Introduction
Alzheimer’s disease (AD), the cause of one of the
most common types of dementia, is a brain disorder affecting the
elderly. It is characterized by the formation of two main protein
aggregates, senile plaques and neurofibrillary tangles, which are
involved in a process leading to progressive neuronal degeneration
and death (1). Several agents have
demonstrated the ability to enhance cognition and global function
in patients with AD. Advances in the understanding of AD
pathogenesis have resulted in the development of numerous compounds
that may modify the disease process. In addition, a wide array of
anti-amyloid and neuroprotective therapeutic approaches are under
investigation (2). Limiting
oxidation and toxicity, reducing Tau phosphorylation and
controlling inflammation may be beneficial disease-modifying
strategies. Moreover, potential neuroprotective and restorative
treatments, such as neurotrophins, neurotrophic factor enhancers
and stem cell-related approaches, are also under investigation
(3).
Neural stem cells (NSCs) may offer an alternative
source for curing patients with AD. Epidermal neural crest stem
cells (EPI-NCSCs) are capable of differentiating into neurons,
astrocytes and oligodendrocytes. Transplantation of EPI-NCSCs into
the hippocampus was demonstrated to result in the generation of
cholinergic neurons that were able to cure memory impairment in a
rat model of AD (4). NSC
transplantation represents an unexplored approach for treating
neurodegenerative disorders associated with cognitive decline, such
as AD. A previous study demonstrated that NSCs ameliorated complex
behavioral deficits associated with widespread AD pathology via
brain-derived neurotrophic factor (BDNF) (5).
Human cellular models of AD pathogenesis would
enable investigation of the candidate pathogenic mechanisms of AD,
and the evaluation and development of novel therapeutic strategies.
Shi et al demonstrated the development of AD pathologies in
cortical neurons that had been generated from human induced
pluripotent stem (iPS) cells derived from patients with Down
syndrome (6). It was identified
that cortical neurons generated from iPS and embryonic stem cells
from patients with Down syndrome developed AD pathologies. These
cortical neurons processed the transmembrane APP protein, resulting
in secretion of the pathogenic peptide fragment amyloid-β42 (Aβ42),
which formed insoluble intra- and extracellular amyloid aggregates.
However, the production of Aβ peptides was blocked by a γ-secretase
inhibitor. In addition, hyperphosphorylated Tau protein, a
pathological hallmark of AD, was localized to cell bodies and
dendrites in iPS cell-derived cortical neurons from patients with
Down syndrome, recapitulating the later stages of the AD pathogenic
process. Furthermore, Yahata et al differentiated human iPS
cells into neuronal cells expressing the forebrain marker, Foxg1
and the neocortical markers, Cux1, Satb2, Ctip2 and Tbr1 (7). The iPS cell-derived neuronal cells
also expressed amyloid precursor protein, as well as β- and
γ-secretase components, and were capable of secreting Aβ into the
conditioned media. Aβ production was inhibited by β- and
γ-secretase inhibitors (GSI) and a nonsteroidal anti-inflammatory
drug. These results indicated that the human iPS cell-derived
neuronal cells expressed functional β- and γ-secretases involved in
Aβ production. However, it remains unclear whether this approach
would be transferable to human patients; additional studies are
required to ensure the safety of cell transplantation into the
brain. Further studies are also needed to improve the effectiveness
of transplants, avoid the potential side-effects, investigate the
mechanisms of AD and determine how cells may assist with the
development of novel treatment agents.
A number of studies have focused on traditional
medicinal plants for the development of novel therapeutic agents
that lack side-effects. Medicinal herbs have long been used in Asia
to treat various neurological diseases, including strokes and
epilepsy (8–10). Panax notoginsenoside Rb1 (PNRb1;
(3β,12β)-20-[(6-O-β-D-glucopyranosyl-β-D-glucopyranosyl)oxy]-12-hydroxydammar-24-en-3-yl
2-O-β-D-glucopyranosyl-β-D-glucopyranoside, is the main bioactive
component of Panax notoginseng, which promotes
neurotransmitter release by modulating phosphorylation of the
synapsis through a cAMP-dependent protein kinase pathway (11). Notoginsenoside has the same
chemical structure as ginsenoside; however, in China, these
molecules are differentiated, as the former is extracted from the
plant Panax notoginseng and the latter is extracted from the
plant Panax ginseng. Panax notoginseng increases memory and
cognitive functions (12), and has
been effectively used to protect neurons and promote functional
rehabilitation in patients following cerebral hemorrhage (13). A previous study has shown that
Panax notoginseng saponins (PNS; key components of Panax
notoginseng) protect against the formation of pathological
lesions of cholinergic neurons in a rat model of AD (14). Modern pharmacological studies have
demonstrated that PNS ameliorates and protects against
neuropathological impairment. Furthermore, PNS remarkably improves
spatial learning and memory in rats with AD (15). Moreover, there are four main
components of PNS: Panax notoginsenoside R1 and ginsenosides Rg1,
Rd and Rb1. Ginsenoside Rg1 (the same as Panax notoginsenoside R1)
upregulated brain-derived neurotrophic factor (BDNF) expression and
inhibited Tau protein phosphorylation in the brain slices of a rat
model of AD (16). However, the
proportions of PNRb1 and Panex ginsenoside Rg1 are 30–40 and
25–35%, respectively. Therefore, the present study explored whether
PNRb1 has similar functions to ginsenoside Rg1 in the treatment of
AD.
