Introduction
With rapid industrialization, there has been an
increase in the incidence of thoracolumbar burst fracture (TBF) due
to traffic accidents and falls from a height, which causes an
enormous economic burden on families and society. TBF often occurs
in young males (20–50 years old) who engage in high-risk social
activities. It accounts for ∼45% of spine fractures and is
characterized by an intraspinal occupying block, which is a threat
to neurological function. Spinal cord injury accounts for 30–60% of
TBF cases (1).
The treatment of TBF involves relieving spinal cord
compression in order to restore the diameter of the spinal canal,
followed by reconstructing spinal stability. Surgery is required
for the anatomical reduction of abnormalities, strong fixation and
effective decompression. Surgical methods for TBF include the
anterior approach, the posterior approach and the
anterior-posterior combined approach. With the anterior approach,
the surgery is conducted under direct vision and the intraspinal
compression may be removed directly, with thorough decompression.
Furthermore, intervertebral bone grafting and fusion and internal
fixation may be performed to allow for the reconstruction of spinal
stability and the retention of structural integrity in the
posterior spine, with a high fusion rate. However, one spinal motor
unit is damaged during surgery, combined with trauma and bleeding,
leading to a visceral vascular injury. The posterior approach is
performed when there is serious damage to the posterior spine. The
posterior approach is capable of detecting the spinal cord and
treating other combined intraspinal injuries with a short fixed
segment. Therefore, spinal motor function may be retained to a
large extent. This approach has the advantage of being a short,
simple surgery with little trauma and bleeding. Posterolateral bone
grafting may be performed simultaneously. The restriction in
anterior decompression is the main disadvantage of this approach
(2,3). Incomplete decompression (where the
stenosis remains in the spinal canal), poor restoration of
vertebral height and kyphosis following the removal of implanted
material are common features of the anterior and posterior
approaches. Early degeneration of adjacent segments and lower back
pain due to bone grafting and fusion remain problematic.
Posterior short-segment fixation is a widely
accepted approach for TBF. As the postsurgical vertebral bone
defect may cause a lack of support in the anterior and middle
spine, the failure rate of the surgery remains high. A number of
improved techniques and methods for this approach have been
proposed and applied in clinical research (4,5), but
the efficacies require further observation.
B-mode ultrasound has the advantage of real-time
imaging. Certain researchers use B-mode ultrasound and MRI to
detect imaging signal changes following spinal cord injury. The
difference between the spinal cord surgical injury range and the
abnormal MRI signal range is comparatively studied, and the glial
scar border is preliminarily positioned to determine the scope of
the surgery. Furthermore, according to the real-time imaging
feature of B-mode ultrasound, the glial scar is precisely
positioned and completely resected. Real-time B-mode ultra-sound
has been used for fracture reduction. Under the real-time
assistance of B-mode ultrasound, the decompression and restoration
of vertebral body height and spinal canal morphology are performed.
Short-segment fixation is performed, followed by pedicle screw
placement on the fractured vertebral side and vertebral pedicle
autogenous bone grafting is performed on the other side. Thus, the
‘shell’ phenomenon is avoided (6–8).
Furthermore, unilateral vertebral pedicle fixation and bone
grafting may improve the biomechanical stability of the affected
vertebral body, effectively reconstructing the support in the
anterior and middle spine, reducing the stress load on the fixation
system and preventing early adjacent segment regression, kyphosis
and lumbar back pain.
In this study, under the assistance of real-time
B-mode ultrasound, we performed posterior fenestration of vertebral
lamina and bone grafting of the vertebral body via the pedicle for
patients with TBF. The objective of this study was to investigate
the role of real-time B-mode ultrasound in posterior decompression
and reduction and to observe the signal changes in spinal cord
blood flow in TBF patients.
Materials and methods
General data
Between February 2004 and December 2008, 138 TBF
patients from The Ordos Center Hospital (Ordos, China) were
enrolled in this study. They were divided into group A and group B.
In group A (108 patients), there were 80 males and 28 females aged
between 18–60 years, with an average age of 38 years. The patients
were divided into group A and group B. In group A (108 patients)
there were 80 males and 28 females. In group B (30 patients) there
were 24 males and 6 females. Injury factors were as follows:
traffic accidents, 45 cases; falling from height, 40 cases; and
other injuries, 23 cases. The fracture segments were T12-L4.
