Introduction
Over two-thirds of individuals with spinal cord
injury (SCI) experience the effects of neuropathic pain in their
daily lives. Neuropathic pain is resistant to general analgesic
treatment and is a long-term issue for SCI patients. SCI causes
severe motor and sensory dysfunction, while neuroinflammation is an
important secondary event in the injury cascade. The development of
strategies to minimize this auto-destructive injury is one of the
main aims in the field of SCI research. A number of studies have
demonstrated remarkable protection and functional recovery using
anti-inflammatory reagents in SCI models (1–7).
However, to date, there have been no studies concerning the use of
anti-inflammatory reagents to reduce neuropathic pain following
SCI. The interleukin-6 (IL-6) cytokine is important in mediating
pro-inflammatory damage after SCI (8–10).
Activation of the Janus kinase and signal transducer and activator
of transcription 3 (JAK-STAT3) signaling pathway by IL-6 is an
important mechanism for transducing signals from the cell surface
and is strongly linked to immune/inflammatory reactions (11,12).
Early activation of this pathway occurs most often in spinal
microglia and contributes to the development of neuropathic pain
(13–15). Attenuation of IL-6 activity is
therefore an attractive therapeutic strategy for reducing the
neurological deficits associated with SCI. The present study
reports a significant reduction of neuropathic pain in mice with
SCI following the administration of anti-mouse IL-6 receptor
antibody (MR16-1).
Materials and methods
Experimental procedures
All experiments were approved by the Ethics
Committee for Animal Studies at Yamaguchi University (Ube, Japan)
and were carried out in accordance with the Guidelines for Proper
Conduct of Animal Experiments, Science Council of Japan (June 1,
2006).
MR16-1
The rat anti-mouse IL-6 receptor monoclonal antibody
(MR16-1), a gift from Chugai Pharmaceuticals Co. Ltd., (Tokyo,
Japan), was prepared as described previously (16). An isotype of this antibody is IgG1.
MR16-1 has been shown to bind to the soluble mouse IL-6 receptor
and suppress IL-6-induced cellular responses in a dose-dependent
manner. Other basic characterizations of this antibody have been
described in previously published reports (8).
Animals and surgery
Sixty adult female C57BL/6J mice (10 weeks old) were
obtained from Japan SLC, Inc. (Shizuoka, Japan) and assigned to the
following groups: The MR16-1 group, comprising MR16-1-treated mice
(n=25); the control group, comprising untreated SCI mice (n=25);
and the sham group, comprising mice subjected to laminectomy but
with normal spinal cords (n=10). Mice were anesthetized with an
intraperitoneal injection of ketamine (100 mg/kg) and xylazine (10
mg/kg). Laminectomy was performed at the level of the 10th thoracic
vertebra under a surgical microscope. A contusion SCI model was
produced using an Infinite Horizon (IH)-impactor (PSI Inc.,
Lexington, KY, USA) with an impact force of 60 kdyn (17). Immediately after injury, MR16-1 was
continuously injected for 1–14 days (150μg/day) using Alzet
osmotic pumps (DURECT Corporation., Cupertino, CA, USA).
ELISA analysis of interleukin-6
concentration
Spinal cord tissue (5 mm in length) at the lesion
epicenter was dissected in each group (5 animals per group) at 12,
24 and 72 h after injury. Spinal cord tissue samples to be used for
ELISA analysis were homogenized in radio-immunoprecipitation assay
(RIPA) lysis buffer (500 μl; Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA) and the IL-6 concentration was measured using
a mouse IL-6 ELISA kit (Invitrogen Life Technologies, Carlsbad, CA,
USA) according to the manufacturer’s instructions. The IL-6 levels
were expressed in pg/mg (18).
Assessment of motor function
recovery
The Basso Mouse Scale (BMS) is a validated scale
used to monitor the progress of hind-limb functional recovery
following SCI. The scale ranges from 0 (no ankle movement) to 9
(complete functional recovery) points (19). BMS scores were recorded at 3, 7,
14, 21, 28 and 42 days following SCI by two independent examiners
who were blind to the experimental conditions. Hind-limb motion was
used to assess coordinated movement and stepping. When differences
in the BMS score between the right and left hind limbs were
observed, the average of the two scores was used.
