Introduction
Hepatic fibrosis is a disease characterized by the
abnormal hyperplasia of fibrous connective tissue in the liver, due
to hepatocellular necrosis and inflammatory stimulation. It is the
result of increased synthesis and decreased degradation of the
extracellular matrix (ECM). Hepatic fibrosis is a common
pathological process of all chronic liver diseases, and is the
necessary prerequisite for liver cirrhosis (1). At present, it is mainly considered
that the process of hepatic fibrosis is reversible, and its
prevention and treatment is important for the prevention of liver
cirrhosis (2). In recent years,
studies concerned with the blockade or reversal of hepatic fibrosis
have become an important topic in medicine worldwide (3-5). Due
to increased investigation into the development of hepatic fibrosis
(6-9), considerable progress has been made in
the treatment of hepatic fibrosis with the emergence of novel
synthetic drugs. However, the majority of these drugs are in
clinical trials, with uncertain antifibrotic efficacy and
significant side-effects.
At present, there are a number of single and
compound prescriptions of traditional Chinese medicine that have
demonstrated clear efficacy and marginal side-effects, and it has
been suggested they may serve as potential treatments for hepatic
fibrosis (10,11). Astragalus flavescens, a
compound prescription of Chinese herbal medicine, contains Radix
Astragali, Radix Scutellariae and Flavescent Sophora. Astragalus
flavescens has been utilized in the treatment of chronic liver
disease for >10 years and has shown curative effects. It has
been clinically demonstrated that Astragalus flavescens is
effective in receding jaundice, detumescence, retracting the
spleen, reducing ascites, lowering transaminase activity,
increasing serum albumin levels and improving the prothrombin time.
Clinical studies have indicated that Astragalus flavescens
is able to regulate the immunity and scavenge oxygen free radicals,
as well as exhibiting anti-viral and -tumor effects. In addition,
the prescription for Astragalus flavescens is low in cost
and convenient to use; therefore, it has good developmental value.
The present study investigated the preventative effects of
Astragalus flavescens on liver fibrosis in rats and its
mechanism of action. The study aimed to provide a reliable
experimental basis for the further application of Astragalus
flavescens in the treatment of hepatic fibrosis.
Materials and methods
Experimental groups
A total of 60 male Wistar rats (clean grade; average
weight, 180 g), purchased from the Animal Experimental Center of
the Fourth Military Medical University (Xi’an, China), were
randomly divided into normal control, model control, high-dose
treatment and low-dose treatment groups (n=15 rats per group). This
study was carried out in strict accordance with the recommendations
in the Guide for the Care and Use of Laboratory Animals of the
National Institutes of Health (8th edition, 2011). The animal use
protocol was reviewed and approved by the Institutional Animal Care
and Use Committee (IACUC) of the First Hospital of Xi’an City
(Xi’an, China). In the normal control group, a normal diet and
water were freely available, 0.9% NaCl was administered to the rats
by gavage daily and peanut oil was administered by subcutaneous
injection at a dose of 0.5 ml/100 g on the first day and 0.3 ml/100
g once every 4 days thereafter. In the model control group,
according to a modification of composite factor modeling methods
(12,13), rats were subcutaneously injected
with a mixture of 40% CCl4 and peanut oil at a dose of
0.5 ml/100 g on the first day and 0.3 ml/100 g once every 4 days
thereafter. The rats were fed with freely available compound feed
containing 79.5% pure flour, 20% lard and 0.5% cholesterol, and
water was the only drink. In the high- and low-dose treatment
groups, the modeling method was the same as that in the model
control group; however, the rats were additionally treated with
Astragalus flavescens (2 g crude drug/g powder; Xi’an
Chinese Traditional Medicine Oral Tablet Factory, Xi’an, China) by
gavage, once a day. The dosages were 2 g/100 g weight and 0.5 g/100
g weight, respectively. Eight weeks following the initiation of
treatment, all rats were sacrificed and heart, blood and liver
specimens were obtained.
