Introduction
Burn injuries are frequent in wartime and also in
times of peace. The prevention and therapy of ischemia-reperfusion
injury to the organs, particularly in the intestines, during the
burn shock and recovery process has become a challenging focus of
research. The mucosal cells are an important component of the
intestinal mucosal barrier structure. A previous study has shown
that the apoptosis of mucosal cells is the main form of cell death
occurring with intestinal ischemia-reperfusion, with apoptotic
cells accounting for 80% of the total number of dead cells
(1). Therefore, studies concerning
the apoptosis of cells in the burned intestinal mucosa have gained
an increasing amount of attention.
The heat shock protein 70 (Hsp70) family is the most
conservative and important category of HSPs. Hsp70 is abundant in
the majority of organisms, and is generated most significantly
during cellular stress; therefore, Hsp70 has been studied the most
extensively and in-depth (2).
Hsp70 plays an important role in the protection of the
gastrointestinal mucosa and previous studies have shown that Hsp70
has a variety of important physiological functions (3–7),
including acting as a chaperone, cytoprotection, anti-apoptotic and
anti-oxidation functions and participating in the immune
response.
Caspase-3 is a key enzyme in the caspase family that
is activated by various apoptosis-stimulating factors. Activated
caspase-3 could induce apoptosis via acting on other members in
caspase family (8). Caspase-3 may
directly cleave Bcl-2 into fragments, so that the function of Bcl-2
is changed from the inhibition of apoptosis to the triggering of
apoptosis (9,10). The role of caspase-3 in
ischemia-reperfusion-induced apoptosis is a topic that is gathering
increasing interest.
Burn scholars have proposed and developed a variety
of treatment options that have achieved certain therapeutic
effects. However, for a variety of reasons, including lack of
functional diversity, unstable efficacy, toxicity and high cost,
these treatments have been challenging to apply in the clinic.
Qinghuobaiduyin (QHBDY) is a traditional Chinese
medicine that contains Astragalus membranaceus, Lonicera
japonica, Scutellaria baicalenis Georgi, Ophiopogon
japonicus, Rheum rhabarbarum. QHBDY has been used as a
clinical prescription since 1995 to treat burns due to its
opsonization effect on the immune system and favorable clinical
therapeutic effects (11,12).
Several studies have shown that certain components
of QHBDY have anti-apoptotic effects in certain tissues and organs.
The aim of the present study was to investigate the protective
effect of QHBDY against apoptosis of the intestinal mucosa.
Materials and methods
Materials
The QHBDY was prepared by the Chinese medicine
laboratory of The Third Xiangya Hospital of Central South
University (Changsha, China). The caspase-3 colorimetric assay kit
and Annexin V-FITC and propidium iodide (PI) apoptosis assay kit
were purchased from Kaiji Biotech (Nanjing, China). The antibody
against caspase-3 was obtained from Cell Signaling Technology, Inc.
(Beverly, MA, USA). The anti-Hsp70 antibody was purchased from
Seajet Scientific Inc. (Beijing, China). The anti-GAPDH antibody
was from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). The
cell culture media and reagents were obtained from Invitrogen
(Carlsbad, CA, USA). The IEC-18 cells were from American Type
Culture Collection (ATCC; Baltimore, MD, USA). The Sprague Dawley
(SD) rats were provided by the Experimental Animal Center of The
Third Xiangya Hospital of Central South University. Both normal and
burn serum were collected from the rat heart.
Model construction of severely burned
rats
The animal experiments were approved by the
Institutional Animal Care and Use Committee of Central South
University. The methods for the model construction of severely
burned rats were previously described by Walker and Mason (13). Briefly, 136 healthy SD rats
weighing 180–220 g were divided into three groups randomly: normal
group (n=8), burned group (n=32) and treatment group (n=96).
According to the treatment dosage of QHBDY, the treatment group was
subdivided into groups of 0.5 ml/100 g, 1 ml/100 g or 1.5 ml/100 g
(n=32 for each group). Under anesthesia, the nude skin was scalded
with a 97°C water bath for 18 seconds to cause a 3rd degree burn
encompassing 30% total body surface area (TBSA). The rats were
resuscitated with an intraperitoneal injection of Ringer’s lactate
in the volume of 4 ml/kg/1% TBSA, placed in individual cages and
allowed free access to food and water. The normal group had the
same procedure with the exception that the water temperature was
37°C. The rats in treatment group were given 1.0 g/l QHBDY by oral
gavage twice within 24 h before burning. After burning, 1.0 g/l
QHBDY was administered again within 5 minutes. The burn area
calculation and control was performed according to the rat’s total
body surface area with the formula: Area (cm2) = K ×
W2/3, W is the weight (g), K=10. The desired burn area
was 30% of the TBSA.
Cell culture
IEC-18 cells were maintained in Dulbecco’s modified
Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum at
37°C in a humidified atmosphere of 5% CO2. Serum and
QHBDY were diluted with 1% saline solution (vol/vol) into
appropriate concentrations, which were then added to treat cells
for 24 h
Western blot analysis
Monolayers of IEC-18 cells were washed twice with
ice-cold phosphate-buffered saline (PBS) and lysed by ice-cold
lysis buffer [150 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1% Nonidet
P-40, 2 mM EDTA, 50 mM sodium fluoride, 0.2% SDS, 100 mM sodium
vanadate and 1 mM phenylmethylsulfonyl fluoride]. After 30–60 min
on ice, lysates were cleared of cellular debris by centrifugation
(13,000 × g) for 1 min at 4°C. Protein (50 μg; determined by the
Bradford protein assay) was diluted in 5X SDS-PAGE sample buffer
[150 mM Tris base (pH 6.8), 30% glycerol, 4% SDS, 7.5 mM
dithiothreitol (DTT) and 0.01% bromophenol blue] and separated on
10% SDS polyacrylamide gel. Following electrophoresis, the
separated proteins were transferred to nitrocellulose (NC)
membranes. The membranes were incubated overnight in 5% nonfat milk
in Tris-buffered saline containing 0.1% Tween-20 to saturate the
nonspecific binding sites. The membranes were then incubated with
anti-Hsp70 primary antibody (dilution, 1:400) for 1 h at 37°C. The
protein bands were visualized using horseradish
peroxidase-conjugated secondary antibody (Goat Anti-Rabbit IgG,
Santa Cruz) with a chemiluminescence-based detection system (ECL
western blotting kit; Pierce Biotechnology, Inc. Rockford, IL,
USA). For molecular weight determinations, multicolored protein
markers were used. To verify equal protein loading, membranes were
stripped and reprobed with anti-GAPDH monoclonal antibody
(dilution, 1:1,000). The intensity of protein bands was quantified
using a ScanJet 4C Flatbed Scanner (Hewlett-Packard, Palo Alto, CA,
USA) with NIH Image v1.52 software (http://rsb.info.nih.gov/nih-image/). Lightly exposed
films were used for quantification.
Immunohistochemistry
The tissues from the small intestine were fixed in
10% paraformaldehyde solution, then embedded in paraffin or frozen.