Materials and methods
Wistar rats
Experiments were performed at the Biomedicine
Experimental Center, College of Medicine (The First Affiliated
Hospital of China Medical University, Shenyang, China) from July
2011 to May 2012. The experimental animals were healthy male Wistar
rats (age, 5 weeks; weight, 100–150 g) supplied by the Experimental
Animal Center, College of Medicine (The First Affiliated Hospital
of China Medical University). All animal experiments were conducted
in strict accordance with the National Institutes of Health
guidelines (2011, Eighth Edition) regarding humane treatment for
the care and use of laboratory animals, and were reviewed and
approved by the Animal Studies Committee of The First Affiliated
Hospital of China Medical University.
Traditional Chinese medicine
PNRb1, one of the biologically active ingredients of
Panax notoginseng (molecular formula,
C54H92O23; molecular weight,
1,109.31), was purchased from Nanjing Zelang Medical Technological
Co., Ltd. (Nanjing, China) and demonstrated a purity of ≥98%
(measured by high performance liquid chromatography). In accordance
with a previous method (17),
brain slices from a rat model of AD were pretreated with artificial
cerebrospinal fluid containing 60, 120 and 240 μM PNRb1 as
described below.
Preparation of the AD rat models
In accordance with a previous method (16), rats were anesthetized with 6%
chloral hydrate (400 mg/kg; Nanfang hospital, Guangzhou, China),
decapitated within 1 min and the brain was placed in buffer
solution with 150 mM NaCl, 2 mM CaCl2, 1.2 mM
MgSO4, 0.5 mM KH2PO4, 1.5 mM
K2HPO4 and 10 mM glucose (pH 7.4), for 5 min
at 4°C. Fascia on the brain and unrelated tissues were removed.
Treated brain tissues were fixed on a microtome and sliced into
400-μm-thick sections, each of which contained the cortex and the
hippocampus. Brain slices with low light levels were placed in
6-well plates containing artificial cerebrospinal fluid (100 mm
NaCl, 20 mm NaHCO3, 2.5 mm KCl, 1 mm NaH2PO4,
1 mm MgCl2, 10 mm glucose). Mixed gas (95% O2
and 5% CO2) was continuously added to the artificial
cerebrospinal fluid at 35°C. The brain slices were randomly
assigned to the blank control group, the model group and three
PNRb1 groups (n=10 per group). After 1 h of incubation, PNRb1
(dissolved in analytical grade methanol) was slowly injected using
a microsyringe to the PNRb1 group slices at concentrations of 60,
120 and 240 μM. After 2 h of pretreatment, 1 μM okadaic acid
(Sigma, St. Louis, MO, USA), which was dissolved in dimethyl
sulfoxide, was added to the model and PNRb1 groups for 4 h for
model induction. The blank control group was not administered
okadaic acid or PNRb1.
Extraction of RNA and quantification of
BDNF and Tau mRNA
Total RNA was isolated from brain cells using
QIAshredder and RNeasy mini kits (Qiagen, Inc., Chatsworth, CA,
USA). An initial strand of cDNA was synthesized from 500 ng RNA
extract, in a volume of 20 μl, using AMV reverse transcriptase XL
(Takara Biotechnology Co., Ltd., Dalian, China) and by priming with
random 9-mers, at 42°C for 10 min. The cDNA strand was stored at
20°C until use. The mRNA levels of BDNF and Tau were evaluated by
qPCR. PCR was performed in an ABI Prism 7900 sequence detector
(Applied Biosystems Inc., Foster City, CA, USA) in a final volume
of 20 μl. The PCR mixture contained 10 mM Tris-HCl buffer (pH 8.3),
50 mM KCl, 1.5 mM MgCl2, 0.2 mM dNTP mixture, 0.5 units
Ampli Taq gold enzyme (Applied Biosystems Inc.) and 0.2 M primers.
