250 mg/kg ANCs (0 weeks: 10.8±0.6 ng/ml; 5
weeks: 10.93±0.4 ng/ml).
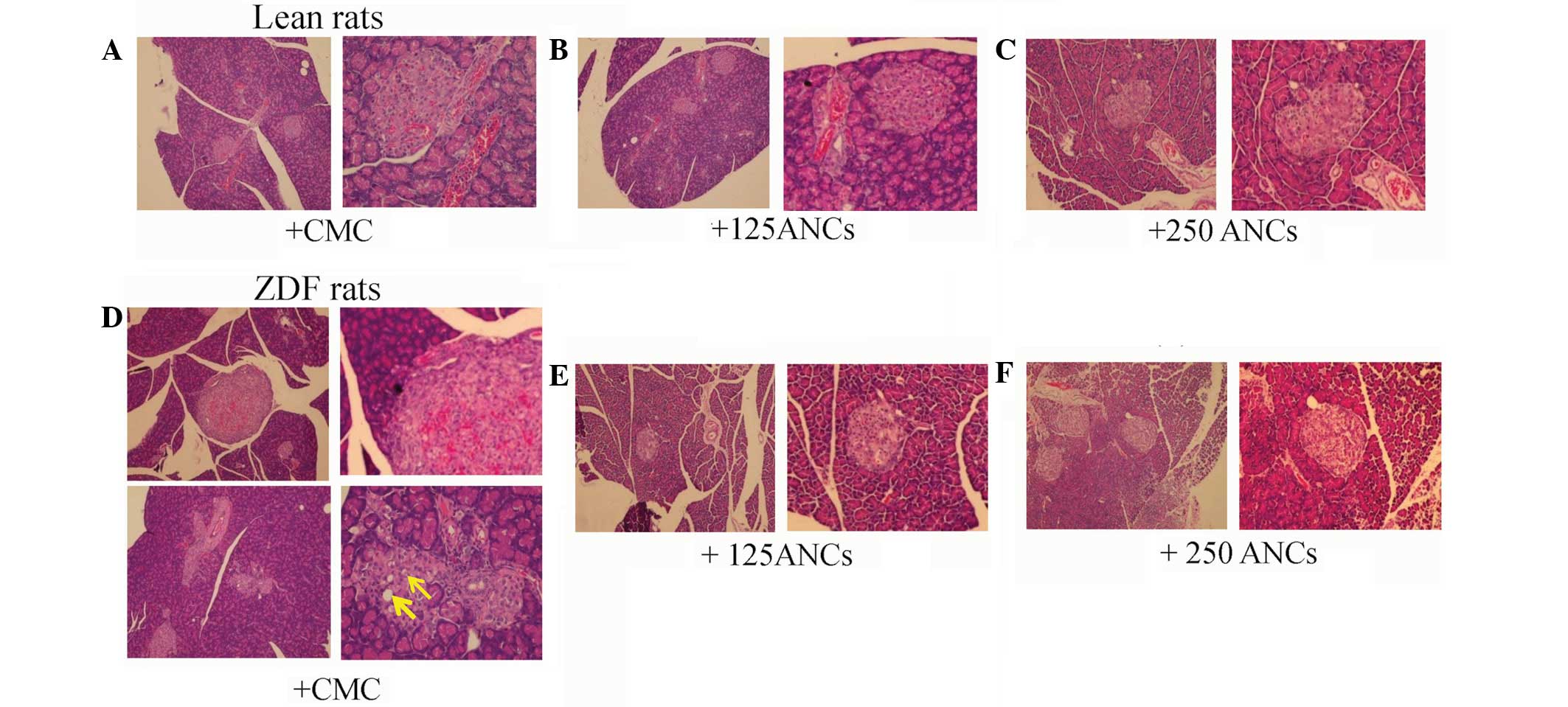
A histological evaluation of the pancreatic islets
of 10-week-old ZDF and lean rats was also conducted. H&E
staining revealed no significant pathological abnormalities in the
islets from the lean rats, which were round or oval with
well-defined boundaries (Fig.