The sections (4-mm thick) were cut from tissue blocks and mounted
on slides, then sections were baked at 60–65°C for 4 h. The slides
were incubated in xylene and graded ethanol to remove the paraffin.
Antigen retrieval was performed with a high temperature and high
pressure citrate buffer. Goat serum was used to block nonspecific
staining and H2O2 to quench endogenous
peroxidase activity. The slides were then incubated with primary
antibody (caspase-3, 1:500; Hsp70, 1:500) for 1 h at 37°C. After
washing with PBS, the secondary antibody (Goat Anti Rabbit IgG-HRP,
Maixin, Inc., Fuzhou, China) was added and the slides were
incubated at 37°C for 1 h. The staining was visualized using a DAB
staining kit (Maixin, Inc.) according to the manufacturer’s
instructions and samples were counterstained with hematoxylin and
eosin (H&E) before viewing under a microscope (Nikon, Tokyo,
Japan). Under high-magnification microscopy, five visual fields
were randomly selected to assess the optical density of positively
immunostained cells. The average gray value was then calculated for
quantitative analysis.
TUNEL assay
The tissue samples were fixed in 10%
paraformaldehyde solution for 24 h, then embedded in paraffin. The
paraffin-embedded tissues were cut into 4-μm sections. TUNEL assays
were performed using the In Situ Cell Death Detection kit (Kaiji
Biotech) according to the manufacturer’s instructions. The number
of apoptotic cells was counted under an optical microscope.
Caspase-3 activity assay
The caspase-3 activity assay was performed using the
caspase-3 activity assay kit according to the manufacturer’s
instructions.
Flow cytometry (FCM)
For measuring the apoptosis rate, cells were
dual-stained with PI and Alexa Fluor 488-Annexin V using an Annexin
V-FITC and propidium iodide (PI) apoptosis assay kit (Kaiji
Biological Inc.) according to the manufacturer’s instructions. The
stained cells were analyzed by FCM (FC 500 MPL system; Beckman
Coulter, Inc., Miami, FL, USA).
Statistical analysis
The data were analyzed for statistical significance
using a Student’s t-test. P<0.05 was considered to indicate a
statistically significant result.
Results
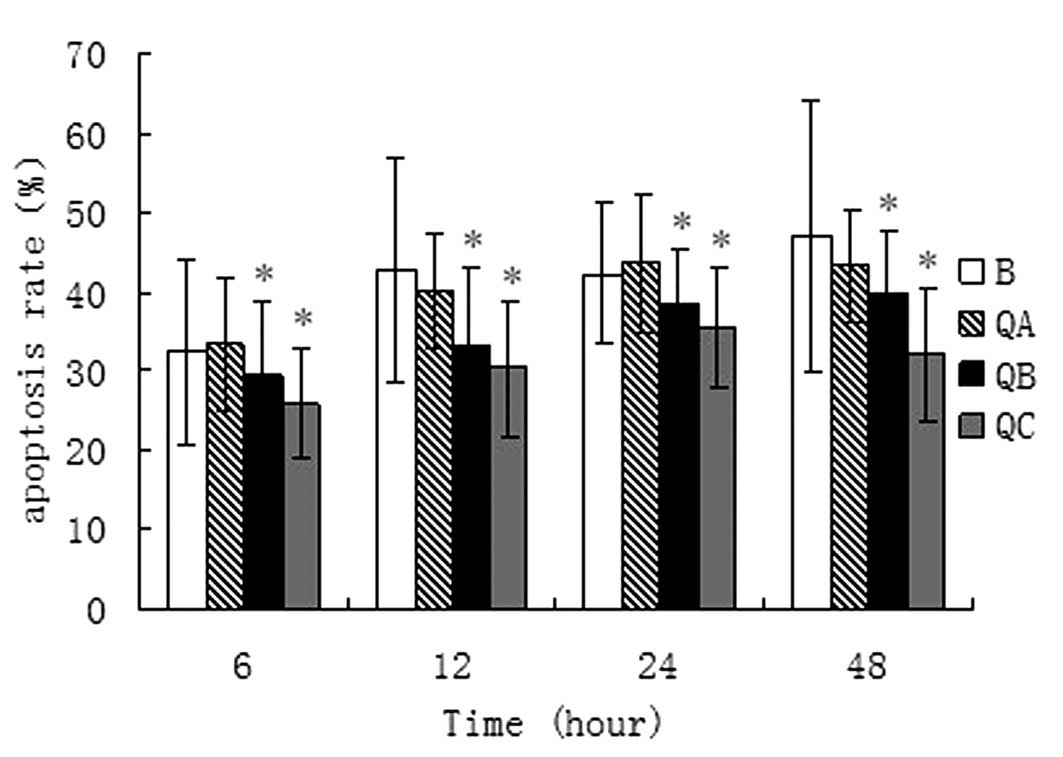
Effect of QHBDY on the mucosal cell
apoptosis rate in the small intestine in burned SD rats
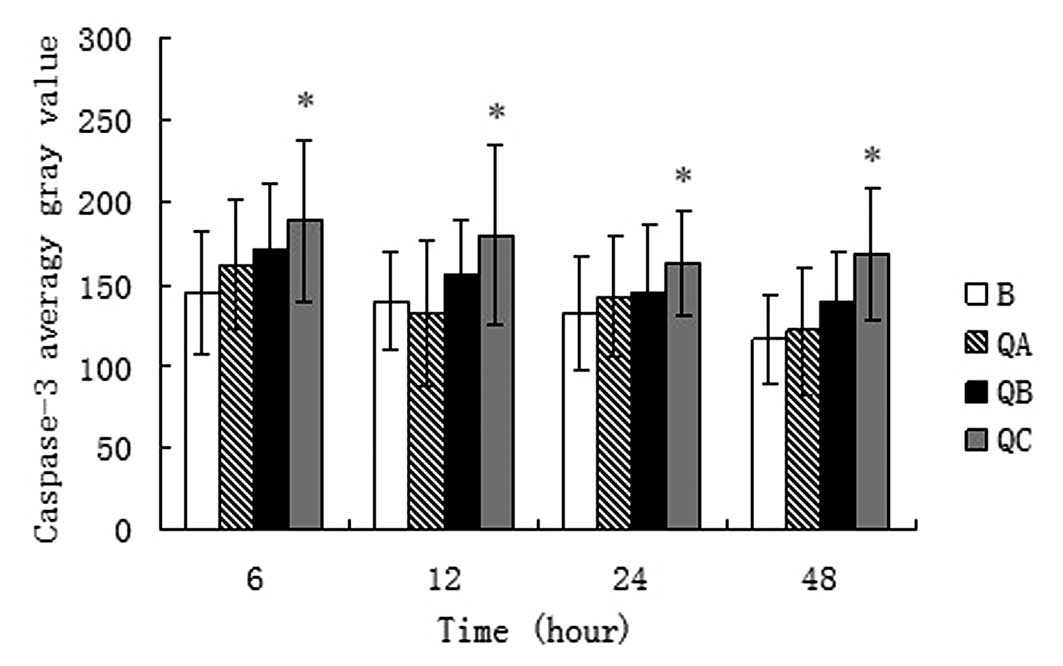
The burned SD rats in the three treatment groups
were treated with QHBDY at dosages of 0.5 ml/100 g (QA group), 1
ml/100 g (QB group) and 1.5 ml/100 g (QC group), and the apoptosis
rate of the mucosa of the small intestine was observed at time
points of 6, 12, 24 and 48 h (Fig.