Assessment of sensory function recovery
and allodynia
Sensory tests were performed 21 and 42 days after
SCI. All behavioral tests were conducted by an experienced
investigator who was blind to the type of intervention. Each hind
paw was tested three times. Paw withdrawal latencies to heat were
measured according to the Hargreaves’ method (20) by applying a standard Plantar test
(Ugo Basile, Comerio, Italy). The animals were placed on a glass
surface and a radiant heat source was positioned under one hind
paw. The latency to paw withdrawal was recorded automatically. Paw
withdrawal thresholds to tactile stimulation were measured
according to the von Frey test using a standard Dynamic Plantar
Aesthesiometer (Ugo Basile). The animals were placed in plexiglass
cages on a wire mesh. The plantar surface of the hind paw was
probed with a von Frey monofilament (21) and the force required for paw
withdrawal was recorded automatically. The von Frey filament and
thermal threshold tests were used to measure mechanical allodynia
and thermal hyperalgesia, respectively.
Histological analysis
Spinal cords were harvested 42 days after surgery.
Mice were anesthetized with an intraperitoneal injection of
ketamine (100 mg/kg) and xylazine (10 mg/kg) and then perfused with
4% paraformaldehyde. A 1-cm length of spinal cord that included the
lesion center was removed and frozen for sectioning. The tissue was
sectioned axially in 10-μm-thick sections. Transverse
sections from the injury epicenter were also stained for myelin
using Luxol fast blue (LFB). LFB-positive areas in which the
density significantly exceeded the threshold of each background
were calculated as the percentage cross-sectional area of residual
tissue. Tissue sections were analyzed using the Cavalieri Probe