4A–C). However, histological examination of the pancreatic
islets from the CMC-treated ZDF rats revealed substantial changes
in islet morphology. In particular, the islets were hypertrophic
and compressed adjacent exocrine tissue, and there was marked
vascular congestion or hemorrhagic degeneration (Fig. 4D, upper panel). Furthermore, the
islets were disorganized, with finger-like projections into the
surrounding exocrine tissue. The degenerated islets also showed
β-cell vacuolation and degeneration (Fig. 4D, lower panel). By contrast, the
histological assessment of pancreatic sections from the ZDF rats
treated with 125 mg/kg ANCs showed a normal distribution of islets
within the exocrine tissue and some β-cell vacuolation (Fig. 4E). Notably, the evaluation of the
pancreatic tissue samples collected from the ZDF rats treated with
250 mg/kg ANCs suggested that this dose had certain protective
effects, since there were fewer abnormal morphological features and
fewer degenerated islets. Additionally, the islets demonstrated a
regular shape with well-defined boundaries (Fig. 4F).
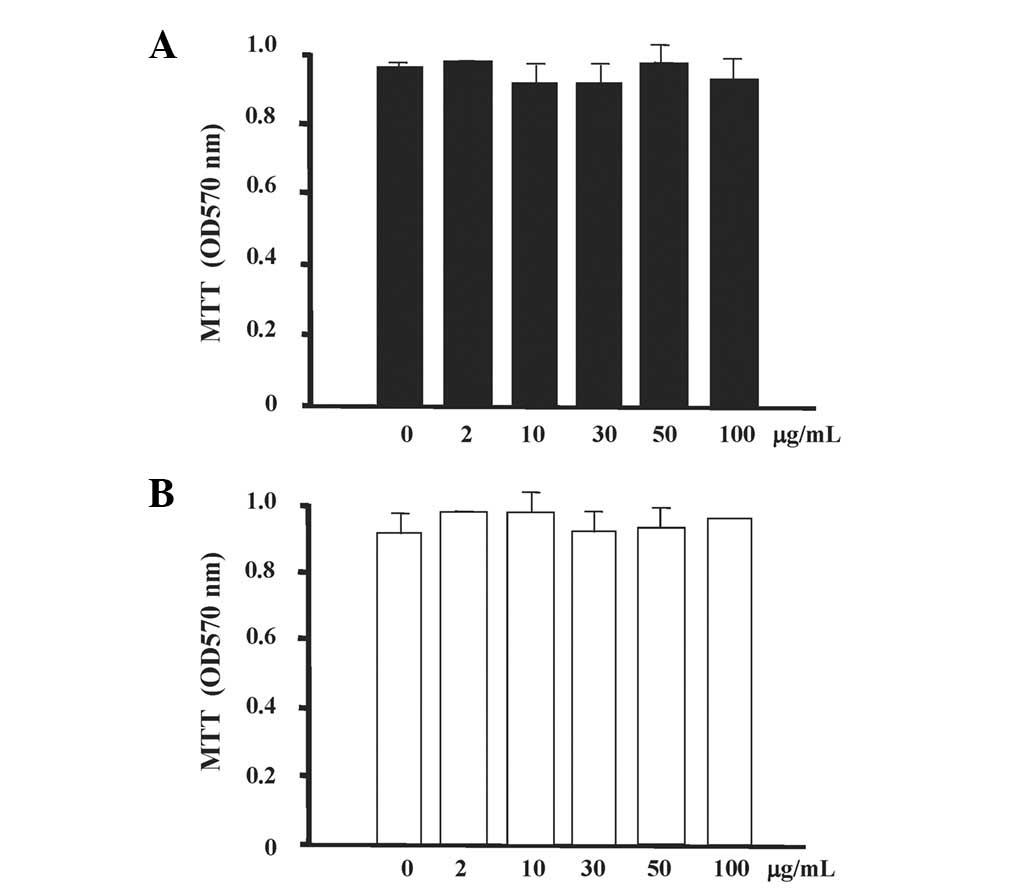
In the cell culture studies, it was observed that
the ANCs did not exert any cytotoxic effects on the murine
macrophages or rat kidney cells (Fig.
5).
Discussion
The results from this study suggest that ANCs
extracted from mulberry fruit exhibit significant anti-diabetic
properties by improving blood glucose levels in ZDF rats as an
animal model of type 2 diabetes. To the best of our knowledge, this
has shown for the first time that ANCs are able to attenuate islet
degeneration in ZDF rats. The results demonstrating that ANCs
reduced blood glucose levels were consistent with those of a prior
study showing that ANCs extracted from black soybean seed coats
exhibited antidiabetic and antioxidative effects in
streptozotocin-induced diabetic rats (22). Furthermore, the administration of
ANCs extracted from Calendula officinalis fruits has been
demonstrated to significantly increase insulin release from
pancreatic β-cells in vitro(8).