The primer and probe sequences for gene amplification were as
follows: BDNF, 5′-GACTCT GGAGAGCGTGAATG-3′ and
5′-CACTCACTAATACTGTCACA-3′; Tau, 5′-GACAAAAAAGCCAAGGGGGC-3′ and
5′-AGGGACGGGGTGCGGGAGCG-3′; and glyceraldehyde 3-phosphate
dehydrogenase (GAPDH), 5′-CCCTTCATTGAC CTCAACTAC-3′ and
5′-CCACCTTCTTGATGTCATCAT-3′. GAPDH was used as the internal
control. The Ampli Taq gold enzyme was activated by heating for 10
min at 95°C, and all genes were amplified by 50 cycles of heating
for 15 sec at 95°C, followed by 1 min at 60°C.
For construction of the standard curves of positive
controls, the total RNA of the primary astrocytes was reverse
transcribed into cDNA and serially diluted in water in five or six
log steps to afford four-fold serial dilutions of cDNA from 100 ng
to 100 pg. These cDNA serial dilutions were stored at −20°C. The
coefficient of linear regression for each standard curve was
calculated, then the cycle threshold value of a sample was
substituted into the formula for each standard curve and the
relative concentration of BDNF and Tau or GAPDH was calculated. To
normalize differences in the volume of total RNA added to each
reaction mixture, GAPDH was used as an endogenous control. Data
represent the average expression of target genes relative to the
expression of GAPDH, from three independent cultures.
Immunoblot analysis
Rat brains were lysed in an ice-cold buffer
containing 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1% (v/v) NP-40, 5
mM EDTA, 5% (v/v) glycerol, 10 μg/ml leupeptin, 10 μg/ml aprotonin,
1 mmol/l phenylmethylsulfonyl fluoride and 1 mM
Na3VO4, using a polytron, and the lysates
were then sonicated. The samples were diluted in water (1:4) and
their protein concentrations were determined using the Bradford
method with affinity-purified bovine serum albumin (Sigma) as the
standard. Samples of 10 g were dissolved in Laemmli sample buffer
(Bio-Rad, Hercules, CA, USA), separated on 12% acrylamide gel and
transferred to polyvinylidene difluoride (PVDF) membranes.
Subsequently, the blots were blocked with normal goat serum
antibody, incubated in rabbit anti-rat phosphorylated Tau protein
and BDNF polyclonal antibody (1:1,000 and 1:600, respectively;
Boster, Wuhan, China) at 4°C overnight, then washed in
phosphate-buffered saline with 0.1% Triton X-100, three times for
15 min each. As an internal control to determine whether equal
quantities of protein had been loaded onto the gel, the PVDF
membranes were stripped and re-probed with antitubulin (T5168;
Sigma). Blots were then incubated with goat anti-rabbit antibody
conjugated to horseradish peroxidase (Sigma) or mouse anti-mouse
antibody conjugated to horseradish peroxidase. Immunoreactive bands
were visualized by enhanced chemiluminescence (ECLplus kit; GE
Healthcare Life Sciences, Shanghai, China) and quantified by
densitometry with ImageJ software, version 1.45 (National
Institutes of Health, Bethesda, MD, USA) according to the
manufacturer’s instructions.
Statistical analysis
The association between PNRb1 concentration and Tau
or BDNF protein levels in the different groups was compared by
one-way analysis of variance, followed by the post hoc test of
Fisher’s protected least significant difference. Spearman’s rank
correlation coefficient was used to identify the strength of the
correlation between the relative expression levels of Tau or BDNF
and PNRb1 treatment concentrations. Online software was used to
compute the Spearman’s rank correlation and the two-sided P-value
(18). The ordinary scatterplot
and scatterplot between the ranks of X and Y were also generated.
P<0.05 was considered to indicate a statistically significant
difference.