Hepatic fibrosis indices
The hepatic fibrosis indices, specifically, type III
precollagen (PC III), type IV collagen (C IV), hyaluronic acid (HA)
and laminin (LN), in the rat serum were detected by specific
personnel in the isotope department using the radioimmunoassay
method.
Liver tissue specimen observation
Rat liver tissue specimens were stained with
Masson’s trichrome (14,15), followed by observation under a
light microscope. The collagen surface density in the liver tissue
was calculated as follows: Collagen surface density (%) = (collagen
area/viewed area) × 100.
Hepatic fibrosis-related factors
Liver tissue paraffin sections were prepared and
dewaxed, enzyme closure was performed using with 3% hydrogen
peroxide and antigen retrieval with citrate buffer. After closing
non-specific sites using non-immune goat serum, primary antibody
(rabbit anti-rat TGF-β1 polyclonal antibody, rabbit anti-rat
PDGF-BB polyclonal antibody and rabbit anti-rat CTGF polyclonal
antibody; Wuhan Boster Biological Technology, Ltd., Wuhan, China)
with 1:50 dilution using PBS was added, followed by incubation at
4°C overnight. After adding polymer enhancer and PBS washing, 50
μl of horseradish peroxidase-labeled secondary antibody
polymer was added by drops to each section, followed by incubation
at 37°C for 30 min and 3 PBS washes. After coloration,
counter-stain and mounting, the sections were observed in Q550CW
image acquisition and analysis system (Leica Science Lab, Berlin,
Germany). PBS replacing primary antibody was used as a blank,
normal serum replacing secondary antibody was used as a negative
control. No coloration was regarded as a negative result. TGF-β1,
CTGF and PDGF were stained in the cytoplasm and cytomembrane. The
brown or dark brown granular staining was defined as a positive
result, and the staining significantly darker than background or no
background staining referred to positively stained cells. Ten
visual fields of each section were selected and the ratio of
positive cell area to liver visual field area
(μm2/μm2) was
calculated. The broad-spectrum immunohistochemistry EliVision™ plus
kit was provided by Fuzhou Maixin Biotechnology Development Co.,
Ltd. (Fuzhou, China).
Statistical analysis
Data are expressed as the mean ± standard deviation.
Statistical analysis was performed using SPSS software, version
11.5 (SPSS, Inc., Chicago, IL, USA). The Student’s t-test was used
to analyze the differences between two groups, and single factor
analysis of variance and Student-Newman-Keuls tests were conducted
for comparisons among multiple groups. P<0.05 and P<0.01 were
considered to indicate a statistically significant difference.
Results
Comparison of serum hepatic fibrosis
indices
The serum levels of hepatic fibrosis indices in the
model control and low- and high-dose treatment groups were
significantly increased compared with those in the normal control
group (P<0.05), and those in the two treatment groups were
significantly lower than those in the model control group
(P<0.05; Table I). The levels
of PC III, C IV and LN in the high-dose treatment group were
significantly lower than those in the low-dose treatment group
(P<0.05); however, no significant difference was identified in
the HA levels between the two treatment groups.
 | Table I.Comparisons of serum hepatic fibrosis
indices in different groups. |
Table I.
Comparisons of serum hepatic fibrosis
indices in different groups.
| Groups | No. of rats | Hepatic fibrosis
indices (μg/l)
|
|---|
| PC III | C IV | HA | LN |
|---|
| Normal control | 15 | 13.20±1.12 | 4.90±0.62 | 104.36±25.30 | 27.46±6.56 |
| Model control | 15 | 40.01±0.52a | 20.56±0.23a | 315.20±98.39a | 70.11±10.02a |
| Low-dose
treatment | 15 |
34.20±0.82a,b |
14.52±0.42a,b |
185.20±18.21a,b |
59.54±7.58a,b |
| High-dose
treatment | 15 |
19.56±0.98a–c |
7.98±0.91a–c |
137.25±19.89a,b |
48.59±9.82a–c |
Morphological changes
The collagen surface densities in the model control
and low- and high-dose treatment groups were significantly
increased compared with that in the normal control group
(P<0.05; Table II). In
addition, the collagen surface density in the two treatment groups
was significantly lower than that in the model control group
(P<0.05). Moreover, the collagen surface density in the
high-dose treatment group was significantly lower than that in the
low-dose treatment group (P<0.05).