In the present study, the chromatogram of the
purified product following acid hydrolysis of the ethanol extract
revealed that cyanidin 3-O-glucoside (51.4%) and
cyanidin-3-rutinoside (45.3%) were the major ANCs present in Thai
Morus alba L. The minor ANCs, which comprised 3.3% of the
total ANCs, were pelargonidin 3-O-glucoside and pelargonidin
3-O-rutinoside. These results were consistent with those revealed
in the study by Qin et al(23), although the ANC content differed,
most likely due to differences between mulberry species and
cultivars, as well as differences in extraction, separation,
purification and analysis between the two studies.
Lean ZDF rats have been demonstrated to be less
sensitive to exogenous glucose-induced hyperglycemia (23). The ZDF rats in the present study
exhibited marked hyperglycemia at 7 weeks of age and their blood
glucose levels continued to increase with age. These results were
consistent with those of a previous study in which diabetes
occurred spontaneously in male rats aged ~6 weeks, and was
associated with hyperphagia, polyuria and polydipsia (24). It was also revealed that the β-cell
mass decreased by 51% from 9 to 12 weeks of age (24). In rats aged 6–12 weeks, the β-cell
mass is not able compensate for insulin resistance, resulting in
compensatory β-cell proliferation (25).
In a previous study, treatment with the ANC cyanidin
3-O-glucoside reduced the body weight and fat accumulation in
visceral adipose and liver tissues of KK-Ay mice by improving
triglyceride metabolism and regulating lipoprotein lipase activity
(26). In another study, aqueous
mulberry extract exhibited anti-obesity effects by upregulating
hepatic peroxisome proliferator-activated receptor α and carnitine
palmitoyltransferase-1 expression, and reducing fatty acid synthase
and 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase
expression (14). However, in the
present study, the ANC extract from mulberry fruit did not promote
reductions in body weight. In fact, the dose of 250 mg/kg ANCs
resulted in a certain level of weight gain in the ZDF rats from the
age of 9 weeks (P>0.05), without changes in food intake. The
differences in results may be due to differences in the polyphenols
contained in the extracts or their free radical scavenging
properties and mechanisms of action.
To the best of our knowledge, this study has
demonstrated for the first time that mulberry fruit extract
contains abundant cyanidin 3-O-glucoside (~28 mg/g of crude ANC
extract), with the highest ANC dose (250 mg/kg body weight)
containing ~7 mg cyanidin 3-O-glucoside. Blood glucose levels were
66% lower and insulin levels were 27% higher in the ZDF rats
treated with 250 mg/kg ANCs than in those treated with CMC between
5 and 10 weeks of age. In addition, the consumption of ANCs did not
affect glycemia in lean rats. The maximum dose of ANCs used in this
study was derived from the cyanidin 3-O-glucoside concentration (10
mg/kg) used in a prior study (11). To date, there are limited data on
the mechanisms of ANCs with regard to insulin-mediated glucose
uptake. Certain studies have shown that cyanidin 3-O-glucoside from
black beans significantly upregulated glucose transport 4 (GLUT4)
expression, induced adipocyte differentiation and glucose uptake
in vitro(27), and
prevented insulin resistance and pancreatic apoptosis in
streptozotocin-induced diabetic rats (28).
In the present study, the islets of the lean rats
showed normal histological features. By contrast, there were marked
morphological changes, including islet hypertrophy and cellular
degeneration, in the CMC-treated ZDF rats. These pathological
observations were consistent with those of earlier studies showing
pancreatic islet hypertrophy in ZDF rats (29). By the time diabetes is diagnosed,
β-cells attempt to secrete sufficient insulin to overcome the
insulin resistance in a process that involves islet hyperplasia.