Results
BDNF expression is upregulated by PNRb1
in the AD rat model
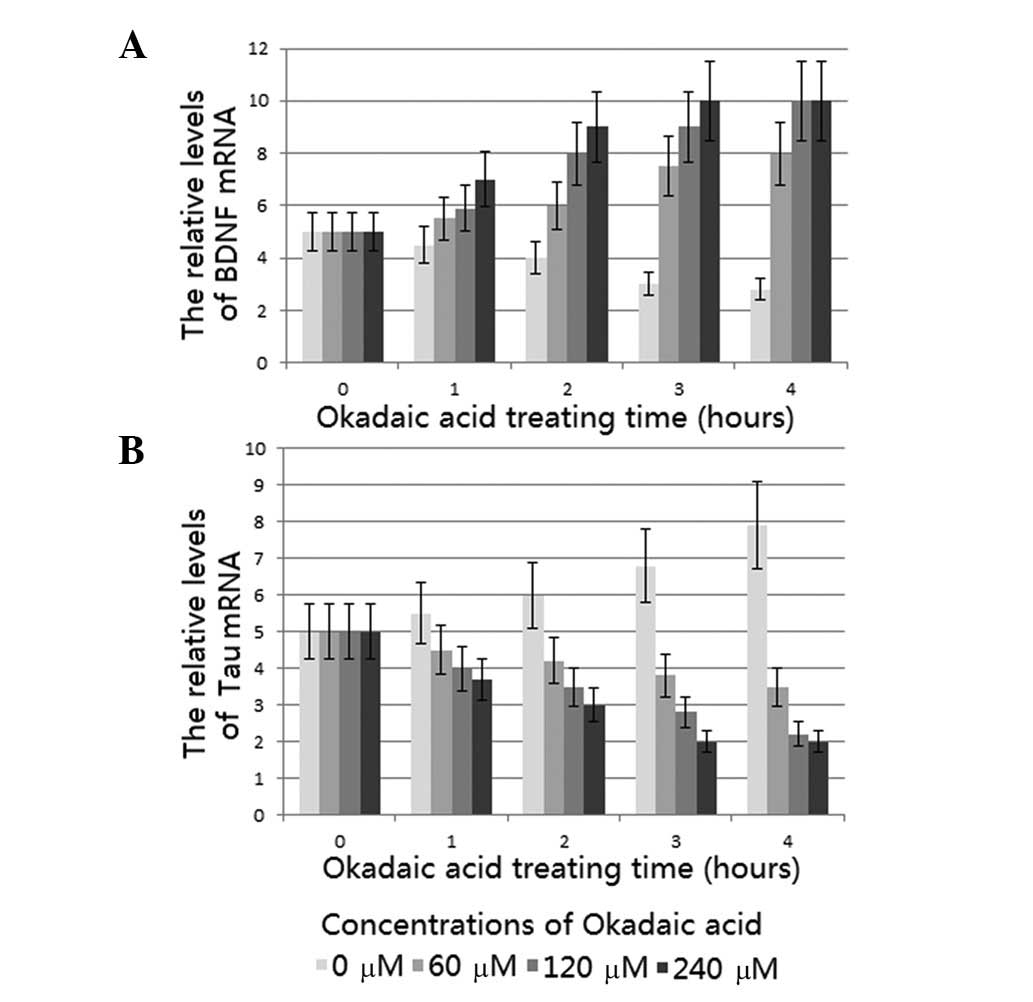
qPCR analysis demonstrated that PNRb1 induced a
significant concentration- and time-dependent increase in the BDNF
mRNA level compared with that of the model group, which is
consistent with the effect of ginsenoside Rg1 (16). The levels of BDNF mRNA were
greatest when the tissues were treated with 240 μM PNRb1 for 3 h
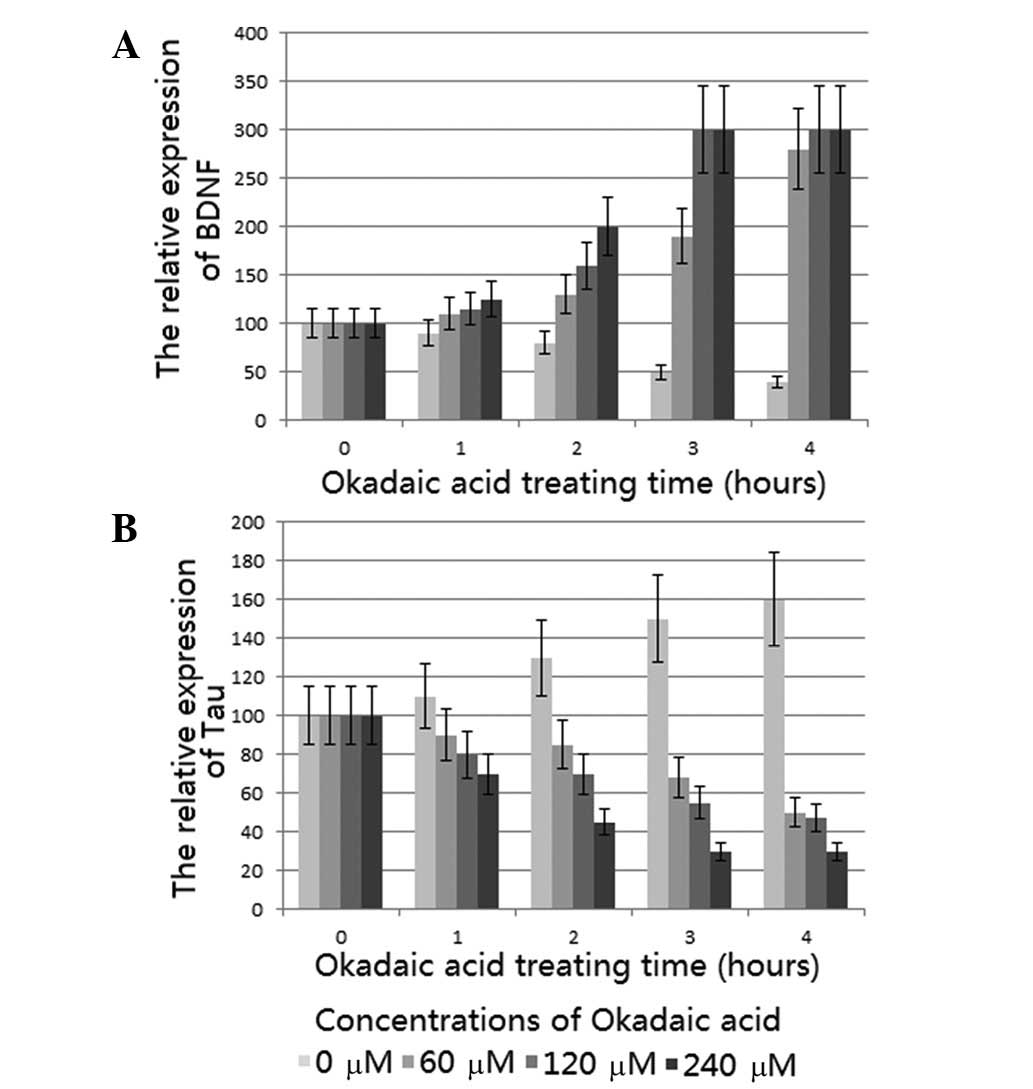
(Fig. 1A). Corresponding results
were also demonstrated in the immunoblot analysis (Fig. 2A); thus, the findings of the qPCR
and immunoblot analysis were consistent. BDNF protein expression
increased due to the increase in BDNF mRNA.
Phosphorylated Tau protein is
downregulated by PNRb1 in the AD rat model
This study examined the effects of PNRb1 on
phosphorylated Tau protein levels in the AD rat model. qPCR
analysis showed that PNRb1 induced a significant concentration- and
time-dependent reduction of the Tau mRNA level compared with that
of the model group, which is consistent with the reported effect of
ginsenoside Rg1 (16). Tissues
treated with 240 μm PNRb1 for 3 h demonstrated the lowest levels of
Tau mRNA (Fig. 1B). Corresponding
results were also demonstrated in the immunoblot analysis (Fig. 2B) and therefore, the immunoblot
analysis results were consistent with the results from the PCR
analysis. Phosphorylated Tau protein expression decreased as the
Tau mRNA levels were reduced.
BDNF and phosphorylated Tau protein are
strictly modulated by PNRb1 in the AD rat model
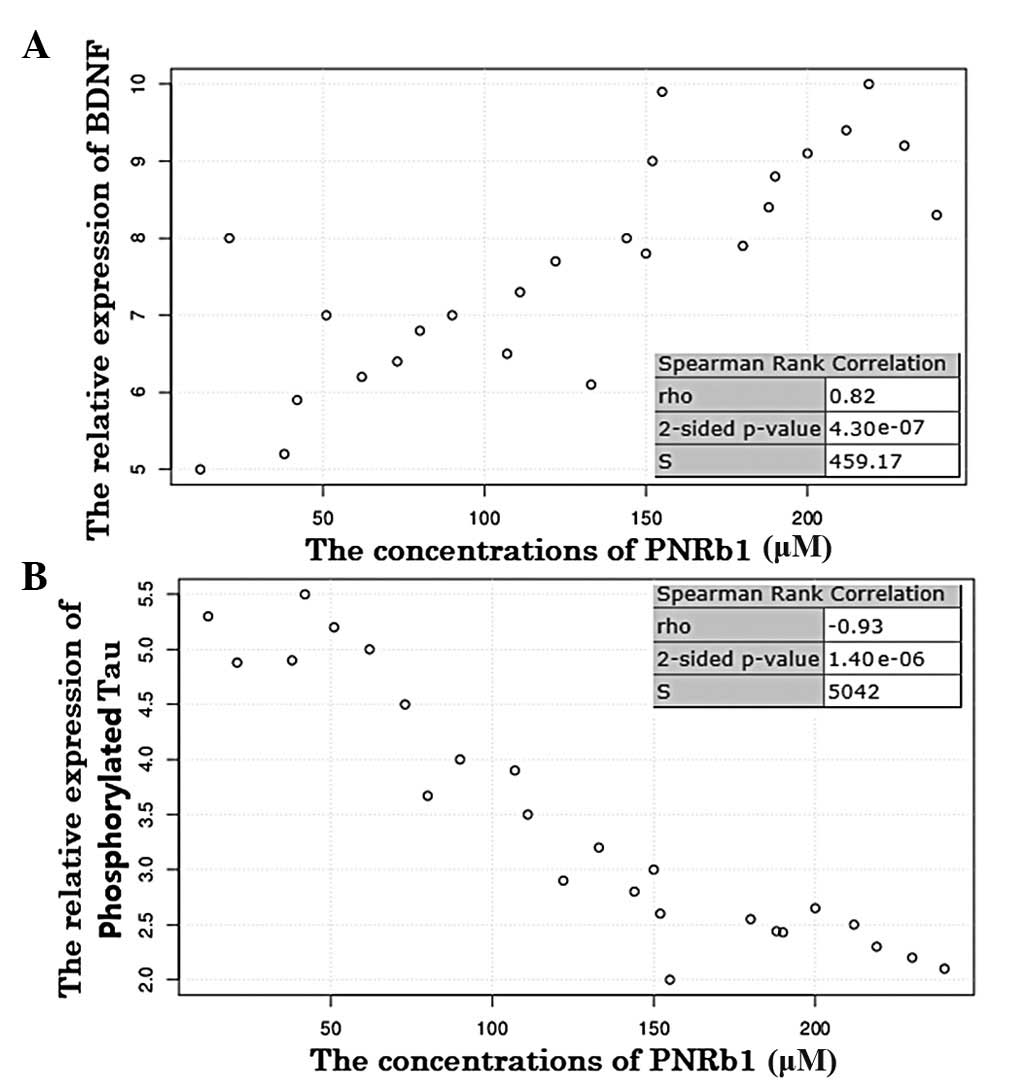
The Spearman’s rank correlation coefficient showed
that BDNF protein expression and PNRb1 treatment concentrations
were significantly and positively correlated in the AD rat model
(P<0.001; Fig. 3A). The