1). As shown in Fig. 2, the
apoptosis rates in the treatment groups were lower than in the
burned group (B group), and the apoptosis rates in the 1 ml/100 g
and 1.5 ml/100 g groups were statistically different from that of
the burned group (P<0.05).
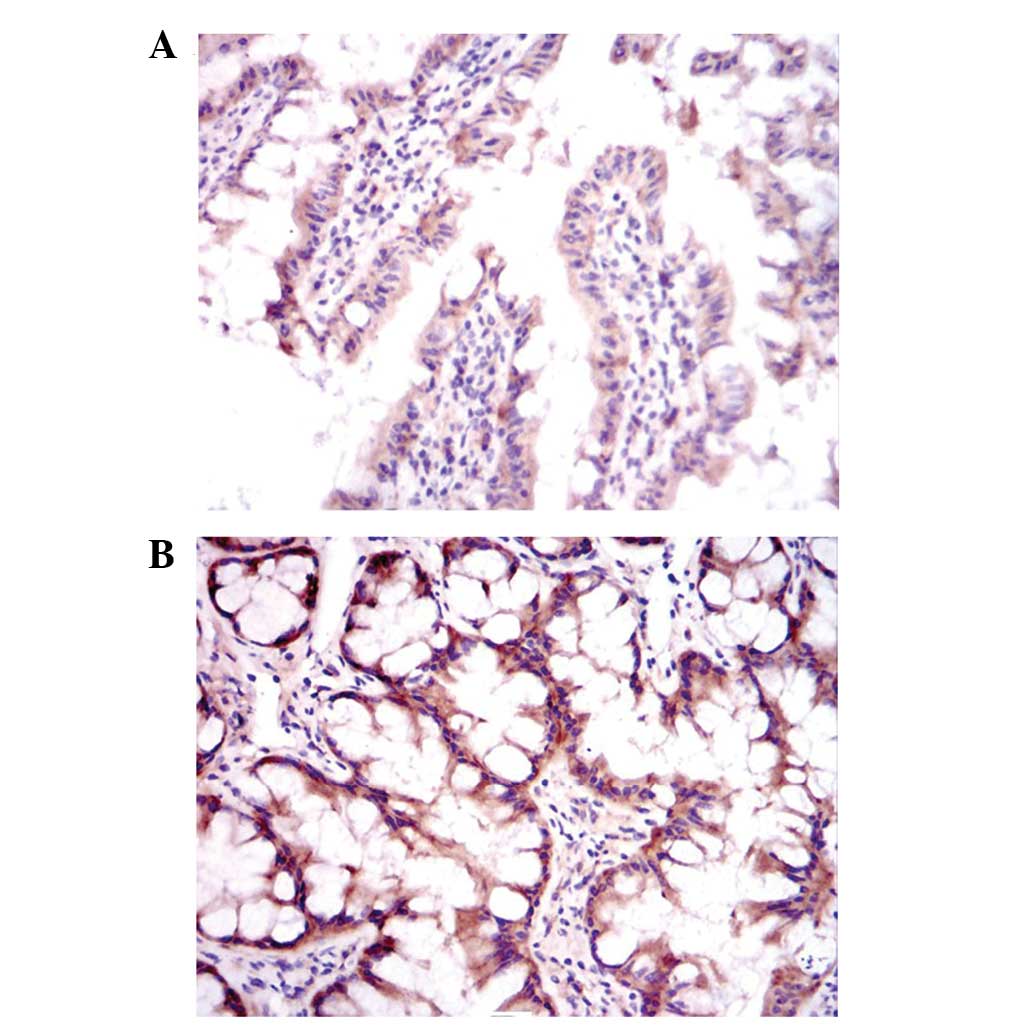
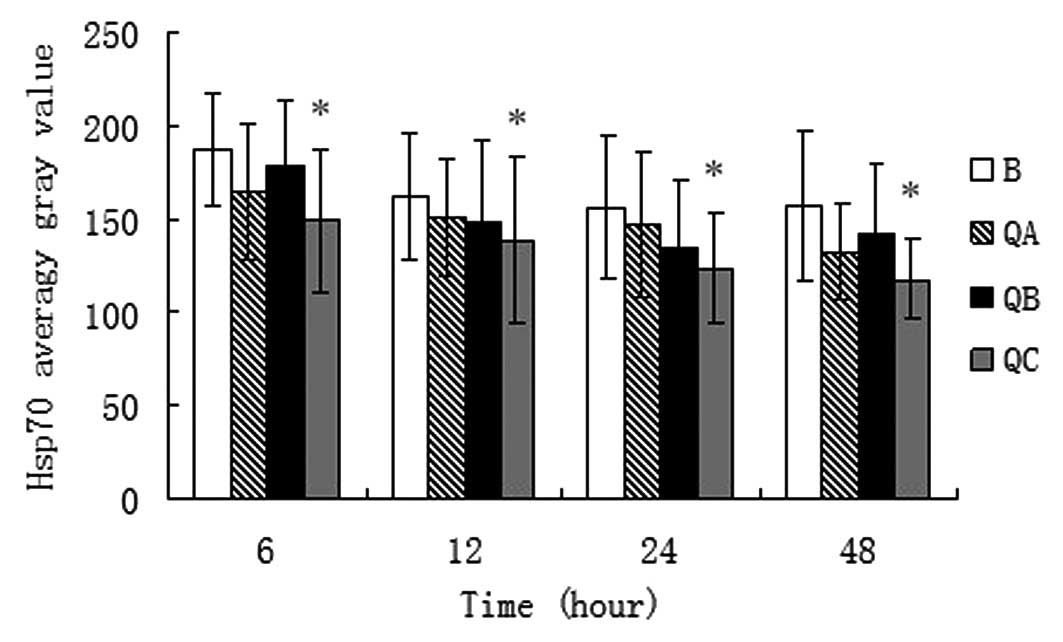
Effect of QHBDY on Hsp70 expression at
the protein level in the small intestines of burned SD rats
The burned SD rats in three treatment groups were
treated with QHBDY at dosages of 0.5, 1 and 1.5 ml/100 g. Tissues
were collected from the small intestine following treatment times
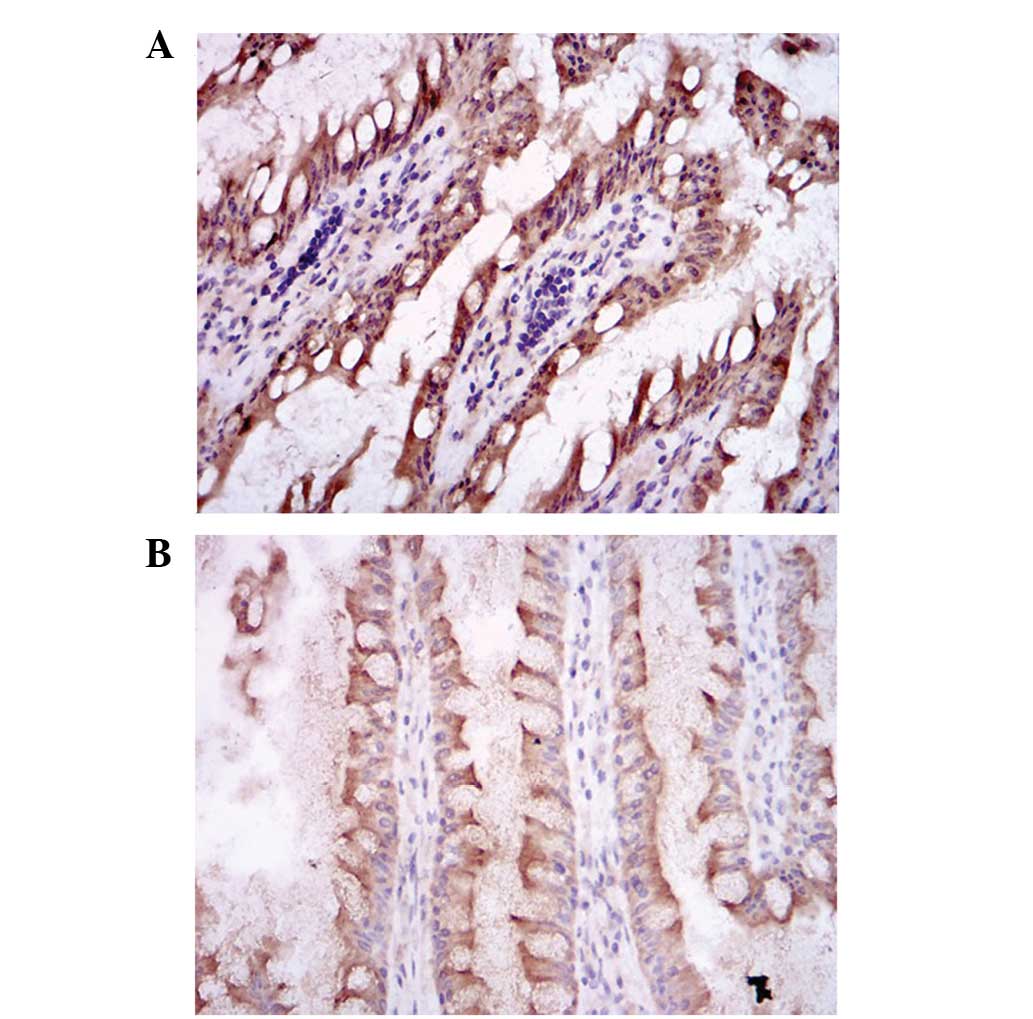
of 6, 12, 24 and 48 h for immunohistochemical staining (Fig. 3). The expression level of Hsp70