 | Table II.Comparisons of collagen surface
density in different groups. |
Table II.
Comparisons of collagen surface
density in different groups.
| Group | No. of rats | Collagen surface
density (%) |
|---|
| Normal control | 15 | 4.83±2.78 |
| Model control | 15 | 24.31±3.55a |
| Low-dose
treatment | 15 |
18.02±3.64a,b |
| High-dose
treatment | 15 |
7.97±1.06a–c |
Expression of hepatic fibrosis-related
factors
The expression levels of the hepatic
fibrosis-related factors TGF-β1, CTGF and PDGF-BB in the different
groups are shown in Table III. The
expression levels of the three hepatic fibrosis-related factors in
the model control group and the two treatment groups were
significantly increased compared with those in the normal control
group (P<0.05). Additionally, the expression levels of these
factors in the two treatment groups were significantly lower than
those in the model control group (P<0.05).
 | Table III.Expression levels of hepatic
fibrosis-related factors in different groups. |
Table III.
Expression levels of hepatic
fibrosis-related factors in different groups.
| Group | No. of rats | Hepatic
fibrosis-related factors (μm2/μm2)
|
|---|
| TGF-β1 | CTGF | PDGF-BB |
|---|
| Normal control | 15 | 0.10±0.02 | 0.03±0.01 | 0.05±0.02 |
| Model control | 15 | 0.61±0.05a | 0.80±0.02a | 0.61±0.06a |
| Low-dose
treatment | 15 |
0.30±0.08a,b |
0.11±0.05a,b |
0.41±0.10a,b |
| High-dose
treatment | 15 |
0.13±0.08a,b |
0.05±0.09a,b |
0.18±0.01a,b |
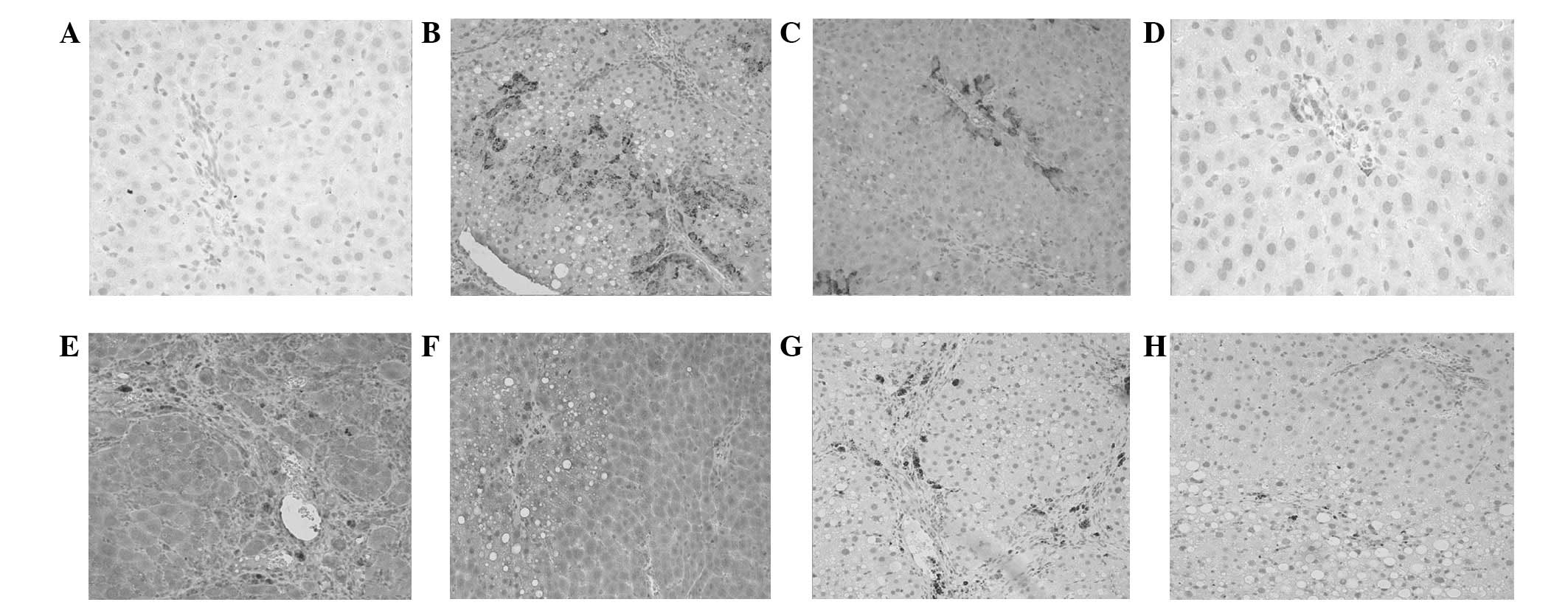
Expression of TGF-β1 was predominantly identified in
the cytoplasm, and infrequently observed in the cytomembrane. The
normal control group demonstrated weak expression of TGF-β1 in the
cytoplasm of liver cells, with the distribution focused in the
portal area and surrounding the central vein wall (Fig. 1A). TGF-β1 expression in the model
control group was significantly increased compared with that of the
normal control group, and the majority of the expression was
observed in the portal area, central vein wall, proliferated
fibrous septum, liver sinus wall, small bile duct cells,
inflammatory cells and fatty and hepatic cells with steatosis and
hydropic degeneration (Fig. 1B).
The expression of TGF-β1 in the low- and high-dose treatment groups