association between the two variables suggests that BDNF protein
expression was upregulated by PNRb1 in the progression of AD.
By contrast, the Spearman’s rank correlation
coefficient showed that phosphorylated Tau protein expression and
PNRb1 concentration were significantly and inversely correlated in
the AD model (P<0.001; Fig.
3B). The inverse correlation between the two variables suggests
that phosphorylated Tau protein expression was downregulated by
PNRb1 in the progression of AD. Therefore, PNRb1 may be used for
the prevention of AD, as it inhibited the phosphorylation of Tau
and upregulated the expression of BDNF in the AD model.
Discussion
An imbalance in the protein kinase and phosphatase
system induces Tau protein phosphorylation, resulting in the
formation of an abnormally phosphorylated Tau protein (19,20).
Cis-trans prolyl isomerization, particularly following
phosphorylation, has revealed that cis p-Tau is an early pathogenic
conformation that leads to Tau pathology and memory loss in
patients with AD (21).
Phosphorylated Tau protein participates in the formation of
neurofibrillary tangles, resulting in the occurrence of AD.
Moreover, the number of neurofibrillary tangles is strongly
associated with the degree of dementia in patients with AD
(22–24). Okadaic acid, a protein
phosphatase-2A inhibitor, is known to enhance Tau phosphorylation,
Aβ deposition and neuronal death, which are the pathological
hallmarks of AD (25). AD may be
detected by investigating the high expression levels of
phosphorylated Tau protein (21,26).
The results of the immunoblot analysis demonstrated
that phosphorylated Tau protein expression was increased in the AD
model group compared with that of the blank control group, which
suggests that Tau protein may be an important target during the