protein was measured using the analysis software of the microscope.
As shown in Fig. 4, there was a
statistical difference in the level of Hsp70 protein expression
between the 1.5 ml/100 g treatment group and the burned group
(P<0.05) at 6, 12, 24 and 48 h. However, no differences were
identified between the other two treatment groups and the burn
group.
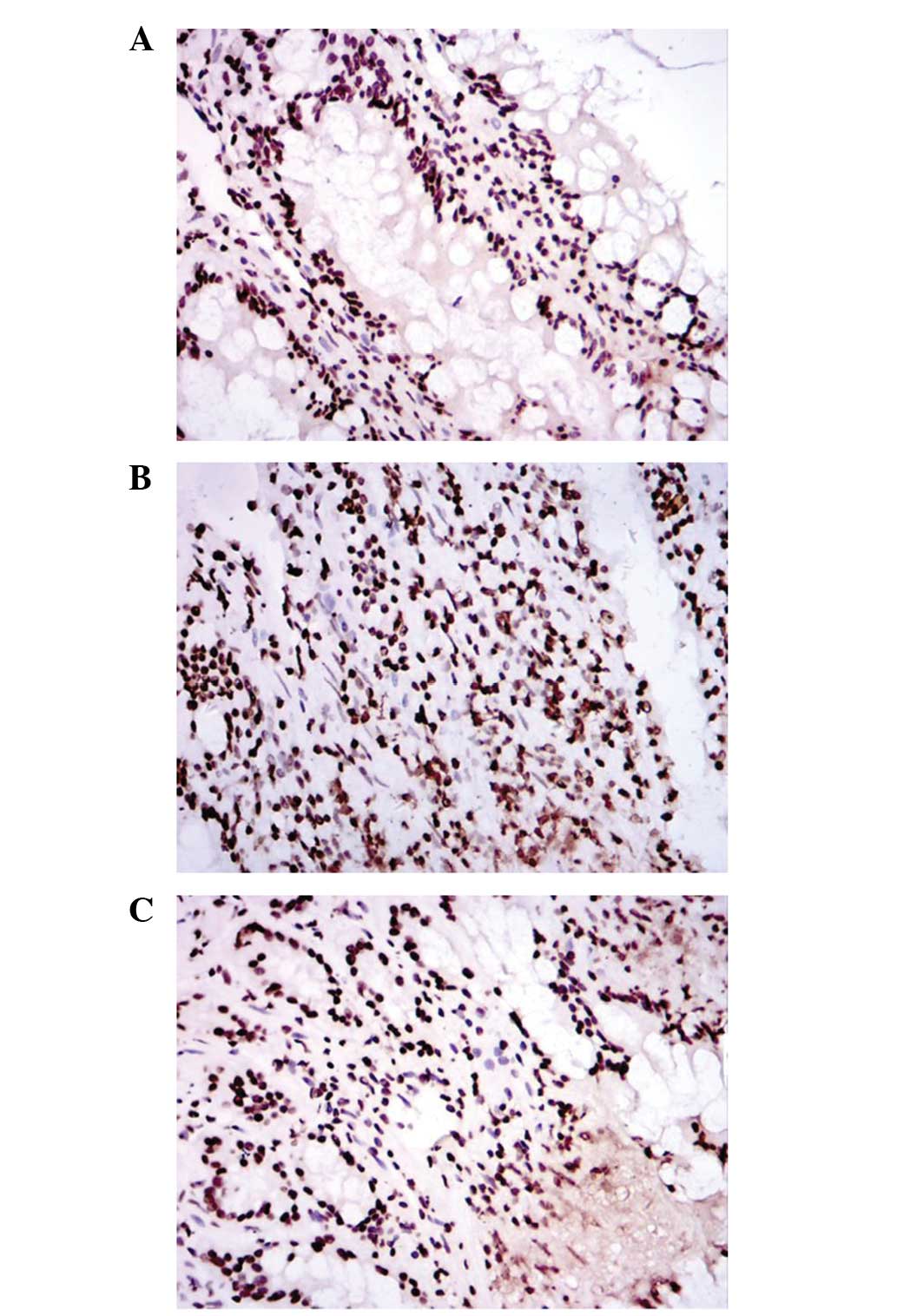
Effect of QHBDY on caspase-3 expression
at the protein level in the small intestines of burned SD rats
At 6, 12, 24 and 48 h after the treatment of the
burned SD rats with QHBDY at three different dosages, the tissues
were collected from the small intestine to investigate the effect
of QHBDY on caspase-3 expression (Fig.