was significantly reduced compared with that of the model control
group, and was mainly evident in the portal area, proliferated
fibrous septum and inflammatory cells. Furthermore, the level of
TGF-β1 expression in the high-dose treatment group was lower
(Fig. 1C).
Expression of CTGF was observed in the cytoplasm and
cytomembrane (as brown granules). In the normal control group, CTGF
expression was negligible (Fig.
1D). By contrast, in the model control group, CTGF expression
was significantly increased; the protein was predominantly
identified in the spindle cells with processes and branches in the
fibrosis portal area and fibrous septa [activated hepatic stellate
cells (HSCs)], and inflammatory cells (Fig. 1E). The level of CTGF expression in
the two treatment groups was significantly lower than that in the
model control group, and that in the high-dose treatment group was
the lowest (Fig. 1F).
Similarly to CTGF, PDGF-BB was expressed in the
cytoplasm and cytomembrane. In the normal control group, no PDGF-BB
expression was observed in hepatic cells and weak PDGF-BB
expression was identified in the vascular wall, portal area and
interstitial cells. In the model control group, the number of
PDGF-BB-positive granules was significantly increased. Hyperplasia
was most evident in the fibrous septa and portal area, with
specific distribution in the infiltration area of inflammatory
cells (Fig. 1G). The PDGF-BB
expression level in the low- and high-dose treatment groups was
significantly decreased compared with that of the model control
group (Fig. 1H shows the
expression in the high-dose treatment group).
Discussion
Modern Chinese medicine considers liver blood stasis
to be the main pathogenesis of hepatic fibrosis, of which the
essence is fibrous tissue hyperplasia, degeneration and
microcirculation disturbance. Therefore, activating blood and
dissolving stasis is important for the treatment of hepatic
fibrosis (16,17). Astragalus flavescens is
proposed to be involved in activating blood and dissolving stasis,
strengthening body resistance, supplementing qi, heat-clearing and
detoxifying, removing dampness and receding jaundice. It may
provide multi-channel, -level and -target prevention of and
treatment for hepatic fibrosis.