okadaic acid induction of excessive phosphorylation. Following
PNRb1 pretreatment, phosphorylated Tau protein expression was
significantly lower than that in the model group. Therefore, PNRb1
was most effective at reducing phosphorylated Tau protein
expression.
BDNF is critical in synaptic plasticity and memory
processes (27,28). BDNF signaling in the central nuclei
of the amygdala and insular cortex, is involved in the
consolidation of conditioned taste aversion memory. The
differential and spatial-specific roles of BDNF in memory
consolidation and reconsolidation suggest that dissociative
molecular mechanisms underlie these processes, which may provide
novel targets for manipulating newly encoded and reactivated
memories without causing universal amnesia (29). It has been proposed that BDNF may
protect neurons of the nervous circuitry in patients with AD
(30). The BDNF mRNA levels and
protein content have been demonstrated to be decreased in the
hippocampus and cortex of patients with AD (31). The significant reduction in BDNF
expression results in progressive atrophy of the cholinergic system
in the basal forebrain and Tau protein phosphorylation in the
brains of patients with AD (32),
suggesting that BDNF downregulation may be a mechanism of inducing
AD.
In the present study, okadaic acid was added to
artificial cerebrospinal fluid that was used to incubate rat brain
slices. This resulted in diminished BDNF expression in the model
group compared with that of the blank control group, which is
consistent with decreased BDNF expression in the brains of patients
with AD (31). Therefore, it was
demonstrated that okadaic acid inhibited BDNF expression. Increased
BDNF expression in the brain may improve neuronal survival
(33,34), resulting in a delay in or
prevention of AD progression.