(Stereo Investigator 64-bit software; MBF Bioscience, Williston,
VT, USA) (22).
Electrophysiological evaluation
Sensory evoked potentials (SEPs) were recorded from
the MR16-1 (n=10), control (n=10) and sham (n=5) groups 42 days
after SCI. SEPs following sciatic nerve electrical train
stimulation were recorded from the sensory cortex of the brain in
mice anesthetized with ketamine. A ground electrode was inserted
subcutaneously between the stimulating and recording electrodes and
a constant current stimulus (S) of 0.1 msec duration and 2.0 mA
intensity was applied at a rate of 5.7 Hz to the hind paw. At a
band-width of 10–3,000 Hz, a total of 200 traces were averaged and
replicated (23). SEP peak latency
and amplitude were measured from the start of S to the peak of the
first positive peak (P1).
Statistical analysis
All data in this study are expressed as the mean ±
SEM. ELISA data and electrophysiological latency data were analyzed
using a one-way ANOVA. The BMS and histological data were analyzed
using a two-way ANOVA for repeated measures. Significant ANOVA
results were followed by post-hoc Bonferroni analysis. Sensory
variables showed normal distribution in the Kolmogorow-Smirnow
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
ELISA data
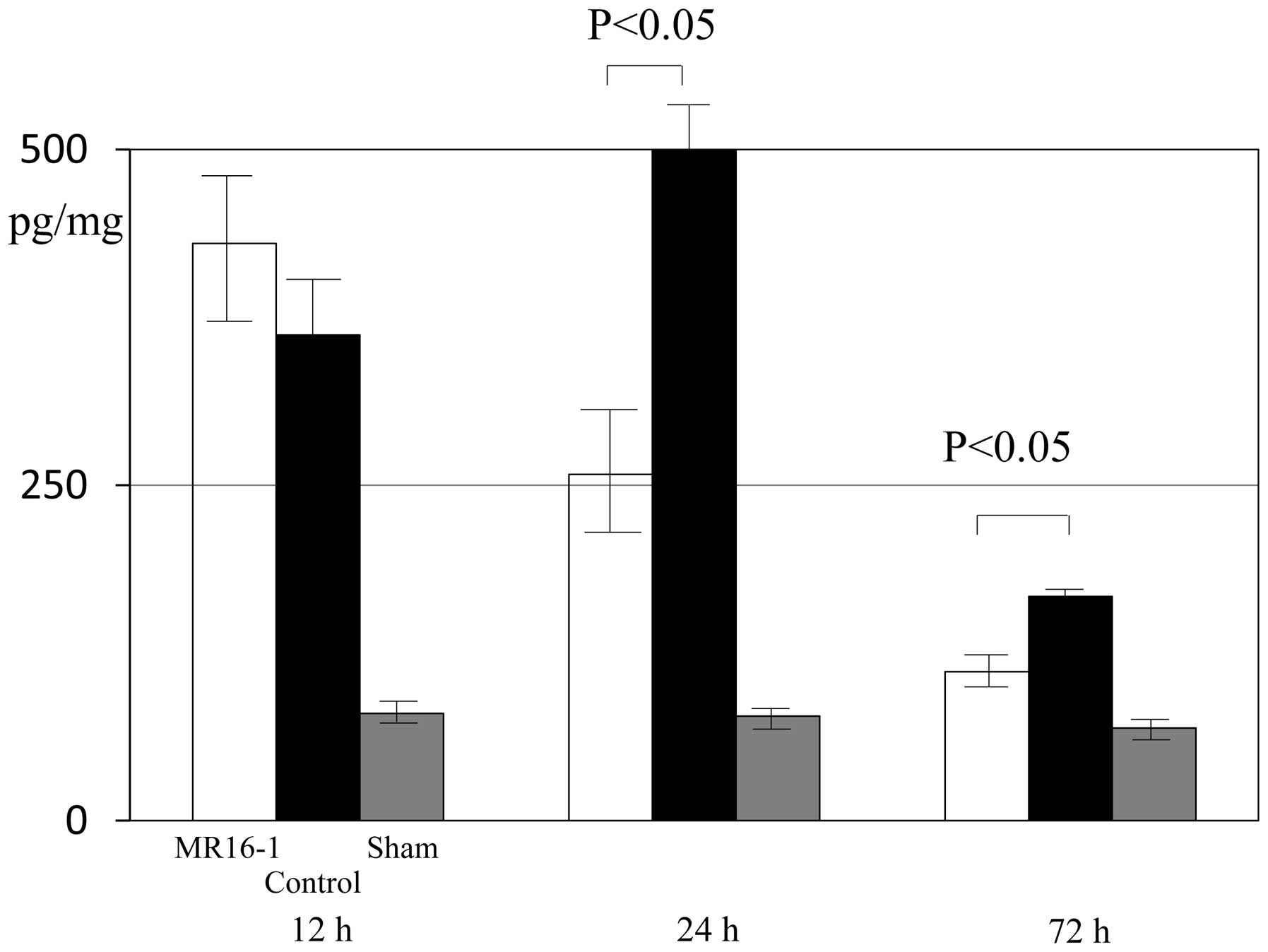
At 12 h after SCI, the expression levels of IL-6
were 430.6±66.8, 362.1±42.3 and 80.0±12.0 pg/mg in the MR16-1,
control and sham groups, respectively. No significant differences
in the expression levels of IL-6 in the spinal cord were identified
between the MR16-1 and control groups 12 h after SCI. At 24–72 h
after SCI, the expression of IL-6 in injured spinal cord tissue was
significantly lower in the MR16-1 group than in the control group
(Fig. 1).