Previous studies have demonstrated that, during the
process of hepatic fibrosis, HSCs are activated and transformed
into myofibroblast-like cells (18). This is important in the formation
of hepatic fibrosis. Although there are a variety of cells involved
in the induction of hepatic fibrosis, activated HSCs are the main
cells that promote the deposition of a large quantity of ECM
(19–22). The initiation and activation of
HSCs are mediated by TGF-β1, which is an intermediary agent between
HSC initiation and hepatic fibrosis (23), and is one of the most important
factors determining hepatic fibrosis (24-26).
During hepatic fibrosis, TGF-β1 initiates and maintains the
activation of HSCs in a paracrine and autocrine manner, and
regulates the cell proliferation. Therefore, it is able to promote
collagen gene transcription and ECM proliferation, and inhibit the
synthesis and secretion of proteolytic enzymes, thus resulting in a
reduction in ECM degradation. Once hepatic fibrosis is initiated,
it continues and the gradually increased fibrosis results in
cirrhosis. Therefore, the inhibition of TGF-β1 production or the
blocking of its biological activity may inhibit the activation of
HSCs. This is considered to be one of the most promising methods of
antifibrotic therapy (27). It has
been proposed that TGF-β1 is also involved in normal physiological
activities, such as immune suppression and the inhibition of cell
proliferation (28,29). Therefore, when using drugs to
inhibit TGF-β1 expression in antifibrotic therapy, controlled doses
are necessary.
CTGF is a downstream response element of TGF-β1 and
a central channel for the activation of HSCs (30). CTGF inhibitors are able to
selectively block the fibrogenic effect of TGF-β1, and this role of
CTGF is more likely to influence the occurrence of fibrosis
(30). Therefore, CTGF inhibitors
may be used for the effective prevention and treatment of hepatic
fibrosis (31). The present study
demonstrated that following treatment with Astragalus
flavescens, the hyperplasia of collagen fibers in rats with
hepatic fibrosis was significantly reduced, and the expression
levels of TGF-β1 and CTGF in the fibrous septum were significantly
inhibited. These results indicate that Astragalus flavescens
is able to reduce the expression of TGF-β1 and CTGF, inhibit the
activation and proliferation of HSCs, and prevent the continued
amplification effect of cell activation. In addition, it may reduce
the generation of CTGF, thus selectively blocking the fibrogenic
channel of TGF-β1.
At present, it is considered that PDGF-BB is the
most effective mitogenic factor for inducing HSC proliferation and
the related signal transduction. PDGF-BB promotes the activation
and division of HSCs and stimulates collagen synthesis (32–34).
The current study identified that the localization of PDGF
distribution was consistent with the sites at which HSC and
collagen deposition are present. The expression levels of PDGF in
the low- and high-dose treatment groups were significantly lower
than that in the model control group. The results indicate that
Astragalus flavescens inhibited the expression of PDGF and
the proliferation and activation of HSCs, and may therefore prevent
the formation of hepatic fibrosis.
Acknowledgements
This study was supported by the Xi’an
Science and Technology Project (Spark Program Technology Fund;
grant no. SF200212).
References
|
1.
|
Crabb DW: Pathogenesis of alcoholic liver
disease: newer mechanisms of injury. Keio J Med. 48:184–188. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Povero D, Busletta C, Novo E, et al: Liver
fibrosis: a dynamic and potentially reversible process. Histol
Histopathol. 25:1075–1091. 2010.PubMed/NCBI
|
|
3.
|
Svegliati Baroni G, D’Ambrosio L, Ferretti
G, et al: Fibrogenic effect of oridative stress on rat hepatic
stellate cells. Hepatology. 27:720–726. 1998.PubMed/NCBI
|
|
4.
|
Hellerbrand C, Stefanovic B, Giordano F,
Burchardt ER and Brenner DA: The role of TGF-β1 in initiating
hepatic stellate cell activation in vivo. J Hepatol. 30:77–87.
