Introduction
The formation of fibrous joint adhesions following
surgery or trauma severely restricts functional recovery and is a
major problem in the field of orthopedics. The efficacy of certain
pharmaceutical agents in the prevention of adhesion formation,
following local administration, has been examined (1–4).
These attempts have achieved limited success and thus adhesion
formation remains an unsolved problem. A number of selective and
nonselective cyclooxygenase (COX)-2 enzyme inhibitors have been
shown to reduce adhesion formation via inhibition of inflammatory
responses involving polymorphonuclear leukocytes, macrophages,
fibrin, fibroblasts and new blood vessel formation. However, as a
result of their limited effects, COX-2 inhibitors have rarely been
applied clinically (5–11).
Celecoxib, a selective COX-2 inhibitor, has
previously been shown to produce a maximal reduction in
intra-abdominal adhesion formation compared with ibuprofen and
other nonselective COX-2 inhibitors (12,13).
In addition to its COX-2 inhibitory effects, and unlike other COX
enzyme inhibitors, celecoxib also possesses antiangiogenic and
antifibroblastic properties (7,14).
These additional properties may contribute to the greater
efficiency of celecoxib in reducing adhesion formation compared
with nonselective COX-2 enzyme inhibitors. Therefore, celecoxib may
provide a promising therapy for joint adhesions, as all types of
adhesions are considered to arise through the same mechanism. The
aim of the present study was to demonstrate marked inhibition of
joint adhesion formation by oral administration of celecoxib. A
rabbit model that accurately mimicked the features of human joint
adhesion was created and the inhibitory effects of celecoxib on the
formation of adhesions were assessed in this model. The effects
were investigated from a number of aspects, including joint flexion
contracture angle, macroscopic appearance, histology and collagen
content. To provide a positive comparison, ibuprofen was also