Degenerating islet cells show cytoplasmic vacuolation, possibly
resulting from autodigestion following cell death (25). In the present study, the
histological assessment of the pancreatic islets from the ZDF rats
demonstrated that 250 mg/kg ANCs attenuated the degenerative
changes in the majority of the rats. Furthermore, the ANC extract
prevented marked reductions in the plasma insulin levels in these
rats. These effects may be coupled with enhanced hepatic/peripheral
tissue glucose uptake. It was not possible to clarify the mechanism
from the current results. Further studies are required to identify
the mechanisms of action of ANCs using isolated islets or β-cells
to examine whether ANCs have direct effects on insulin
secretion.
In conclusion, our results suggest that the ANC
extract of mulberry fruit is an effective anti-diabetic agent with
marked glucose-lowering effects that prevents the progressive
decline in insulin secretion. Although ANCs may protect against
β-cell damage, further studies are required to examine the
pharmacokinetics and the molecular basis for the pharmacological
activity of ANCs on insulin resistance and glucose handling in the
management of diabetes mellitus. Long-term studies are required to
confirm the present results and to establish the durability of the
improvements in glucose levels.
Acknowledgements
This study was supported in part by a grant from the
SENSHIN Medical Research Foundation. The authors would like to
thank Ms. Pornpen Dararat, Dr Yuko Nawa, Dr Fumiyo Masuda, Ms.
Tomoka Nagasato, Ms. Mika Yamamoto, Ms. Nobue Uto and all the staff
at the Department of Laboratory and Vascular Medicine, Kagoshima
University (Kagoshima, Japan) and the Department of Pharmacology,
Faculty of Dentistry, Mahidol University (Bangkok, Thailand), for
their assistance with the experiments.
References
|
1
|
Jun H, Bae HY, Lee BR, et al: Pathogenesis
of non-insulin-dependent (type II) diabetes mellitus (NIDDM) -
genetic predisposition and metabolic abnormalities. Adv Drug Deliv
Rev. 35:157–177. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rosak C: The pathophysiologic basis of
efficacy and clinical experience with the new oral antidiabetic
agents. J Diabetes Complications. 16:123–132. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jennings AM, Wilson RM and Ward JD:
Symptomatic hypoglycemia in NIDDM patients treated with oral
hypoglycemic agents. Diabetes Care. 12:203–208. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xia X, Ling W, Ma J, et al: An
anthocyanin-rich extract from black rice enhances atherosclerotic
plaque stabilization in apolipoprotein E-deficient mice. J Nutr.
136:2220–2225. 2006.PubMed/NCBI
|
|
5
|
Wallace TC: Anthocyanins in cardiovascular
disease. Adv Nutr. 2:1–7. 2011. View Article : Google Scholar
|
|
6
|
Wang LS and Stoner GD: Anthocyanins and
their role in cancer prevention. Cancer Lett. 269:281–290. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Grace MH, Ribnicky DM, Kuhn P, et al:
Hypoglycemic activity of a novel anthocyanin-rich formulation from
lowbush blueberry, Vaccinium angustifolium Aiton.
Phytomedicine. 16:406–415. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jayaprakasam B, Vareed SK, Olson LK and
Nair MG: Insulin secretion by bioactive anthocyanins and
anthocyanidins present in fruits. J Agric Food Chem. 53:28–31.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Duthie G and Crozier A: Plant-derived
phenolic antioxidants. Curr Opin Clin Nutr Metab Care. 3:447–451.
2000. View Article : Google Scholar
|
|
10
|
Kaewkaen P, Tong-Un T, Wattanathorn J, et
al: Mulberry fruit extract protects against memory impairment and
hippocampal damage in animal model of vascular dementia. Evid Based
Complement Alternat Med. 2012:2635202012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Andallu B, Kumar AV and Varadacharyulu NC:
Oxidative stress in streptozocin-diabetic rats: Amelioration by
mulberry (Morus Indica L.) leaves. Chin J Integr Med. Dec
22–2012.(Epub ahead of print).