5). The results show that QHBDY at the dosage of 1.5 ml/100 g
was able to decrease the expression of caspase-3 following
treatment for 6, 12, 24 and 48 h compared with that in the burned
group (Fig. 6).
Effect of burn serum on caspase-3
activity and IEC-18 cell apoptosis rate
In order to study the effect of burn serum on
caspase-3 protein activity, burn serum was added to IEC-18 cells
for 24 h at three different concentrations (1:150, 1:100 and 1:80),
or normal serum (1:100) was added to IEC-18 cells as a control. As
shown in Fig. 7, following
treatment with burn serum, the relative activity of caspase-3
protein increased; the relative activity of caspase-3 protein in
the normal serum group was 2.48±0.63, but in the three burn serum
treatment groups, the relative caspase-3 activities were 3.34±0.79,
6.40±1.75 and 9.45±2.56 at 1:150, 1:100 and 1:80 concentrations,
respectively. There were statistically significant differences
between the three burn serum treatment groups and the normal serum
treatment group, and the relative caspase-3 activity in the 1:80
burn serum treatment group was the most elevated compared with that
in the normal serum group (P<0.01).
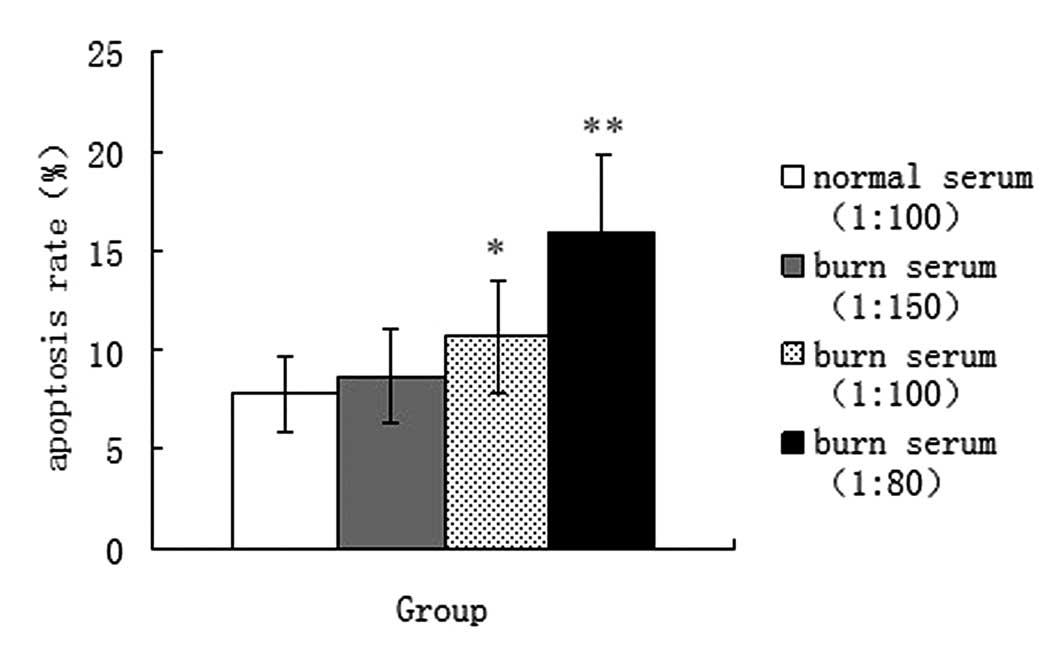
The cell apoptosis rates in the four groups of cells
were compared by FCM. Fig. 8 shows
that following treatment with burn serum, the cell apoptosis rate
increased; the cell apoptosis rate in the normal serum group was
7.75±1.85%, but in three burn serum treatment groups, the cell
apoptosis rates were 8.64±2.36, 10.7±2.86 and 15.89±3.98% at 1:150,
1:100 and 1:80 concentrations, respectively. The cell apoptosis
rates in the 1:100 burn serum treatment groups were significantly
different from that in the normal serum treatment group
(P<0.05), and the difference was most evident between the 1:80
burn serum treatment and normal serum groups (P<0.01).
Effect of QHBDY on caspase-3 activity and
IEC-18 cell apoptosis rate
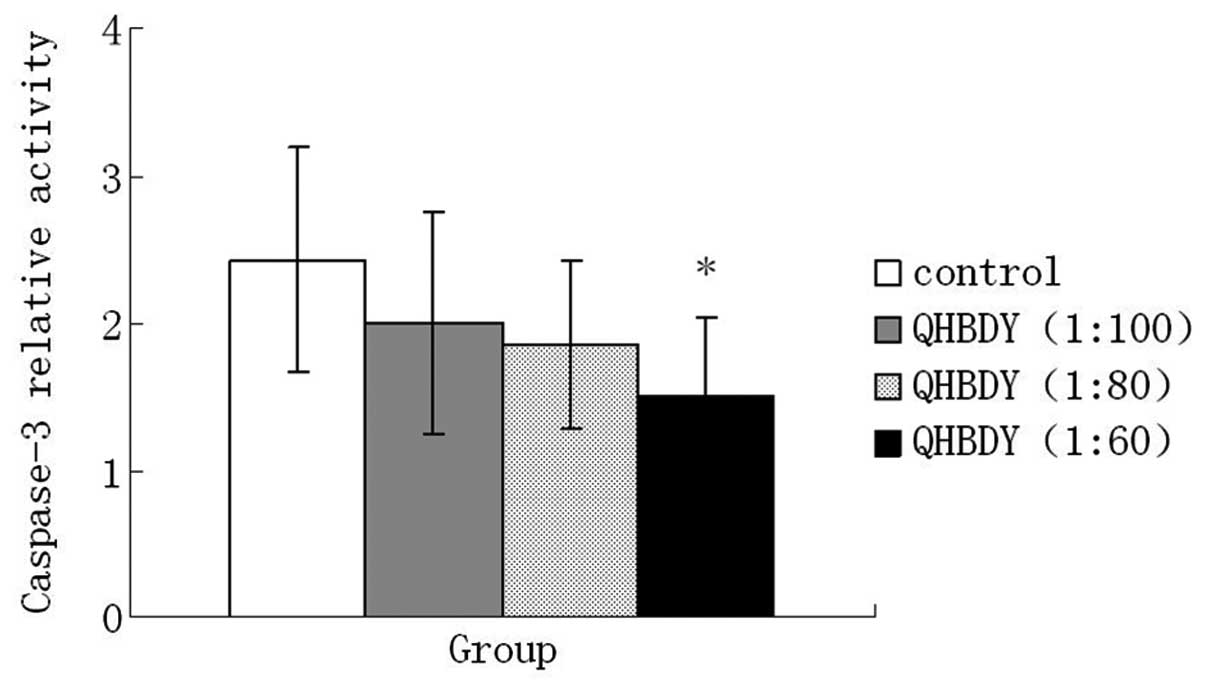
Three different concentrations of QHBDY (1:100, 1:80
and 1:60) were used to treat IEC-18 cells for 24 h, and 1% saline
solution was selected as control. In this experiment, the results
indicate that QHBDY was able to decrease the relative activity of
caspase-3. There was a statistically significant difference in
caspase-3 activity between the 1:60 treatment group and the control
(P<0.05; Fig. 9).