administered in this study due to its maximal antiadhesion effect
among the nonselective COX-2 inhibitors (13,15).
Materials and methods
Animal model
A total of 60 female New Zealand White rabbits
(weight, 2.5–3.0 kg; age, 3–4 months) were purchased from the
Shanghai Laboratory Animal Center (Chinese Academy of Sciences,
Shanghai, China). All experimental procedures were approved by the
Research Ethics Committees of the Sixth Affiliated People’s
Hospital of Shanghai Jiaotong University (Shanghai, China). The
rabbits were randomly assigned to one of the following three groups
(n=20 per group): Celecoxib, ibuprofen and control groups. Using
intravenous pentobarbital sodium (Dainihon, Osaka, Japan) to
achieve anesthesia, the left knee joint was prepared for surgery
under aseptic conditions. Following a skin incision, the knee was
opened using a lateral parapatellar approach, and the medial and
lateral sides of the femoral condyle were exposed. A partial
capsulotomy and synovectomy were performed using an osteotome and
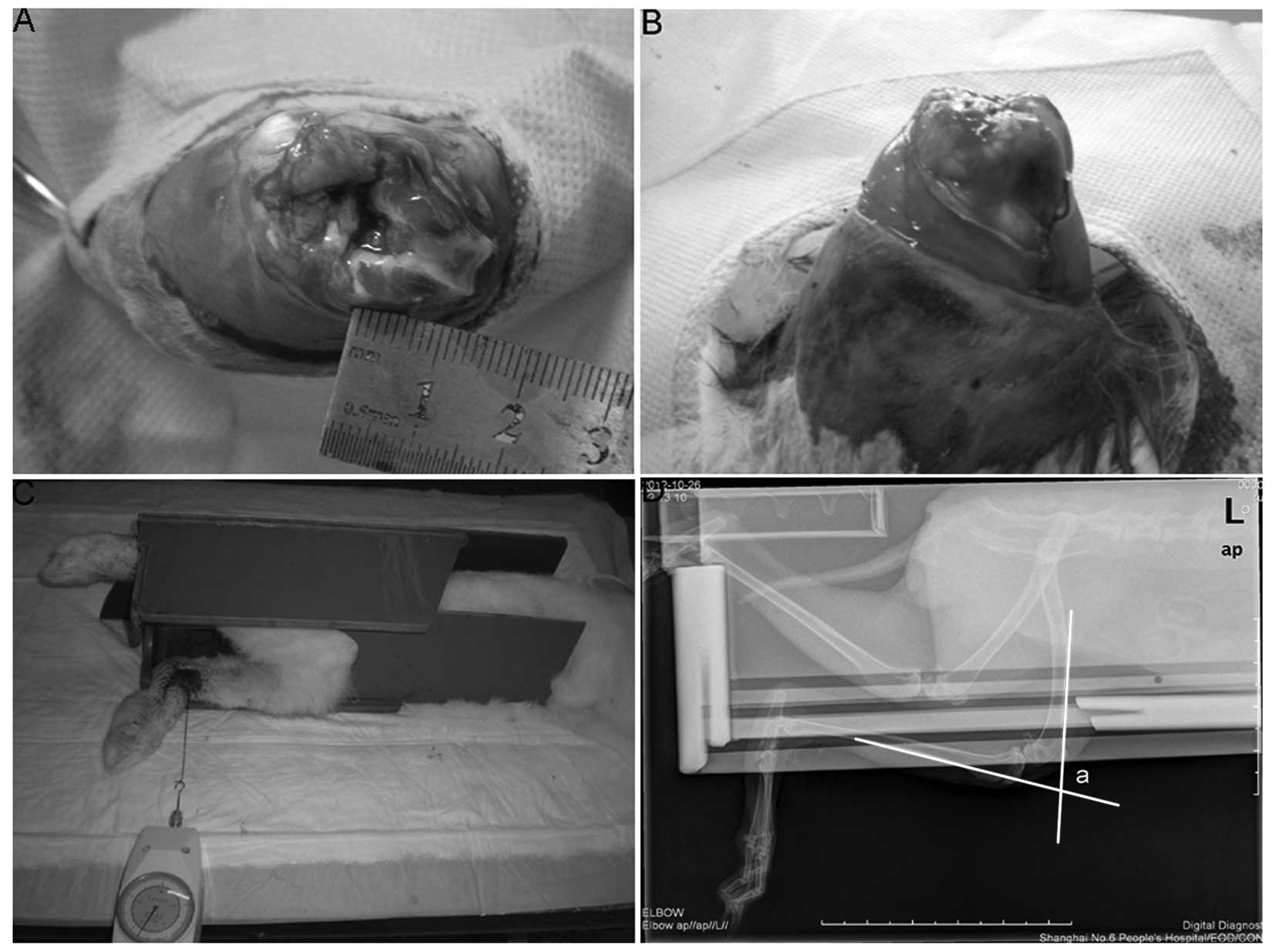
an osteochondral portion of the condyle (~5×10 mm) was removed to
ensure the underlying cancellous bone was exposed (Fig. 1A). Each limb that was subjected to
surgery underwent knee joint immobilization at 140° of flexion with
a Kirschner wire (Zimmer, Shanghai, China) for 30 days (Fig. 1B). Rabbits were housed in
individual cages in which motion was not limited. They had access
to food and water ad libitum.
Drug treatment
Following recovery from anesthesia, all rabbits were
administered prophylactic cefazolin and buprenorphine (Shanghai
Pharma, Shanghai, China) for pain management as required. In
addition, the rabbits in the two treatment groups received oral
celecoxib (2.86 mg/kg twice a day; Pfizer, Shanghai, China) or
ibuprofen (3.81 mg/kg three times a day; GSK, Shanghai, China),
commencing on the day of surgery, for 30 days. The rabbits in the
control group did not receive oral celecoxib or ibuprofen.