At 24 h following SCI, the expression levels of IL-6
were 258.0±44.7, 503.3±24.0 and 78.0±12.0 pg/mg in the MR16-1,
control and sham groups, respectively. At 72 h after SCI, the
expression levels of IL-6 were 111.2±6.9, 166.4±5.0 and 77.0±11.5
pg/mg in the MR16-1, control and sham groups, respectively. Between
72 h and 2 weeks following SCI, no significant differences were
identified in the expression levels of IL-6 in the spinal cord
among the three groups.
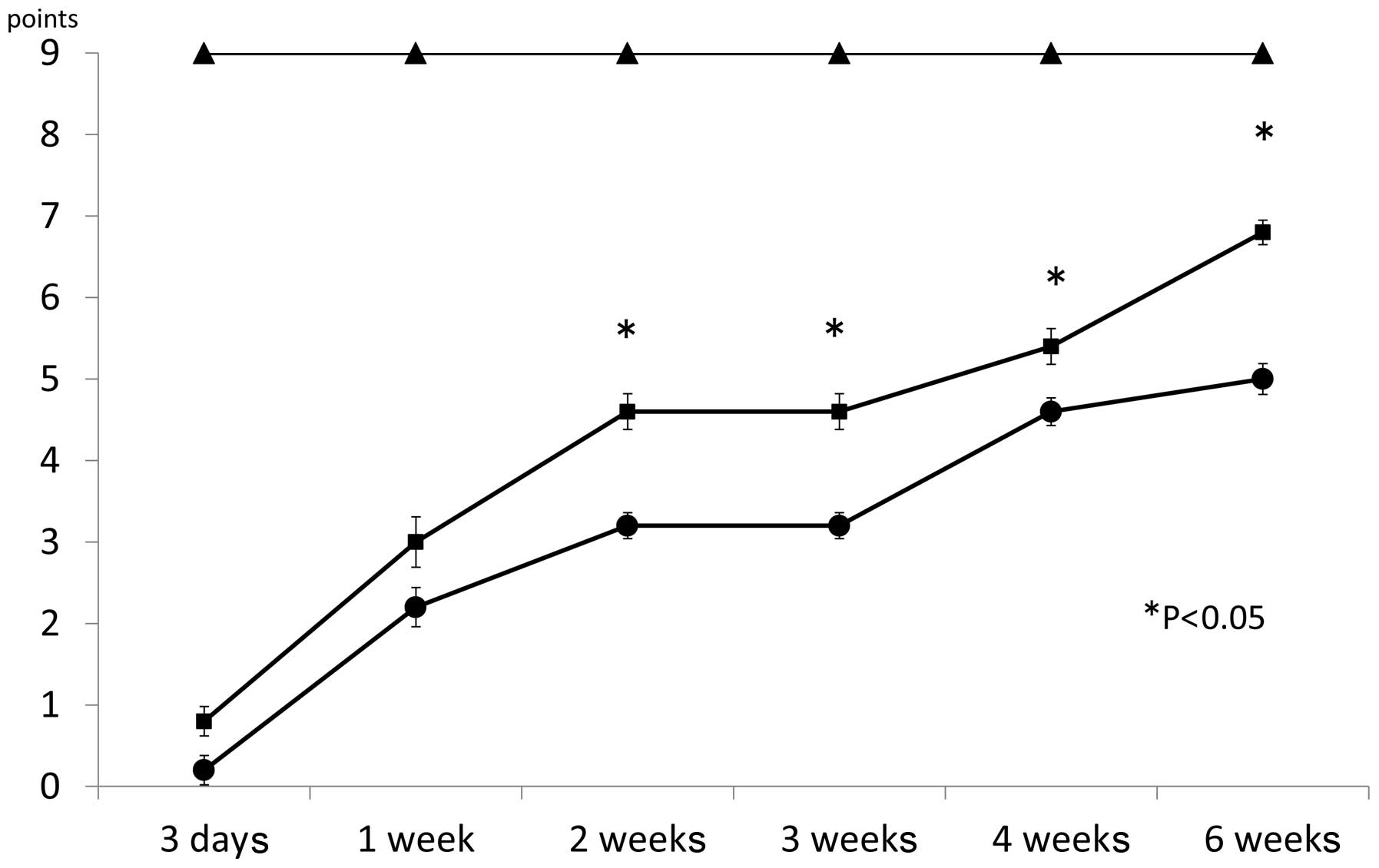
Motor function
Mice injected with MR16-1 showed continuous recovery
of motor function. Three days after surgery, the BMS score was
0.8±0.18 for the MR16-1 group, 0.2±0.18 for the control group and
9.0±0.00 for the sham group (Fig.
2). One week after surgery, the BMS score was 3.0±0.31 for the
MR16-1 group, 2.2±0.24 for the control group and 9.0±0.00 for the
sham group. Two weeks after surgery, the BMS score was 4.6±0.22 for
the MR16-1 group, 3.2±0.16 for the control group and 9.0±0.00 for
the sham group. Three weeks after surgery, the BMS score was
4.6±0.22 for the MR16-1 group, 3.2±0.16 for the control group and
9.0±0.00 for the sham group. Four weeks after surgery, the BMS
score was 5.4±0.22 for the MR16-1 group, 4.6±0.17 for the control
group and 9.0±0.00 for the sham group. Six weeks after surgery, the
BMS score was 6.8±0.15 for the MR16-1 group, 5.0±0.19 for the
control group and 9.0±0.00 for the sham group. Between 2 and 6 week
after SCI, the BMS scores between the MR16-1 and sham groups were
significantly different.
The BMS scores for the MR16-1 and control groups
indicated gradual recovery one week after SCI. However, between 2
and 6 weeks following MR16-1 treatment, mice in the MR16-1 group
showed a marked recovery compared with those in the control group
(Fig. 2).