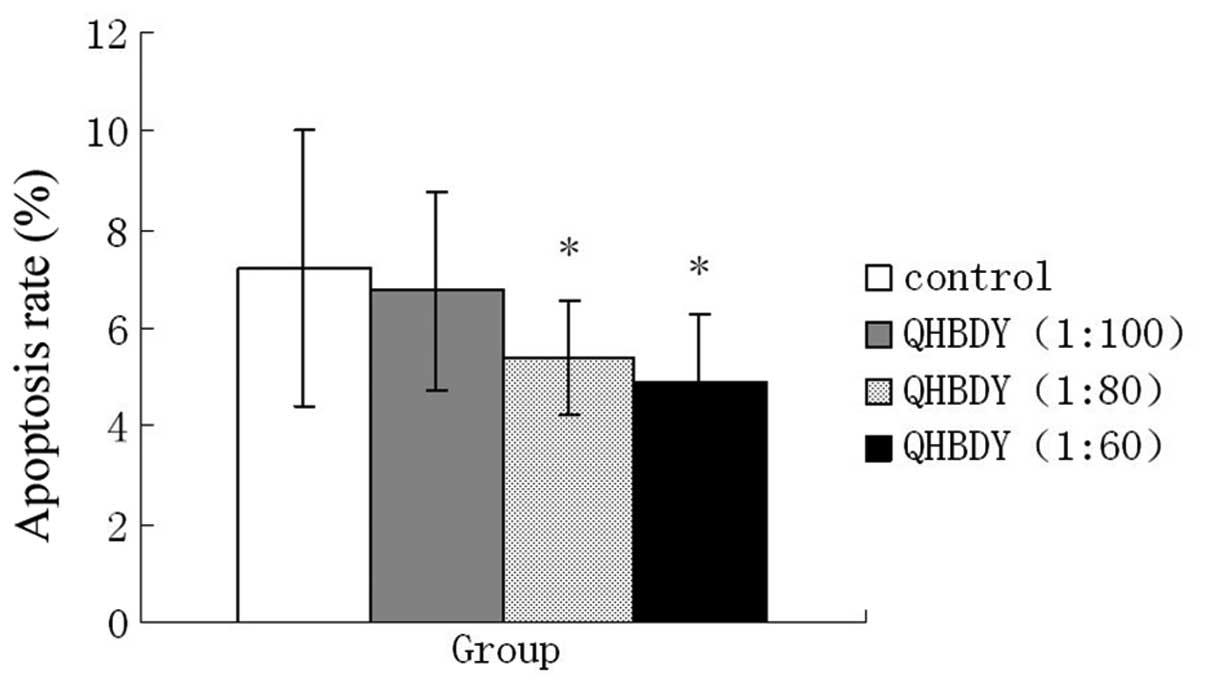
As shown in Fig.
10, treatment with QHBDY decreased the IEC-18 cell apoptosis
rates; the cell apoptosis rate in the 1:80 and 1:60 treatment
groups were statistically significantly different from the control
(P<0.05).
Effect of co-treatment with QHBDY and
burn serum on caspase-3 activity and apoptosis rate
According to the experimental results of caspase-3
relative activity and IEC-18 apoptosis rate, burn serum at a
concentration of 1:80 and QHBDY at a concentration of 1:60 were
selected to treat IEC-18 cells together for 24 h. The results show
that compared with the burn serum treatment group, the caspase-3
relative activity in the co-treatment group decreased and there was
a statistically significant difference between the two groups
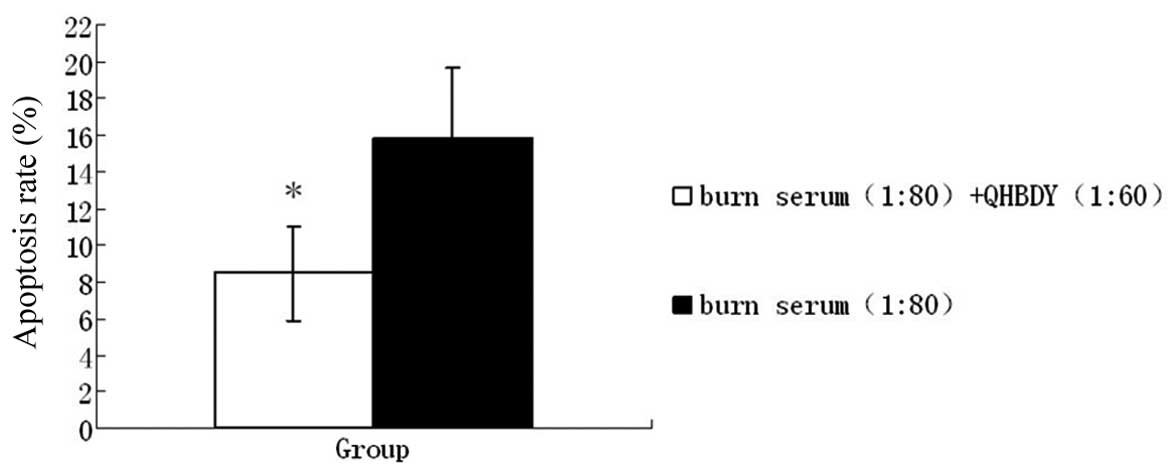
(P<0.05; Fig. 11). The cell
apoptosis rate was also statistically different between the
co-treatment group and the burn serum treatment group (P<0.05);
the cell apoptosis rate in the co-treatment group was lower than
that in the burn serum treatment group (Fig. 12).