BDNF has a high molecular weight. If orally
administered, exogenous BDNF may be easily damaged by gastric acid.
However, with other means of peripheral administration, BDNF is not
able to cross the blood brain barrier. Therefore, promoting the
production or release of endogenous BDNF may be an effective
treatment for patients with AD. In the current study it was
demonstrated that PNRb1, in addition to reducing phosphorylated Tau
protein expression in the AD model and potentially slowing down the
progression of AD, also upregulated BDNF expression and contributed
to the production or release of endogenous BDNF.
To the best of our knowledge, the present study was
the first to demonstrate the inverse expression pattern between
BDNF and phosphorylated Tau, which was modulated by PNRb1. In the
progression of AD, BDNF is upregulated by PNRb1 and phosphorylated
Tau protein is downregulated by PNRb1, suggesting that PNRb1 may be
used for the prevention of AD.
References
|
1
|
Maccioni RB, Muñoz JP and Barbeito L: The
molecular bases of Alzheimer’s disease and other neurodegenerative
disorders. Arch Med Res. 32:367–381. 2001.
|
|
2
|
Yu MS, Leung SK, Lai SW, et al:
Neuroprotective effects of anti-aging oriental medicine Lycium
barbarum against β-amyloid peptide neurotoxicity. Exp Gerontol.
40:716–727. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Singh S, Kushwah AS, Singh R, Farswan M
and Kaur R: Current therapeutic strategy in Alzheimer’s disease.
Eur Rev Med Pharmacol Sci. 16:1651–1664. 2012.
|
|
4
|
Esmaeilzade B, Nobakht M, Joghataei MT, et
al: Delivery of epidermal neural crest stem cells (EPI-NCSC) to
hippocamp in Alzheimer’s disease rat model. Iran Biomed J. 16:1–9.
2012.PubMed/NCBI
|
|
5
|
Blurton-Jones M, Kitazawa M,
Martinez-Coria H, et al: Neural stem cells improve cognition via
BDNF in a transgenic model of Alzheimer disease. Proc Natl Acad Sci
USA. 106:13594–13599. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shi Y, Kirwan P, Smith J, MacLean G, Orkin
SH and Livesey FJ: A human stem cell model of early Alzheimer’s
disease pathology in Down syndrome. Sci Transl Med. 4:124–129.
2012.
|
|
7
|
Yahata N, Asai M, Kitaoka S, et al:
Anti-Aβ drug screening platform using human iPS cell-derived
neurons for the treatment of Alzheimer’s disease. PLoS One.
6:e257882011.
|
|
8
|
Kim H: Neuroprotective herbs for stroke
therapy in traditional eastern medicine. Neurol Res. 27:287–301.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pearl PL, Drillings IM and Conry JA: Herbs
in epilepsy: evidence for efficacy, toxicity, and interactions.
Semin Pediatr Neurol. 18:203–208. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schachter SC: Botanicals and herbs: a
traditional approach to treating epilepsy. Neurotherapeutics.
6:415–420. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xue JF, Liu ZJ, Hu JF, Chen H, Zhang JT
and Chen NH: Ginsenoside Rb1 promotes neurotransmitter release by
modulating phosphorylation of synapsins through a cAMP-dependent
protein kinase pathway. Brain Res. 1106:91–98. 2006.PubMed/NCBI
|
|
12
|
Chuang CM, Hsieh CL, Lin HY and Lin JG:
Panax Notoginseng Burk attenuates impairment of learning and memory
functions and increases ED1, BDNF and beta-secretase immunoreactive
cells in chronic stage ischemia-reperfusion injured rats. Am J Chin
Med. 36:685–693. 2008. View Article : Google Scholar
|
|
13
|
Wei SG, Meng LQ and Huang RY: Effect of
Panax notoginseng saponins on serum neuronal specific enolase and
rehabilitation in patients with cerebral hemorrhage. Zhongguo Zhong
Xi Yi Jie He Za Zhi. 27:159–162. 2007.(In Chinese).
|
|
14
|
Zhong Z, Qu Z, Wang N, et al: Protective
effects of Panax notoginseng saponins against pathological lesion
of cholinergic neuron in rat model with Alzheimer’s disease. Zhong
Yao Cai. 28:119–122. 2005.(In Chinese).
|
|
15
|
Zhong Z, Qu Z, Bao Y, Wang N, Zhang F and
Zhang W: Effects of Panax notoginseng saponins in a rat model of
Alzheimer’s disease. Neural Regeneration Research. 3:37–40.
2008.PubMed/NCBI
|
|
16
|
Li X, Li M, Li Y, Quan Q and Wang J:
Cellular and molecular mechanisms underlying the action of
ginsenoside Rg1 against Alzheimer’s. Neural Regeneration Research.