Fracture types by computed tomography (CT) were as follows: type A,
40 cases; type B, 45 cases; and type C, 23 cases. There were ten
cases with traumatic shock, 18 cases with fractures in the pelvis
and other limbs and 95 cases with neurological symptoms. According
to the Frankel grading scale (9),
12 patients were diagnosed as grade A, 20 as grade B, 38 as grade
C, 15 as grade D and 23 as grade E. In group B (30 cases), there
were 22 males and 8 females aged between 20–58 years. Injury
factors were as follows: traffic accidents, 10 cases; falling from
height, 18 cases; and other injuries, 2 cases. The fracture
segments were T12-L4. Fracture types were determined by CT; there
were 12 type A cases, eight type B and ten type C. There were five
cases with traumatic shock, four cases with fracture in the pelvis
and other limbs and 25 cases with neurological symptoms. According
to the Frankel grading scale, three patients were diagnosed as
grade A, seven as grade B, ten as grade C, five as grade D and five
as grade E.
Follow-up was conducted between 18 months and four
years. Presurgical X-ray, CT and MRI examination revealed that
there were varying degrees of traumatic spinal stenosis in all
patients, with spinal cord compression in the injured spine
analyzed using MRI. The criteria for intraspinal fracture piece by
CT were as follows (3,4): type A, volume of fracture piece in
the spinal canal accounted for <30% of the volume of the spinal
canal; type B, the volume of fracture piece in the spinal canal
accounted for 30–50% of the volume of the spinal canal; type C, the
volume of the fracture piece in the spinal canal accounted for
>50% of the volume of the spinal canal. Presurgical and
postsurgical recovery of neurological function was evaluated
according to American Spinal Injury Association (ASIA)standards,
and the spinal decompression range was determined by measuring the
proportion of the encroaching fracture piece in the spinal canal
(spinal stenosis rate) on the CT image. Written informed consent
was obtained from all patients.
Surgical methods
Patients lay in a prone position and following
general anesthesia, a posterior midline incision, centered by the
injured vertebra, was performed to expose the injured vertebra and
adjacent vertebral pedicles. We implanted five screws in one side
of the injured vertebra and the adjacent vertebral pedicles,
respectively. Under fluoroscopy of the C-arm X-ray machine (GE OEC
Flurostar 7900; GE OEC medical system Inc., Salt Lake City, UT,
USA), the insertion length and direction of the pedicle screw were
adjusted. In group A, fenestration of the vertebral lamina was
performed on one side, followed by observing the correlation
between the fracture piece and the spinal dura mater using
real-time B-mode ultrasound (10)
(Aloka Alpha 10; UST-9128 probe, 5 MHz, Aloka Ltd., Co., Tokyo,
Japan; or GE Vivid 7.0, 10S probe, 10 MHz; GE Healthcare Co.,
Nobelsville, IN, USA). The fracture piece was pushed into the
vertebral body using the L-shaped operative tool until the cortical
bone margin of the fracture piece was parallel to the posterior
margin of the vertebral body, followed by longitudinal distraction
of the vertebral body anterior margin. When a successful reduction
was observed under fluoroscopy, fixation was performed. Following
the pedicle bone grafting on the other pedicle of the injured
vertebra column, an autologous iliac bone fragment (or allograft
bone) was implanted into the hollow cavity of the injured vertebra
and then fixed. The decompression status and correlation between
the fracture piece and the posterior margin of the vertebral body
were explored by B-mode ultrasound, until a satisfactory result was
obtained.