1999.
|
|
5.
|
Tsrkamoto H: Is interleukin 10
antifibrogenic in chronic liver injury? Hepatology. 28:1707–1709.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Knittel T, Janneck T, Müller L, Fellmer P
and Ramadori G: Transforming growth factor beta 1-regulated gene
expression of Ito cells. Hepatology. 24:352–360. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Pinzani M, Milani S, Grappone C, Weber FL
Jr, Gentilini P and Abboud HE: Expression of platelet-derived
growth factor in model of acute liver injury. Hepatology.
19:701–707. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Lasky JA, Ortiz LA, Tonthat B, et al:
Connective tissues growth factor mRNA expression is upregulated in
bleomycin-induced lung fibrosis. Am J Physiol. 275:L365–L371.
1998.PubMed/NCBI
|
|
9.
|
Abraham DJ, Shiwen X, Black CM, Sa S, Xu Y
and Leask A: Tumor necrosis factor alpha suppresses the induction
of connective tissue growth factor by transforming growth
factor-beta in normal and scleroderma fibroblasts. J Biol Chem.
275:15220–15225. 2000. View Article : Google Scholar
|
|
10.
|
Chen MM, Lam A, Abraham JA, Schreiner GF
and Joly AH: CTGF expression is induced by TGF- beta in cardiac
fibroblasts and cardiac myocytes: a potential role in heart
fibrosis. J Mot Cell Cardiol. 32:1805–1819. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Sato S, Nagaoka T, Hasegawa M, Tamatani T,
Nakanishi T, Takigawa M and Takehara K: Serum levels of connective
tissue growth factor are elevated in patients with systemic
sclerosis: association with extent of skin sclerosis and severity
of pulmonary fibrosis. J Rheumatol. 27:149–154. 2000.PubMed/NCBI
|
|
12.
|
Guo Y, Wang H and Zhang C: Establishment
of rat precision-cut fibrotic liver slice technique and its
application in verapamil metabolism. Clin Exp Pharmacol Physiol.
34:406–413. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Han DW, Ma XH and Zhao YC: Research of
animal model of hepatic cirrhosis. Shanxi Medical Journal. 4:1–5.
1979.(In Chinese).
|
|
14.
|
Xue KX, Yan HY and Liu TX:
Immunohistochemistry study on collagen type I and III of
sternomastoid muscle in congenital muscular torticollis. Journal of
Xinxiang Medical College. 22:530–532. 2005.(In Chinese).
|
|
15.
|
15. Han TL, Wang M, Hong M, et al: The
comparison and application of masson trichrome and he staining in
histological paraffin sections of teeth in guinea pig. China Animal
Husbandry & Veterinary Medicine. 38:55–57. 2011.(In
Chinese).
|
|
16.
|
Lei N, Zheng SZ and Lu Y: Mechanisms and
research progress of promoting blood circulation for removing blood
stasis herbs in treating hepatic fibrosis. China Journal of TCM and
Pharmacy. 25:265–268. 2010.(In Chinese).
|
|
17.
|
Yang Q, Feng YY and Jiang SL: Experiences
of professor Yao Xi-xian in treating chronic hepatic fibrosis based
on blood stasis theory. China Journal of TCM and Pharmacy.
22:168–171. 2007.(In Chinese).
|
|
18.
|
Luk JM, Zhang QS, Lee NP, et al: Hepatic
stellate cell-targeted delivery of M6P-HSA-glycyrrhetinic acid
attenuates hepatic fibrogenesis in a bile duct ligation rat model.
Liver Int. 27:548–557. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Constandinou C, Henderson N and Iredale
JP: Modeling liver fibrosis in rodents. Methods Mol Med.