Effect of co-treatment with QHBDY and
burn serum on Hsp70 expression at the protein level
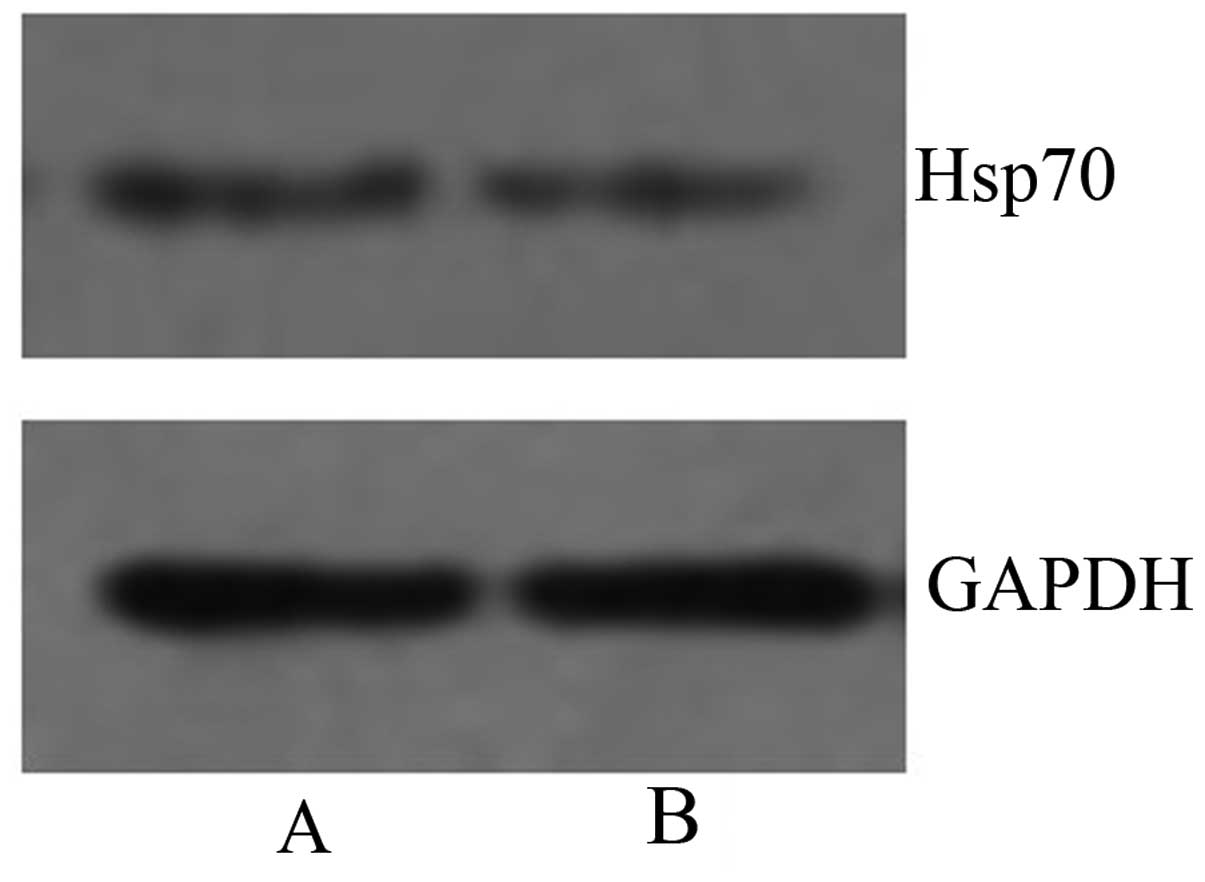
After treatment with burn serum and QHBDY for 24 h,
the IEC-18 cells were collected and protein was extracted in order
to perform western blot analysis. GAPDH was used for
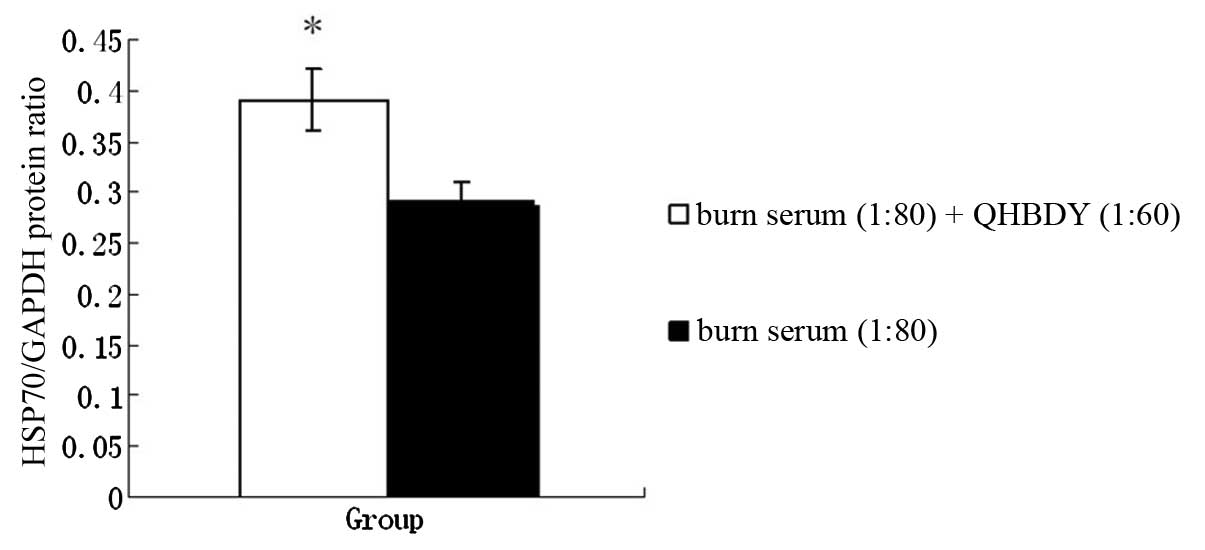
quantification. The western blot images are shown in Fig. 13. The relative optical density of
Hsp70 was 0.29±0.02 in the burn serum group and 0.39±0.03 in the
co-treatment group. There was a statistically significant
difference between the two groups (P<0.05; Fig 14).
Discussion
The probability of infection increases greatly due
to burn injury, particularly large-area burn injury. This makes the
gastrointestinal tract, which is the largest bacterial storage pool
in the body, particularly important. Therefore, investigation of
how to reduce the degree of burn injury of the intestinal mucosa in
the early stage and protect intestinal mucosal barrier function has
become an important area of research. In recent years, studies of
burn injury to intestinal mucosa have increasingly focused on
apoptosis (1,3).
QHBDY consists of Astragalus membranaceus,
Lonicera japonica, Scutellaria baicalenis Georgi,
Ophiopogon japonicus, Rheum rhabarbarum. In previous
studies, clinical and scientific research data have shown the how
the opsonization function of QHBDY affects the humoral and cellular
immunity of severely burned patients and its inhibitory effect on
the expression of inflammatory mediators in the inflammatory
response (14,15). Astragalus significantly inhibits
the increase in tumor necrosis factor-α (TNF-α) levels and neuronal
apoptosis in the brain tissue in rats with focal cerebral
ischemia-reperfusion (16,17). Ophiopogon extract has roles in the
prevention of apoptosis, proliferation and reduction of
intercellular adhesion molecule-1 expression (18). In neural cell primary culture
studies, it was observed that Ophiopogon injection exerted a
neuroprotective effect via the prevention of apoptosis (19–22).
These anti-apoptotic effects for certain tissues and organs may be
attributed to certain components in QHBDY. The current study
focused on the protective effect of QHBDY against intestinal
mucosal apoptosis.
In order to study the anti-apoptotic effect of
QHBDY, a series of experiments were carried out in vivo and
in vitro. An animal model comprising severely burned rats
was constructed, and these rats were divided into two categories:
the burned and treatment groups. The treatment group was subdivided
into three groups according to the dosage of QHBDY. The intestinal
mucosal cell apoptosis rate, the expression of Hsp70 and caspase-3
was analyzed at 6, 12, 24 and 48 h after treatment. The TUNEL
method was used to evaluate the intestinal mucosal apoptosis rate.