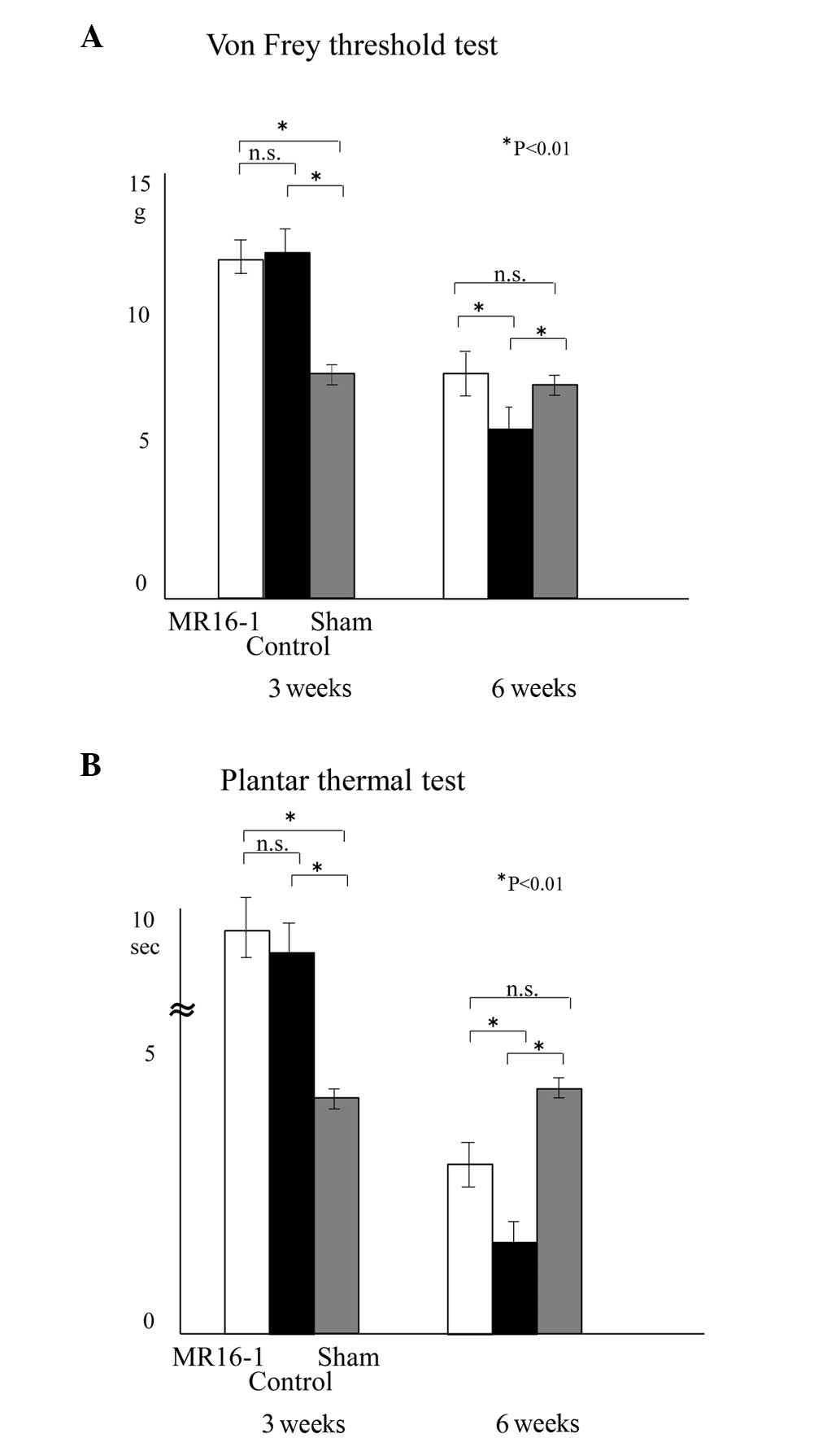
Sensory functional recovery and
prevention of allodynia
The mice that were continuously infused with MR16-1
showed recovery of sensory function (Fig. 3). The sensory scores of the mice in
the von Frey and thermal tests were as follows: in the MR16-1 group
3 weeks after SCI, 11.7±0.64 g and 9.8±0.86 sec, respectively, and
at 6 weeks, 7.0±0.84 g and 2.9±0.37 sec, respectively; in the
control group 3 weeks after SCI, 11.9±0.69 g and 9.2±0.82 sec,
respectively and at 6 weeks, 5.3±1.1 g and 1.7±0.27 sec,
respectively; in the sham group 3 weeks after SCI, 6.9±0.26 g and
4.2±0.23 sec, respectively, and at 6 weeks, 6.4±0.24 g and 4.5±0.21
sec, respectively.
At 3 weeks after SCI, the majority of mice were in
the recovery phase for sensory function. It was not possible to
evaluate the occurrence of allodynia since the majority of mice
were under hypesthesia. Six weeks after SCI, hyperalgesia was
significantly suppressed in the MR16-1 group compared with the
control group. The data for the control group demonstrate the
natural course of sensory recovery in SCI, while the data for the
sham group demonstrate normal sensory evaluation without
neurological deficit.