117:237–250. 2005.PubMed/NCBI
|
|
20.
|
Saile B, Matthes N, Neubauer K, et al: Rat
liver myofibroblasts and hepatic stellate cells differ in
CD95-mediated apoptosis and response to TNF-alpha. Am J Physiol
Gastrointest Liver Physiol. 283:G435–G444. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Friedman SL: Molecular regulation of
hepatic fibrosis, an integrated cellular response to tissue injury.
J Biol Chem. 275:2247–2250. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Nieto N, Greenwel P, Friedman SL, Zhang F,
Dannenberg AJ and Cederbaum AI: Ethanol and arachidonic acid
increase alpha 2(I) collagen expression in rat hepatic stellate
cells overexpressing cytochrome P450 2E1. Role of
H2O2 and cyclooxygenase-2. J Biol Chem.
275:20136–20145. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Zhang C, Zhu Y, Wan J, Xu H, Shi H and Lu
X: Effects of Ginkgo biloba extract on cell proliferation,
cytokines and extracellular matrix of hepatic stellate cells. Liver
Int. 26:1283–1290. 2006.
|
|
24.
|
Kuriyama S, Yokoyama F, Inoue H, et al:
Sequential assessment of the intrahepatic expression of epidermal
growth factor and transforming growth factor-beta1 in
hepatofibrogenesis of a rat cirrhosis model. Int J Mol Med.
19:317–324. 2007.PubMed/NCBI
|
|
25.
|
Kaimori A, Potter J, Kaimori JY, Wang C,
Mezey E and Koteish A: Transforming growth factor-beta1 induces an
epithelial-to-mesenchymal transition state in mouse hepatocytes in
vitro. J Biol Chem. 282:22089–22101. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Gressner AM, Weiskirchen R, Breitkopf K
and Dooley S: Roles of TGF-beta in hepatic fibrosis. Front Biosci.
7:d793–d807. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Tamatani T, Kobayashi H, Tezuka K, et al:
Establishment of the enzyme-linked immunosorbent assay for
connective tissue growth factor (CTGF) and its detection in the
sera of biliary atresia. Biochem Biophys Res Commun. 251:748–752.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Song SL, Gong ZJ, Zhang QR, Huang TX and
Wu SK: Changes and significance of cytokines and ultrastructure of
experimental hepatic fibrosis in rats. Chinese Journal of
Integrated Traditional and Western Medicine on Liver Diseases.
14:28–31. 2004.(In Chinese).
|
|
29.
|
McCartney-Francis NL and Wahl SM:
Transforming growth factor beta: a matter of life and death. J
Leukoc Biol. 55:401–409. 1994.PubMed/NCBI
|
|
30.
|
Saegusa S, Isaji S and Kawarada Y: Changes
in serum hyaluronic acid levels and expression of CD44 and CD44
mRNA in hepatic sinusoidal endothelial cells after major
hepatectomy in cirrhotic rats. World J Surg. 26:694–699. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Blom IE, Goldschmeding R and Leask A: Gene
regulation of connective tissue growth factor: new targets for
antifibrotic therapy? Matrix Biol. 21:473–482. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Chen YX, Lu CH, Xie WF, et al: Effects of
ribozyme targeting platelet-derived growth factor receptor beta
subunit gene on the proliferation and apoptosis of hepatic stellate
cells in vitro. Chin Med J (Engl). 118:982–988. 2005.PubMed/NCBI
|
|
33.
|
Yang L, Zhang CZ and Zhu QJ: Kangxian
ruangan keli inhibits hepatic stellate cell proliferation mediated
by PDGF. World J Gastroenterol. 9:2050–2053. 2003.PubMed/NCBI
|
|
34.
|
Guo J and Friedman SL: Hepatic
fibrogenesis. Semin Liver Dis. 27:413–426. 2007. View Article : Google Scholar
|