In group B, five screws were implanted in one side
of the injured vertebra and the adjacent vertebral pedicles,
respectively. Bone grafting was performed on one side of the
vertebral pedicle of the injured vertebra, with fenestration of the
opposite vertebral lamina. An L-shaped operative tool pushed the
fracture piece into the vertebral body. Once the incision was
cleaned and the drainage tube had been placed, the incision was
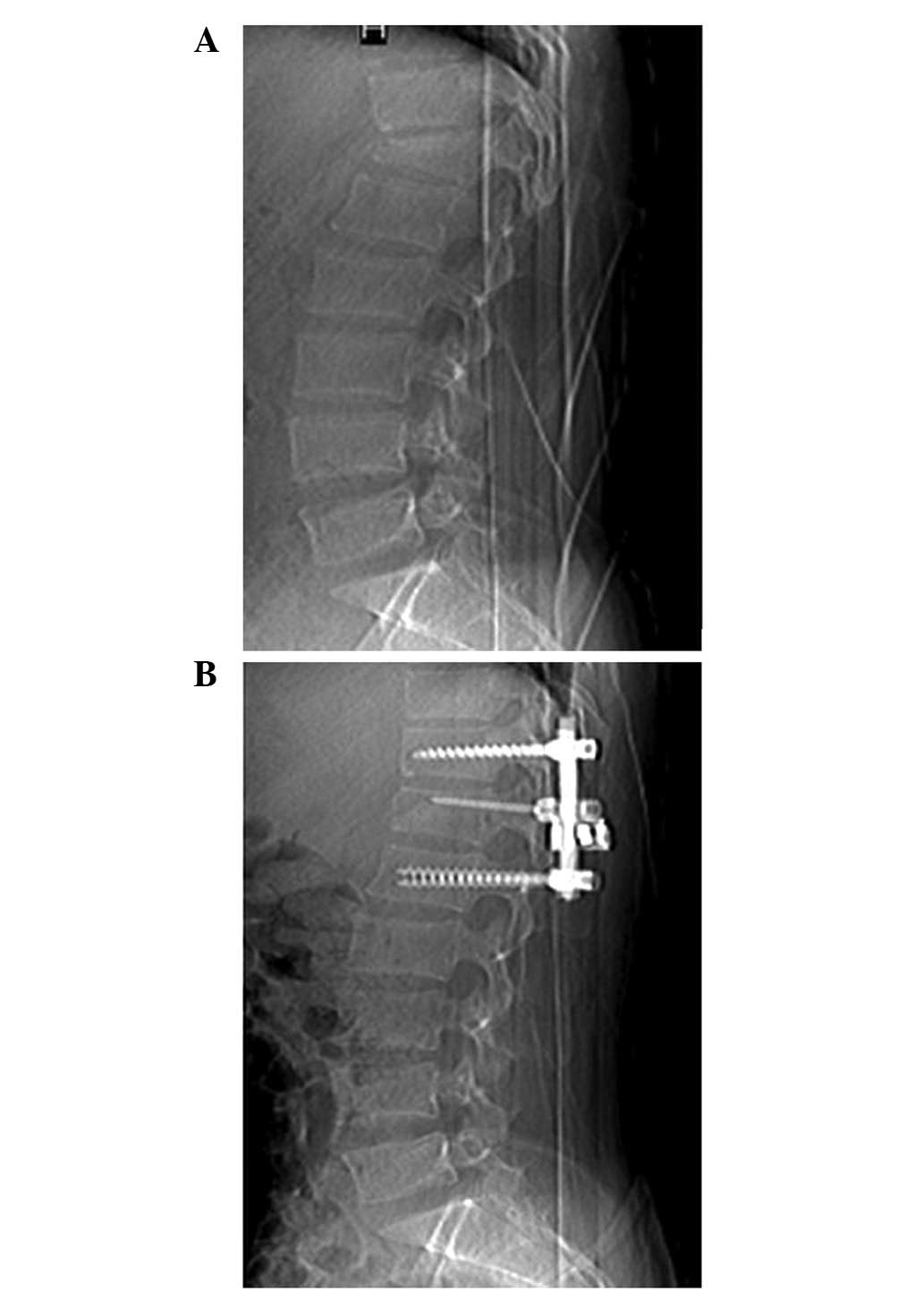
closed. Presurgical and postsurgical X-rays are shown in Fig. 1. The postsurgical CT projections
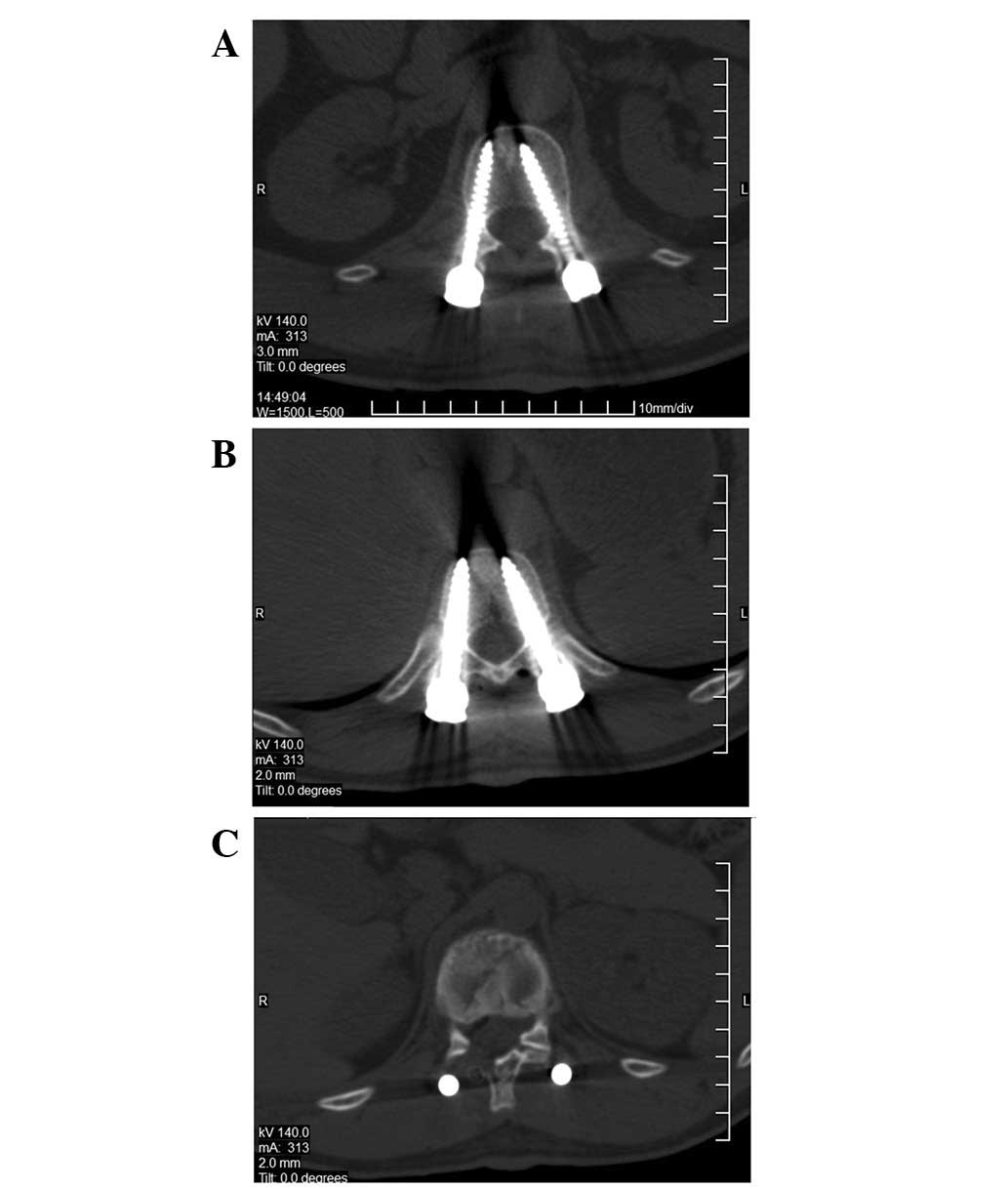
after two weeks are shown in Fig.
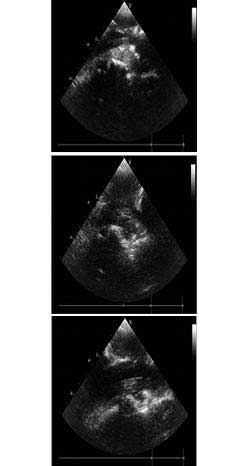
2. The intraoperative B-mode ultrasounds are shown in Fig. 3.
Statistical analysis
Data are expressed as the mean ± SD. Statistical
analysis was performed using SPSS 11.0 statistical software (SPSS,
Chicago, IL, USA). A t-test was used to analyze the differences
between the groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Follow-up results revealed that, in group A, 12
patients were classified as grade A and there were six cases
without neurological recovery. In the other patients, neurological
function increased by 1–3 grades. There were no aggravated spinal
cord injuries or other serious complications. In group B, three
patients were classified as grade A and there were two cases
without neurological recovery. In the other patients, neurological
function increased by 1–3 grades. Table I shows that, in groups A and B, the
postsurgical spinal stenosis rate was significantly lower compared
with the presurgical stenosis rate (P<0.05). The postsurgical
spinal stenosis rate in group B was significantly higher compared
with group A (P<0.05). There was no significant difference in
neurological function recovery between the groups (P>0.05;
Table II).
 | Table I.Comparison of the spinal stenosis rate
between the groups (%). |
Table I.