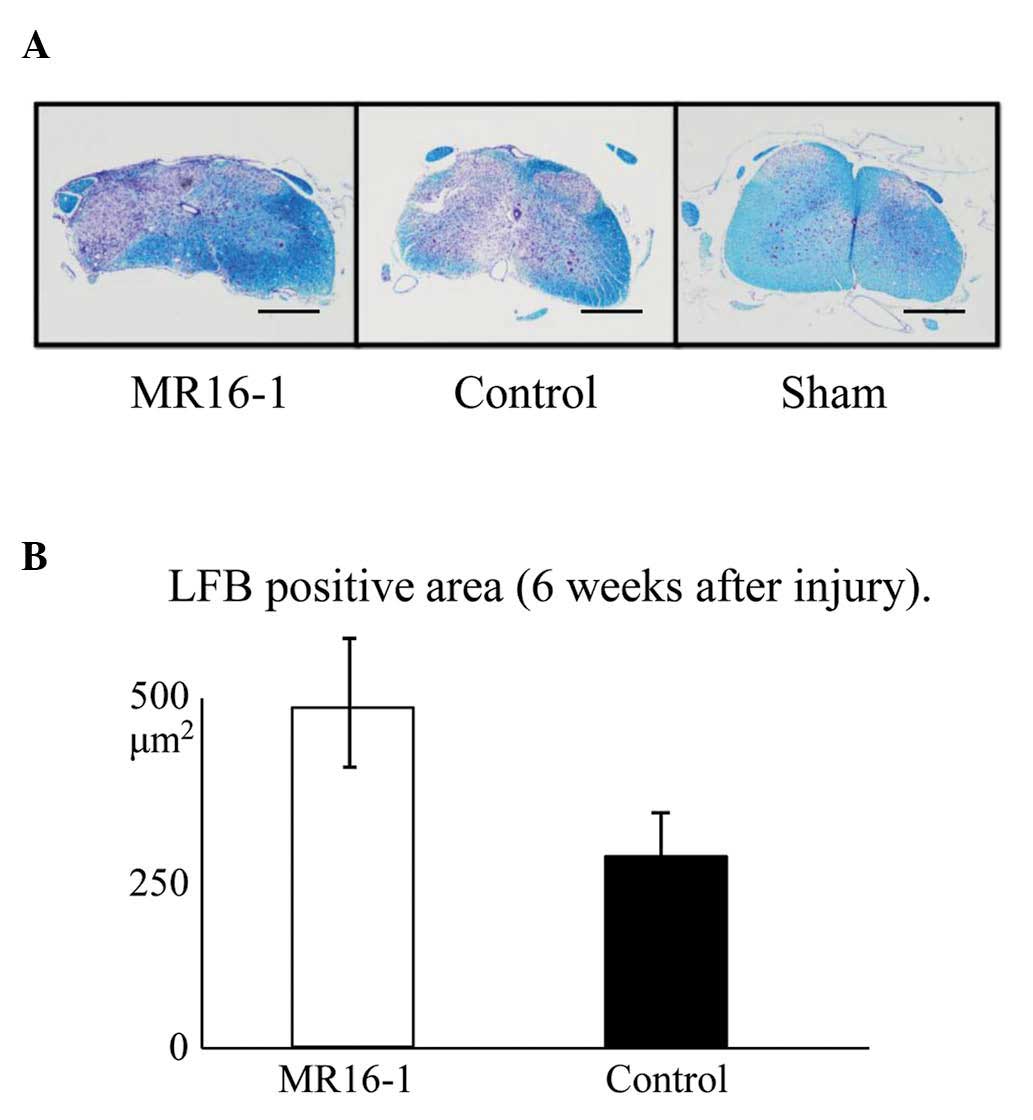
LFB stain
The area of Luxol fast blue-stained tissue,
representing spared myelin sheath, was significantly increased as a
result of treatment with MR16-1 (Fig.
4A).
The LFB-positive area at the center of the axial
section of the SCI was 482±22 μm2 in the MR16-1
group and 263±43 μm2 in the control group
(Fig. 4B), demonstrating
significant preservation in the MR16-1 group.
SEPs
SEP recordings were performed 6 weeks after SCI in
order to provide electrophysiological evidence for the recovery of
sensory function. The latency of the first wave was 7.4±1.1 msec in
the MR16-1 group, 7.7±0.9 msec in the control group and 7.1±0.5
msec in the sham group (no significant differences were
identified). However, the amplitude of the first wave was 9.1±1.9
msec in the MR16-1 group, 4.6±0.8 msec in the control group and
14.1±0.7 msec in the sham group. The amplitude for the MR16-1 group
was significantly lower than that of the sham group, but
significantly higher than that of the control group. MR16-1-treated
SCI mice exhibited suppressed allodynia and increased sensory
function, as determined by electrophysiological measurements.