Macroscopic evaluation
Thirty days subsequent to surgery, eight rabbits in
each group were sacrificed by intravenous administration of an
overdose of pentobarbital sodium (Dainihon) and the Kirschner wires
were removed. The knee joint of each rabbit was exposed via a
parapatellar skin incision and held at 140° of flexion. The
presence and severity of intra-articular adhesions were
semiquantitatively assessed by blinded observers using a severity
score scale of 0–4 (16) as
follows: Grade 0, no adhesions; grade 1, filmy weak adhesions,
easily exfoliated by light preparation with forceps; grade 2, mild
adhesions, easily exfoliated by moderate preparation with forceps;
grade 3, moderately dense adhesions, which may be partly exfoliated
by strong preparation with forceps; grade 4, dense fibrous
adhesions, which may not be exfoliated via strong preparation with
forceps.
Histological evaluation of adhesion
tissues
Following macroscopic evaluation of the adhesions,
intra-articular adhesion tissues were harvested from the knee and
fixed in 10% neutral buffered formalin for one week. Subsequently,
the tissues were embedded in paraffin and 6-μm sections were
prepared. The sections were mounted on silane-coated slides and
stained with hematoxylin and eosin (H&E). The sections were
then observed under a microscope (VanoxAH-2; Olympus, Tokyo,
Japan), and fibrosis, predominant cells and cell densities were
evaluated. Image-Pro Plus (Media Cybernetics, Silver Spring, MD,
USA) was used to analyze cell numbers.
Measurement of the flexion contracture
angle
Following macroscopic and histological evaluations,
the animals remaining in each group were also sacrificed via
administration of an overdose of pentobarbital sodium (Dainihon),
and were immediately subjected to biomechanical evaluations.
Following removal of the Kirschner wire, a thick silk thread was
hooked onto the lower left leg, 10 cm distal from the knee, and an
extension of 5.5 N was applied perpendicular to the tibia by
pulling the thread with a dynamometer (Fig. 1C). The animal was laid on the X-ray
table on its left side and the flexion contracture angle was
determined on a lateral view radiograph of the left knee (Fig. 1D, angle ‘a’). All measurements were
made within 15 min of sacrifice.
Statistical analysis
Statistical analyses of the differences among the
groups were performed using one-way analysis of variance (ANOVA)
and a post-hoc Student-Newman-Keuls test. The data are presented as
the mean ± standard deviation (SD). P<0.05 was considered to
indicate a statistically significant difference. All statistical
analyses were conducted using SPSS 11.0 (SPSS, Inc., Chicago, IL,
USA).
Results
In general, the rabbits adapted to Kirschner wire
immobilization well during the 30 days of the experimental period.
However, of the 60 rabbits used in this study, one animal in the
celecoxib group died on day 7 of unknown causes and two animals
suffered from wound infection (one each from the ibuprofen and
control groups). In addition, the fixation of two animals became
loose in the celecoxib and ibuprofen groups, respectively. These
seven animals mentioned were excluded from the study. All other
animals gained weight and appeared healthy, with no signs of
impaired wound healing.