|
|
12
|
Musabayane CT, Bwititi PT and Ojewole JA:
Effects of oral administration of some herbal extracts on food
consumption and blood glucose levels in normal and
streptozotocin-treated diabetic rats. Methods Find Exp Clin
Pharmacol. 28:223–228. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Du Q, Zheng J and Xu Y: Composition of
anthocyanins in mulberry and their antioxidant activity. J Food
Compost Anal. 21:390–395. 2008. View Article : Google Scholar
|
|
14
|
Peng CH, Liu LK, Chuang CM, Chyau CC,
Huang CN and Wang CJ: Mulberry water extracts possess an
anti-obesity effect and ability to inhibit hepatic lipogenesis and
promote lipolysis. J Agric Food Chem. 59:2663–2671. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen PN, Chu SC, Chiou HL, Kuo WH, Chiang
CL and Hsieh YS: Mulberry anthocyanins, cyanidin 3-rutinoside and
cyanidin 3-glucoside, exhibited an inhibitory effect on the
migration and invasion of a human lung cancer cell line. Cancer
Lett. 235:248–259. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ha US, Bae WJ, Kim SJ, et al: Protective
effect of cyanidin-3-O-β-D-glucopyranoside fraction from mulberry
fruit pigment against oxidative damage in streptozotocin-induced
diabetic rat bladder. Neurourol Urodyn. Nov 25–2012.(Epub ahead of
print).
|
|
17
|
Clark JB, Palmer CJ and Shaw WN: The
diabetic Zucker fatty rat. Proc Soc Exp Biol Med. 173:68–75. 1983.
View Article : Google Scholar
|
|
18
|
Mega C, de Lemos ET, Vala H, et al:
Diabetic nephropathy amelioration by a low-dose sitagliptin in an
animal model of type 2 diabetes (Zucker diabetic fatty rat). Exp
Diabetes Res. 2011:1620922011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu X, Zhao M, Hu J, Zeng S and Bai X:
Correspondence analysis of antioxidant activity and UV-Vis
absorbance of Maillard reaction products as related to reactants.
LWT - Food Science and Technology. 46:1–9. 2012. View Article : Google Scholar
|
|
20
|
Zhang M, Chen H, Li J, Pei Y and Liang Y:
Antioxidant properties of tartary buckwheat extracts as affected by
different thermal processing methods. LWT - Food Science and
Technology. 43:181–185. 2010. View Article : Google Scholar
|
|
21
|
Sutharut J and Sudarat J: Total
anthocyanin content and antioxidant activity of germinated colored
rice. International Food Research Journal. 19:215–221. 2012.
|
|
22
|
Nizamutdinova IT, Jin YC, Chung JI, et al:
The anti-diabetic effect of anthocyanins in streptozotocin-induced
diabetic rats through glucose transporter 4 regulation and
prevention of insulin resistance and pancreatic apoptosis. Mol Nutr
Food Res. 53:1419–1429. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Qin C, Li Y, Niu W, Ding Y, Zhang R and
Shang X: Analysis and characterisation of anthocyanins in mulberry
fruit. Czech J Food Sci. 28:117–126. 2010.
|
|
24
|
Jones HB, Nugent D and Jenkins R:
Variation in characteristics of islets of Langerhans in
insulin-resistant, diabetic and non-diabetic-rat strains. Int J Exp
Pathol. 91:288–301. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pick A, Clark J, Kubstrup C, Levisetti M,
Pugh W, Bonner-Weir S and Polonsky KS: Role of apoptosis in failure
of beta-cell mass compensation for insulin resistance and beta-cell
defects in the male Zucker diabetic fatty rat. Diabetes.
47:358–364. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wei X, Wang D, Yang Y, et al:
Cyanidin-3-O-β-glucoside improves obesity and triglyceride
metabolism in KK-Ay mice by regulating lipoprotein lipase activity.
J Sci Food Agric. 91:1006–1013. 2011.
|
|
27
|
Inaguma T, Han J and Isoda H: Improvement
of insulin resistance by Cyanidin 3-glucoside, anthocyanin from
black beans through the up-regulation of GLUT4 gene expression. BMC
Proc. 5(Suppl 8): P212011. View Article : Google Scholar
|
|
28
|
Nizamutdinova IT, Jin YC, Chung JI, Shin
SC, Lee SJ, Seo HG, Lee JH, Chang KC and Kim HJ: The anti-diabetic
effect of anthocyanins in streptozotocin-induced diabetic rats
through glucose transporter 4 regulation and prevention of insulin
resistance and pancreatic apoptosis. Mol Nutr Food Res.
53:1419–1429. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Janssen SW, Hermus AR, Lange WP, et al:
Progressive histopathological changes in pancreatic islets of
Zucker Diabetic Fatty rats. Exp Clin Endocrinol Diabetes.
109:273–282. 2001. View Article : Google Scholar : PubMed/NCBI
|