Discussion
Chronic pain and allodynia following SCI represent a
therapeutic challenge. To the best of our knowledge, the present
study is the first report demonstrating that MR16-1 suppresses
allodynia and hyperalgesia in mice with SCI. Mice treated with
MR16-1 exhibited only moderate hyperalgesia of the lower limbs.
A previous study reported that the administration of
IL-6 cytokine at lesion sites one day after injury increased the
recruitment and the peak of macrophage, neutrophil and microglial
cell activity at the lesion sites to a greater extent than when
administered 4 days after injury (24). In the present study, the
concentration of IL-6 at the injury site between 24 and 72 h after
SCI was significantly reduced in mice treated with MR16-1. These
mice also exhibited improved locomotor BMS scores from 14 days
after SCI compared with untreated mice. This result suggests that
MR16-1 conferred a neuroprotective effect in SCI mice. This is in
agreement with a previous study in which MR16-1 treatment for SCI
decreased connective tissue formation due to astrogliosis (25). The suppression of gliosis leads to
protection of the myelin sheath in the injured spinal cord
(25). In the present study, an
increase in myelin preservation was also observed, as revealed by
LFB staining at 42 days post-injury. Furthermore, SEPs confirmed
that MR16-1 treatment for SCI led to electrophysiological sensory
recovery. In this study, cord dysfunction at the thoracic level was
revealed by recording hind-limb SEPs following SCI. Potentials
evoked in hind-limbs appeared as well-characterized peaks, and
provide a sensitive and quantitative measure to detect pathological
changes following SCI. This is in accordance with a number of
clinical studies reporting that pathological changes in SEPs are a
sensitive method for the detection of the extent of cord injury
(23). Although this method
primarily represents the function of the somatosensory pathways,
SEPs have been used extensively in neurophysiological assessments
of spinal cord integrity (23,26).
IL-6 is one of the major proinflammatory cytokines
that triggers secondary injury in the pathophysiology of SCI. It is
known to promote the activation and infiltration of macrophages and
microglia, the major inflammatory cells observed in SCI (9). When IL-6 is released, it binds to the
membrane-bound IL-6 receptor (IL-6R) to form an IL-6/IL-6R complex
that associates with the signal transducer, gp130, transmitting a
signal into cells (16). In
addition, the gp130-JAK/STAT pathway promotes the differentiation
of astrocytes. These cells produce a chondroitin sulfate
proteoglycan (CSPG) that forms a glial scar. Therefore, the
overexpression of IL-6 enhances inflammation and tissue injury
(25). By contrast, previous
studies using gene-knockout animals have revealed that the
excessive inhibition of IL-6 signaling is detrimental to functional
recovery, since it inhibits axonal regeneration or causes failed
gliosis, which implies that IL-6 may also have a beneficial
function in spinal cord repair (9,25).
Based on these findings and the theory that a blockade of IL-6
signaling may reduce the extent of post-SCI inflammatory damage,
previous researchers have administered MR16-1 following SCI and
demonstrated reduced inflammation, decreased astrogliosis and
enhanced tissue sparing, leading to improved functional
recovery.
In conclusion, the present results suggest that
continuous blockade of IL-6 signaling following SCI reduces
damaging inflammatory activity, thus promoting functional and
sensory recovery. A humanized antibody against human IL-6 receptor
(MRA; tocilizumab) is already in clinical use for the treatment of
rheumatoid arthritis. This drug may also represent a novel option
for the treatment of human SCI.
References
|
1.
|
Donnelly DJ and Popovich PG: Inflammation
and its role in neuroprotection, axonal regeneration and functional
recovery after spinal cord injury. Exp Neurol. 209:378–388. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Jones TB, McDaniel EE and Popovich PG:
Inflammatory-mediated injury and repair in the traumatically
injured spinal cord. Curr Pharm Des. 11:1223–1236. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Popovich PG and Jones TB: Manipulating
neuroinflammatory reactions in the injured spinal cord: back to
basics. Trends Pharmacol Sci. 24:13–17. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Schwab JM, Brechtel K, Mueller CA, et al:
Experimental strategies to promote spinal cord regeneration - an
integrative perspective. Prog Neurobiol. 78:91–116. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Thuret S, Moon LD and Gage FH: Therapeutic
interventions after spinal cord injury. Nat Rev Neurosci.