It was observed that the apoptosis rates in the 1 ml/100 g and 1.5
ml/100 g groups were lower than in the burned group at 6, 12, 24
and 48 h. This suggests QHBDY was able to downregulate intestinal
mucosal cell apoptosis to exert its protective effect. In addition,
tissues from the small intestine were collected for
immunohistochemical analysis to compare Hsp70 and caspase-3
expression at the protein level. The results showed that the
expression of Hsp70 in the 1.5 ml/100 g group was higher than that
in the burned group, and the expression of caspase-3 in the 1.5
ml/100 g group was lower than the burned group. These results
further demonstrate the anti-apoptotic effect of QHBDY.
The anti-apoptotic role of QHBDY in cells was also
investigated in vitro. Burn serum was added to IEC-18 cells
to model the state of burning. According to our previous
experiments, a drug concentration of 1:60 and a time point of 24 h
were selected for the treatment of IEC-18 cells together with burn
serum. The cell apoptosis rate, protein expression of Hsp70 and
caspase-3 activity were analyzed prior to and following drug
treatment. FCM results showed that the cell apoptosis rate in the
drug treatment group was lower than in the burn serum group. This
illustrated the anti-apoptotic effect of QHBDY. From western blot
experiments, it was observed that the level of Hsp70 protein
expression was higher in the drug treatment group than in the burn
serum group. Caspase-3 activity decreased following treatment with
QHBDY. These results further demonstrate the anti-apoptotic role of
QHBDY in a cell model.
Therefore, it may be concluded that QHBDY may play
an important role in the prevention of apoptosis at the animal and
cellular levels.
Acknowledgements
This study was supported by a grant from the Hunan
Provincial Natural Science Foundation (08JJ5012).
Abbreviations:
|
QHBDY
|
qinghuobaiduyin
|
|
HSP
|
heat shock protein
|
|
FCM
|
flow cytometry
|
References
|
1
|
Itoh H, Yagi M, Fushida S, et al:
Activation of immediate early gene, c-fos, and c-jun in the rat
small intestine after ischemia/reperfusion. Transplantion.
69:598–604. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang L, Zhou Y, Peng J, Zhang Z, Jiang DJ
and Li YJ: Role of endogenous nitric oxide synthase inhibitor in
gastric mucosal injury. Can J Physiol Pharmacol. 86:97–104. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kumar V, Mohanty MK and Kanth S: Fatal
burns in Manipal area: a 10 year study. J Forensic Leg Med. 14:3–6.
2007.PubMed/NCBI
|
|
4
|
Shinozawa Y: Fluid management and care for
multiple organ dysfunction syndrome in patients with extensive
burns. Nippon Geka Gakkai Zasshi. 106:736–739. 2005.(In
Japanese).
|
|
5
|
Duncan DJ, Hopkins PM and Harrison SM:
Negative inotropic effect of tumour necrosis factor-alpha and
interleukin-1beta are ameliorated by alfentanil in rat ventricular
myocytes. Br J Pharmacol. 150:720–726. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin WJ, Wang DL and Pan YQ: Depression
research of psychoneuroimmunology: the role of cytokines. Adv
Psychol Sci. 16:404–410. 2008.(In Chinese).
|
|
7
|
Cheng C, Li Z, Huang W, et al: The
influence of the large military exercise on the soldiers’ some
immune-endocrine function. Med J Chin PLA. 32:189–190. 2007.(In
Chinese).
|
|
8
|
Mishima S, Yukioka T, Matsuda H and
Shimazaki S: Mild hypotension and body burns synergistically
increase bacterial translocation in rats consistent with a ‘two-hit
phenomenon’. J Burn Care Rehabil. 18:22–26. 1997.PubMed/NCBI
|
|
9
|
Zhang HY, Lv NH, Xie Y, et al: Protection
of heat shock preconditioning on acute gastric mucosal lesion in
scalded rats and its mechanism. Zhonghua Shao Shang Za Zhi.
23:58–61. 2007.(In Chinese).
|
|
10
|
Odashima M, Otaka M, Jin M, et al:
Induction of a 72-kDa heat-shock protein in cultured rat gastric
mucosal cells and rat gastric mucosa by zinc L-carnosine. Dig Dis
Sci. 47:2799–2804. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Muñoz J, Albillos A, Pérez-Páramo M, Rossi
I and Alvarez-Mon M: Factors mediating the hemodynamic effects of
tumor necrosis factor-alpha in portal hypertensive rats. Am J
Physiol. 276:G687–G693. 1999.PubMed/NCBI
|
|
12
|
Suemasu S, Tanaka K, Namba T, Ishihara T,
Katsu T, Fujimoto M, Adachi H, Sobue G, Takeuchi K, Nakai A and
Mizushima T: A role for HSP70 in protecting against
indomethacin-induced gastric lesions. J Biol Chem. 284:19705–19715.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Walker HL and Mason AD Jr: A standard
animal burn. J Trauma. 8:1049–1051. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bromberg Z, Raj N, Goloubinoff P,
Deutschman CS and Weiss YG: Enhanced expression of 70-kilodalton
heat shock protein limits cell division in a sepsis-induced model
of acute respiratory distress syndrome. Crit Care Med. 36:246–255.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wilmore DW and Robinson MK: Metabolism and
nutrition support. Surgical Basic Science. Fischer JE: 7th Edition.
Mosby Press Co; St Louis, MO: pp. 1251993
|
|
16
|
Wang LC: Research progress on chemical
compositions and efficacy of Lonicera spp. J Anhui Agric
Sci. 37:2036–2037. 2009.(In Chinese).
|
|
17
|
Wang L, Cui B and Zhang H: Pharmacological
research progress on Lonicera spp. Chin J Anim Husbandry Vet
Med. 34:91–95. 2007.(In Chinese).
|
|
18
|
He X and Lan R: Pharmacological action and
clinical application of honeysuckle. Lishizhen Med Mat Med Res.
15:865–867. 2004.(In Chinese).
|
|
19
|
Gong ZZ, Zheng YX, Zheng NG, et al:
Anti-oxidative effect of honeysuckle in vivo: an experimental
study. Prac J Med Pharm. 23:584–585. 2006.(In Chinese).
|
|
20
|
Kang OH, Choi JG, Lee JH and Kwon DY:
Luteolin isolated from the flowers of Lonicera japonica
suppresses inflammatory mediator release by blocking NF-κB and
MAPKs activation pathways in HMC-1 cells. Molecules. 15:385–398.
2010.PubMed/NCBI
|
|
21
|
Xiang Z and Ning Z: Scavenging and
antioxidant properties of compound derived from chlorogenic acid in
South-China honeysuckle. LWT - Food Sci Technol. 41:1189–1203.
2008. View Article : Google Scholar
|
|
22
|
Schierenbeck KA: Japanese honeysuckle
(Lonicera japonica) as an invasive species; history,
ecology, and context. Crit Rev Plant Sci. 23:3912004.
|