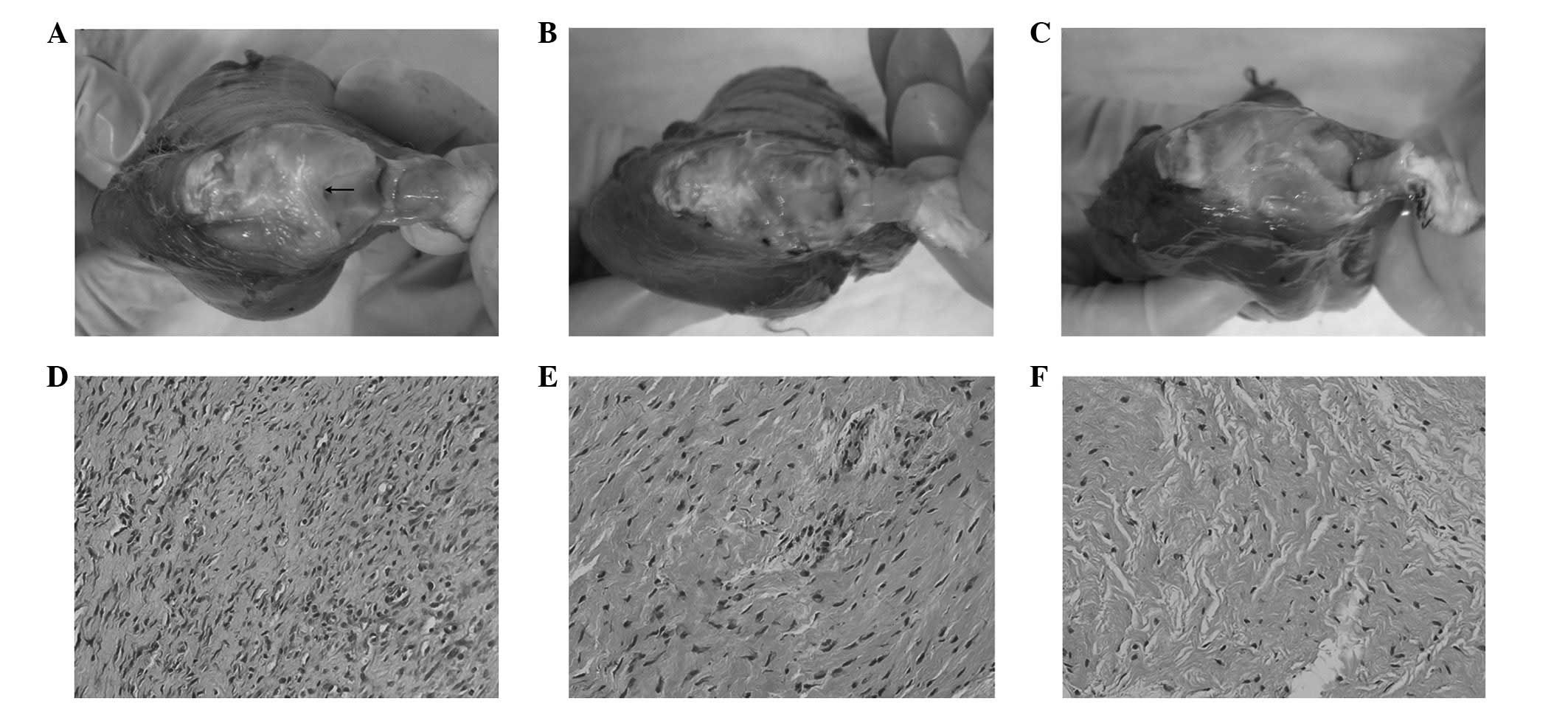
At 30 days following surgery, thick fibrous
adhesions had developed in the knees of the rabbits in the control
group (Fig. 2A). By contrast, the
adhesions in the two treatment groups were few, soft, weak and
easily stretched (Fig. 2B and C).
Furthermore, fewer adhesions were observed in the rabbits treated
with celecoxib compared with those treated with ibuprofen (Fig. 2C). Accordingly, the adhesion scores
in the ibuprofen (0.001<P<0.0025) and celecoxib (P<0.001)
groups were significantly lower than those in the control group
(Table I). Moreover, the adhesion
scores were significantly lower in the celecoxib group than those
in the ibuprofen group (Table I;
0.025<P<0.05).
 | Table IAdhesion scores of the rabbits in the
control and treatment groups. |
Table I
Adhesion scores of the rabbits in the
control and treatment groups.