Comparison of the spinal stenosis rate
between the groups (%).
| Group | Type | CT fracture type
|
|---|
| A | B | C |
|---|
| A | Presurgical | 18.9±3.5 | 39.8±4.4 | 56.6±4.2 |
| Postsurgical | 2.5±2.3 | 1.9±2.3 | 1.2±3.5 |
| B | Presurgical | 19.2±3.3 | 38.0±4.8 | 55.8±3.2 |
| Postsurgical | 6.4±1.2 | 5.9±1.9 | 8.4±4.2 |
 | Table II.Comparison of neurological function
recovery between two groups. |
Table II.
Comparison of neurological function
recovery between two groups.
| Group | Type | Frankel grade
|
|---|
| A | B | C | D | E |
|---|
| A | Presurgical | 12 | 20 | 38 | 15 | 23 |
| Follow-up | 6 | 10 | 12 | 20 | 60 |
| B | Presurgical | 3 | 7 | 10 | 5 | 5 |
| Follow-up | 2 | 3 | 4 | 6 | 15 |
Discussion
Although the necessity for decompression of fracture
pieces encroaching on the spinal canal in TBF patients has been a
controversial topic in recent years, the majority of researchers
believe that effective spinal canal decompression is important for
the recovery of spinal cord function, and it is the current
treatment principle for spine and spinal cord injury. It has been
proposed that spinal cord injuries do not correlate positively with
the state of spinal cord compression under imaging examination, but
are dependent upon the degree of injury (11,12).
Cases with light epidural compression but severe spinal cord injury
are often observed clinically. However, evidence has suggested that
the degree of spinal cord compression demonstrated by imaging
correlates closely with the recovery of spinal cord function.
This study demonstrated that spinal canal
decompression may be performed to treat TBF with spinal cord
injury, particularly for incomplete spinal cord injuries.
Therefore, it remains a method of therapy, even for TBF with clear
spinal cord compression. The posterior approach may detect the
spinal cord and treat other combined intraspinal injuries using a
short fixed segment. It has the advantage of being a short, simple
surgery with little trauma and bleeding. For patients with multiple
traumas, particularly those accompanied by multiple physical
injuries, the posterior approach is particularly advantageous.
Without considering the recovery of neurological function, it may
accelerate the treatment of other injuries. However, some fracture
piece remains after the posterior approach, which interferes with
the spinal cord.
There are three methods for performing fracture
piece reduction or decompression in the posterior approach
(13–15): i) The indirect reduction method.
Traction is reduced on the fractured vertebral body using equipment
without direct decompression. The fracture piece was pressed into
the fractured vertebral body using the elastic tension of the
posterior longitudinal ligament. This method is simple, but there
is a poor level of reduction; ii) the fracture piece is not
completely removed, but it is pushed into the fractured vertebral
body using a bone punch. This method is relatively simple with less
bleeding. However, for a big fracture piece (sagittally occupying
>50%), exposing the fracture piece is difficult and may cause
spinal cord injury during the separation process; iii) annular or
subannular decompression, combined with separating, cutting and
removal of the fracture piece. This method has a good decompression
effect, and is capable of restoring the volume of the spinal canal
to its maximum. In this method, the fracture piece may be separated
and cut layer by layer without exposing the top of the spinal
canal. It has little effect on the spinal cord and is not limited
by the duration following injury (16–18).
Greater intraoperative bleeding and a higher skill requirement are
disadvantages of this method. Furthermore, the range of resection
is greater than the normal posterior border of the fractured
vertebral body, resulting in a loss of bone mass (19–21).