7:2860–2866. 2012.PubMed/NCBI
|
|
17
|
Li X, Liu Y, Yuan HF and Quan QK: Effects
of gensenoside Rg1 on tau protein phosphorylation induced by
okadaic acid in rat brain slices. Zhong Xi Yi Jie He Xue Bao.
8:955–960. 2010.(In Chinese).
|
|
18
|
Wessa P: Spearman Rank Correlation
(v1-0.1) in Free Statistics Software (v1.1.23-r7). Office for
Research Development and Education. http://www.wessa.net/rwasp_spearman.wasp/uri.
Accessed June 27, 2013
|
|
19
|
Li L, Liu Z, Liu J, et al: Ginsenoside Rd
attenuates beta-amyloid-induced tau phosphorylation by altering the
functional balance of glycogen synthase kinase 3beta and protein
phosphatase 2A. Neurobiol Dis. 54:320–328. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sontag JM, Nunbhakdi-Craig V, White CL
III, Halpain S and Sontag E: The protein phosphatase PP2A/Bα binds
to the microtubule-associated proteins Tau and MAP2 at a motif also
recognized by the kinase Fyn: Implications for tauopathies. J Biol
Chem. 287:14984–14993. 2012.
|
|
21
|
Nakamura K, Zhou XZ and Lu KP: Distinct
functions of cis and trans phosphorylated tau in Alzheimer’s
disease and their therapeutic implications. Curr Mol Med.
15–Nov;2012.(Epub ahead of print).
|
|
22
|
Rosenmann H, Meiner Z, Geylis V, Abramsky
O and Steinitz M: Detection of circulating antibodies against tau
protein in its unphosphorylated and in its neurofibrillary
tangles-related phosphorylated state in Alzheimer’s disease and
healthy subjects. Neurosci Lett. 410:90–93. 2006.PubMed/NCBI
|
|
23
|
Bancher C, Brunner C, Lassmann H, et al:
Accumulation of abnormally phosphorylated tau precedes the
formation of neurofibrillary tangles in Alzheimer’s disease. Brain
Res. 477:90–99. 1989.
|
|
24
|
Wang JZ, Grundke-Iqbal I and Iqbal K:
Kinases and phosphatases and tau sites involved in Alzheimer
neurofibrillary degeneration. Eur J Neurosci. 25:59–68. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yoon SY, Choi JE, Kweon HS, et al: Okadaic
acid increases autophagosomes in rat neurons: Implications for
Alzheimer’s disease. J Neurosci Res. 86:3230–3239. 2008.PubMed/NCBI
|
|
26
|
Voss K, Koren J III and Dickey CA: The
earliest tau dysfunction in Alzheimer’s disease? Tau phosphorylated
at s422 as a toxic seed. Am J Pathol. 179:2148–2151. 2011.
|
|
27
|
Tota S, Goel R, Pachauri SD, et al: Effect
of angiotensin II on spatial memory, cerebral blood flow,
cholinergic neurotransmission, and brain derived neurotrophic
factor in rats. Psychopharmacology (Berl). 226:357–369. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Scaini G, Comim CM, Oliveira GM, et al:
Chronic administration of branched-chain amino acids impairs
spatial memory and increases brain-derived neurotrophic factor in a
rat model. J Inherit Metab Dis. 30–Oct;2012.(Epub ahead of
print).
|
|
29
|
Wang Y, Zhang TY, Xin J, et al:
Differential involvement of brain-derived neurotrophic factor in
reconsolidation and consolidation of conditioned taste aversion
memory. PLoS One. 7:e499422012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nagahara AH, Merrill DA, Coppola G, et al:
Neuroprotective effects of brain-derived neurotrophic factor in
rodent and primate models of Alzheimer’s disease. Nat Med.
15:331–337. 2009.
|
|
31
|
Mufson EJ, Counts SE, Fahnestock M and
Ginsberg SD: Cholinotrophic molecular substrates of mild cognitive
impairment in the elderly. Curr Alzheimer Res. 4:340–350. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Laske C, Stellos K, Hoffmann N, et al:
Higher BDNF serum levels predict slower cognitive decline in
Alzheimer’s disease patients. Int J Neuropsychopharmacol.
14:399–404. 2011.PubMed/NCBI
|
|
33
|
Allen SJ, Watson JJ, Shoemark DK, Barua NU
and Patel NK: GDNF, NGF and BDNF as therapeutic options for
neurodegeneration. Pharmacol Ther. 138:155–175. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cardenas-Aguayo Mdel C, Kazim SF,
Grundke-Iqbal I and Iqbal K: Neurogenic and neurotrophic effects of
BDNF peptides in mouse hippocampal primary neuronal cell cultures.
PLoS One. 8:e535962013.PubMed/NCBI
|