7:628–643. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Lee HL, Lee KM, Son SJ, Hwang SH and Cho
HJ: Temporal expression of cytokines and their receptors mRNAs in a
neuropathic pain model. Neuroreport. 15:2807–2811. 2004.PubMed/NCBI
|
|
7.
|
Siddall P, Xu CL and Cousins M: Allodynia
following traumatic spinal cord injury in the rat. Neuroreport.
6:1241–1244. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Okada S, Nakamura M, Mikami Y, et al:
Blockade of interleukin-6 receptor suppresses reactive astrogliosis
and ameliorates functional recovery in experimental spinal cord
injury. J Neurosci Res. 76:265–276. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Mukaino M, Nakamura M, Yamada O, et al:
Anti-IL-6-receptor antibody promotes repair of spinal cord injury
by inducing microglia-dominant inflammation. Exp Neurol.
224:403–414. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Lacroix S, Chang L, Rose-John S and
Tuszynski MH: Delivery of hyper-interleukin-6 to the injured spinal
cord increases neutrophil and macrophage infiltration and inhibits
axonal growth. J Comp Neurol. 454:213–228. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Hirano T, Nakajima K and Hibi M: Signaling
mechanisms through gp130: a model of the cytokine system. Cytokine
Growth Factor Rev. 8:241–252. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Cattaneo E, Conti L and De-Fraja C:
Signalling through the JAK-STAT pathway in the developing brain.
Trends Neurosci. 22:365–369. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Dominguez E, Rivat C, Pommier B, Mauborgne
A and Pohl M: JAK/STAT3 pathway is activated in spinal cord
microglia after peripheral nerve injury and contributes to
neuropathic pain development in rat. J Neurochem. 107:50–60. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Yamauchi K, Osuka K, Takayasu M, et al:
Activation of JAK/STAT signalling in neurons following spinal cord
injury in mice. J Neurochem. 96:1060–1070. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Dominguez E, Mauborgne A, Mallet J,
Desclaux M and Pohl M: SOCS3-mediated blockade of JAK/STAT3
signaling pathway reveals its major contribution to spinal cord
neuroinflammation and mechanical allodynia after peripheral nerve
injury. J Neurosci. 30:5754–5766. 2010. View Article : Google Scholar
|
|
16.
|
Okazaki M, Yamada Y, Nishimoto N,
Yoshizaki K and Mihara M: Characterization of anti-mouse
interleukin-6 receptor antibody. Immunol Lett. 84:231–240. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Scheff SW, Rabchevsky AG, Fugaccia I, Main
JA and Lumpp JE Jr: Experimental modeling of spinal cord injury:
characterization of a force-defined injury device. J Neurotrauma.
20:179–193. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Nakajima Y, Osuka K, Seki Y, et al:
Taurine reduces inflammatory responses after spinal cord injury. J
Neurotrauma. 27:403–410. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Basso DM, Fisher LC, Anderson AJ, Jakeman
LB, McTigue DM and Popovich PG: Basso Mouse Scale for locomotion
detects differences in recovery after spinal cord injury in five
common mouse strains. J Neurotrauma. 23:635–659. 2006. View Article : Google Scholar
|
|
20.
|
Hargreaves K, Dubner R, Brown F, Flores C
and Joris J: A new and sensitive method for measuring thermal
nociception in cutaneous hyperalgesia. Pain. 32:77–88. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Kim HY, Wang J, Lu Y, Chung JM and Chung
K: Superoxide signaling in pain is independent of nitric oxide
signaling. Neuroreport. 20:1424–1428. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Chen KB, Uchida K, Nakajima H, et al:
Tumor necrosis factor-α antagonist reduces apoptosis of neurons and
oligodendroglia in rat spinal cord injury. Spine (Phila Pa 1976).
36:1350–1358. 2011.
|
|
23.
|
Cho N, Nguyen DH, Satkunendrarajah K,
Branch DR and Fehlings MG: Evaluating the role of IL-11, a novel
cytokine in the IL-6 family, in a mouse model of spinal cord
injury. J Neuroinflammation. 9:1342012. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Klusman I and Schwab ME: Effects of
pro-inflammatory cytokines in experimental spinal cord injury.
Brain Res. 762:173–184. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Guerrero AR, Uchida K, Nakajima H, et al:
Blockade of interleukin-6 signaling inhibits the classic pathway
and promotes an alternative pathway of macrophage activation after
spinal cord injury in mice. J Neuroinflammation. 9:402012.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Chandran AP, Oda K, Shibasaki H and
Pisharodi M: Spinal somatosensory evoked potentials in mice and
their developmental changes. Brain Dev. 16:44–51. 1994. View Article : Google Scholar : PubMed/NCBI
|