For TBF with serious collapse following posterior
vertebral distraction and reduction, the trabecular bone and
nucleus pulposus of the fractured vertebral body are not completely
restored, with the existence of the ‘shell’ phenomenon (22,23).
In this study, for the 108 patients in group A, real-time B-mode
ultrasound assisted posterior decompression and reduction were
performed and the morphological changes to spine were observed. For
the 30 patients in group B, posterior fenestration combined with
pushing the fracture piece into the fractured vertebral body using
the L-shaped operative tool was performed. Certain researchers use
B-mode ultrasound and MRI in order to detect the imaging signal
changes following spinal cord injury (24). The difference between the spinal
cord surgical injury range and the MRI abnormal signal range is
comparatively studied, and the glial scar border is preliminarily
positioned. However, according to the real-time imaging feature of
B-mode ultrasound, the glial scar is precisely positioned and
ultimately completely resected. In group A of this study, the
real-time B-mode ultrasound detected and directed the reduction of
the fracture piece and observed the pulsation and change in blood
flow of the spinal cord. In group B, post-surgical CT observation
revealed that the reduction effect of the fracture piece was
reduced compared with group A, with a significantly higher spinal
stenosis rate. During the observation period, the neurological
function recoveries in the groups were not significantly different,
which may be due to the small sample size.
In conclusion, neurological deficits following
spinal cord injury may cause various levels of somatic dysfunction,
with paraplegia in severe cases, which leads to an enormous
economic burden on families and society (25,26).
The selection of suitable surgical methods contributes to the
rehabilitation of the patient and a reduction in postsurgical
complications to the maximum extent. For TBF treatment, posterior
decompression and internal fixation assisted by real-time B-mode
ultrasound may shorten hospitalization time, reduce mortality,
protect the spinal motor function unit, prevent early spinal
degeneration and is the most suitable method for the posterior
approach.
References
|
1.
|
Bohlman HH, Kirkpatrick JS, Delamarter RB
and Leventhal M: Anterior decompression for late pain and paralysis
after fractures of the thoracolumbar spine. Clin Orthop Relat Res.
300:24–29. 1994.PubMed/NCBI
|
|
2.
|
Eberl R, Kaminski A, Müller EJ and Muhr G:
Importance of the cross-sectional area of the spinal canal in
thoracolumbar and lumbar fractures. Is there any correlation
between the degree of stenosis and neurological deficit? Orthopade.
32:859–864. 2003.(In German).
|
|
3.
|
Xu BS, Tang TZ, Ni CF, Yang HL, Xu YZ and
Bao ZH: Clinical application of short-segment pedicle instrument
and vertebroplasty for thoracolumar fractures. Chinese Journal of
Trauma. 5:264–266. 2003.
|
|
4.
|
McDonough PW, Davis R, Tribus C and
Zdeblick TA: The management of acute thoracolumbar burst fractures
with anterior corpectomy and Z-plate fixation. Spine. 29:1901–1908.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Heary RF, Salas S, Bono CM and Kumar S:
Complication avoidance: thoracolumbar and lumbar burst fractures.
Neurosurg Clin N Am. 17:377–388. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Dai LY, Jiang LS and Jiang SD:
Anterior-only stabilization using plating with bone structural
autograft versus titanium mesh cages for two- or three-column
thoracolumbar burst fractures: a prospective randomized study.
Spine (Phila Pa 1976). 34:1429–1435. 2009. View Article : Google Scholar
|
|
7.
|
Radcliff K, Kepler CK, Rubin TA, et al:
Does the load-sharing classification predict ligamentous injury,
neurological injury, and the need for surgery in patients with
TBFs?: Clinical article. J Neurosurg Spine. 16:534–538. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Kim MS, Eun JP and Park JS: Radiological
and clinical results of laminectomy and posterior stabilization for
severe thoracolumbar burst fracture: surgical technique for
one-stage operation. J Korean Neurosurg Soc. 50:224–230. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Standard for neurologic and functional
class of spinal cord injury. Chicago: American Spinal Injury
Association; 1992
|
|
10.
|
Lu FF, Chen Q, Zhong SY, et al: Precise
localization of glial scar boundary in chronic spinal cord injury
in dogs. Chinese Journal of Neurology. 5:489–492. 2009.
|
|
11.
|
Li Q, Liu Y, Chu Z, Chen J and Chen M:
Treatment of thoracolumbar fractures with transpedicular
intervertebral bone graft and pedicle screws fixation in injured
vertebrae. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 25:956–959.
2011.(In Chinese).
|
|
12.
|
Alpantaki K, Bano A, Pasku D, et al:
Thoracolumbar burst fractures: a systematic review of management.
Orthopedics. 33:422–429. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Parker JW, Lane JR, Karaikovic EE and
Gaines RW: Successful short-segment instrumentation and fusion for
thoracolumbar spine fractures: a consecutive 41/2-year series.
Spine (Phila Pa 1976). 25:1157–1170. 2000.PubMed/NCBI
|
|
14.
|
Marco RA, Meyer BC and Kushwaha VP:
Thoracolumbar burst-fractures treated with posterior decompression
and pedicle screw instrumentation supplemented with
balloon-assisted vertebroplasty and calcium phosphate
reconstruction. Surgical technique. J Bone Joint Surg Am. 92:67–76.
2010.
|
|
15.
|
Mohanty SP, Bhat SN and Ishwara-Keerthi C:
The effect of posterior instrumentation of the spine on canal
dimensions and neurological recovery in thoracolumbar and lumbar
burst fractures. Musculoskelet Surg. 95:101–106. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Liao JC, Fan KF, Keorochana G, Chen WJ and
Chen LH: Transpedicular grafting after short-segment pedicle
instrumentation for thoracolumbar burst fracture: calcium sulfate
cement versus autogenous iliac bone graft. Spine (Phila Pa 1976).
35:1482–1488. 2010.
|
|
17.
|
Afzal S, Akbar S and Dhar SA: Short
segment pedicle screw instrumentation and augmentation
vertebroplasty in lumbar burst fractures: an experience. Eur spine
J. 17:336–341. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Li Q, Li XZ, Liu Y, et al: Treatment of
thoracolumbar fracture with pedicle screws at injury level: a
biomechanical study based on three-dimensional finite element
analysis. Eur J Orthop Surg Traumatol. Sep 19–2012.(Epub ahead of
print).
|
|
19.
|
Jindal N, Sankhala SS and Bachhal V: The
role of fusion in the management of burst fractures of the
thoracolumbar spine treated by short segment pedicle screw
fixation: a prospective randomised trial. J Bone Joint Surg Br.
94:1101–1106. 2012.PubMed/NCBI
|
|
20.
|
Ugras AA, Akyildiz MF, Yilmaz M, Sungur I
and Cetinus E: Is it possible to save one lumbar segment in the
treatment of thoracolumbar fractures? Acta Orthop Belg. 78:87–93.
2012.PubMed/NCBI
|
|
21.
|
Eno JJ, Chen JL and Mitsunaga MM: Short
same-segment fixation of thoracolumbar burst fractures. Hawaii J
Med Public Health. 71:19–22. 2012.PubMed/NCBI
|
|
22.
|
Riaz-ur-Rehman, Azmatullah, Azam F,
Mushtaq and Shah M: Treatment of traumatic unstable thoracolumbar
junction fractures with transpedicular screw fixation. J Pak Med
Assoc. 61:1005–1008. 2011.PubMed/NCBI
|
|
23.
|
Ge CM, Wang YR, Jiang SD and Jiang LS:
Thoracolumbar burst fractures with a neurological deficit treated
with posterior decompression and interlaminar fusion. Eur Spine J.
20:2195–2201. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Yang W, Zhao X, Wang Q, et al: Application
of the real-time monitoring with ultrasonography in the vertebral
and spinal cord operation. Chin J Med Ultrasound. 12:2139–2142.
2010.
|
|
25.
|
Mavrogenis A, Tsibidakis H, Papagelopoulos
P, et al: Posterior transpedicular decompression for thoracolumbar
burst fractures. Folia Med (Plovdiv). 52:39–47. 2010.PubMed/NCBI
|
|
26.
|
Marino RJ, Ditunno JF Jr, Donovan WH and
Maynard F Jr: Neurologic recovery after traumatic spinal cord
injury: data from the Model Spinal Cord Injury Systems. Arch Phys
Med Rehabil. 80:1391–1396. 1999. View Article : Google Scholar : PubMed/NCBI
|