| Group | Number | Adhesion scores | P-valuea | P-valueb |
|---|
| Control | 8 | 3.6±0.4 | NA | NA |
| Ibuprofen | 8 | 1.9±0.3 |
0.001<P<0.0025 | NA |
| Celecoxib | 8 | 1.4±0.3 | P<0.001 |
0.025<P<0.05 |
Histologically, the adhesion tissues in the control
group were dense, thick and fibrous (Fig. 2D), while those in the ibuprofen
group and particularly in the celecoxib group were loose and thin
with sparse fiber formation (Fig. 2E
and F). In all three groups, the predominant cells were
considered to be fibroblasts and few inflammatory cells were
observed. The cell densities in the celecoxib (range, 117–130;
mean, 124; P<0.001) and the ibuprofen (range, 151–165; mean,
158; 0.001<P<0.0025) groups were significantly lower than
those in the control group (range, 220–239; mean, 229; Table II). Moreover, the cell densities
were lower in the celecoxib group than those in the ibuprofen group
(Table II;
0.025<P<0.05).
 | Table IICell densities of the rabbits in the
control and treatment groups. |
Table II
Cell densities of the rabbits in the
control and treatment groups.
| Group | Number | Total cells (per
hpf) | P-valuea | P-valueb |
|---|
| Control | 8 | 229±19 | NA | NA |
| Ibuprofen | 8 | 158±13 |
0.001<P<0.0025 | NA |
| Celecoxib | 8 | 124±9 | P<0.001 |
0.025<P<0.05 |
As exhibited in Table
III, the flexion contracture angles in the control group ranged
between 112 and 125° (mean, 120°). Despite the differences between
the two treatment groups, the contracture angles in the celecoxib
group (range, 21–32°; mean, 26.4°; P<0.001) and the ibuprofen
group (range, 40–48°; mean, 44.2°; 0.001<P<0.0025) were
significantly lower than those in the control group. Moreover, the
contracture angles were significantly lower in the celecoxib group
than in the ibuprofen group (0.025<P<0.05).
 | Table IIIContracture angles of the rabbits in
the control and treatment groups. |
Table III
Contracture angles of the rabbits in
the control and treatment groups.
| Group | Number | Contracture angles
(°) | P-valuea | P-valueb |
|---|
| Control | 11 | 120.0±11.2 | NA | NA |
| Ibuprofen | 9 | 44.2±4.4 |
0.001<P<0.0025 | NA |
| Celecoxib | 9 | 26.4±3.4 | P<0.001 |
0.025<P<0.05 |
Discussion
Adhesion development is a process that involves
tissue fibrosis, with inflammatory responses involving
polymorphonuclear leukocytes, macrophages, fibrin, fibroblasts and
new blood vessel formation. The fibroblasts in adhesions express
COX-2 enzymes, while those in non-adhesion-bearing areas do not
(17). Consequently, certain
selective and nonselective COX-2 enzyme inhibitors may, to an
extent, inhibit adhesion formation. In addition, the formation of
all types of adhesions is partly dependent on angiogenesis
(18,19), and there is experimental evidence
indicating that COX-2-induced prostaglandins may modulate
fibroblast growth factor- and vascular endothelial growth
factor-induced angiogenesis (20).
Thus, the COX-2 inhibitor, celecoxib, may selectively inhibit the
angiogenesis associated with newly forming adhesions via a
COX-2-based mechanism (8), thereby
producing a reduction in intra-abdominal adhesion formation
(13).
In view that the same mechanism underlies the
formation of different adhesion types, celecoxib may represent a
promising therapy for joint adhesions. In the present study, the
inhibitory effects of celecoxib on the formation of joint adhesions
were demonstrated in vivo from different aspects, including
adhesion score, histology, collagen content and joint contracture
angle. The results of the histological and biochemical analyses of
the adhesion tissues were closely correlated with those of the
macroscopic and biomechanical evaluations. For example, collagen
content is one of the key factors determining the mechanical
strength of repair tissues (21).
The collagen type ratio is another determinant of tissue strength,
and a higher proportion of type III collagen is assumed to decrease
the strength by reducing the fibril diameter, which is closely
correlated with the strength of connective tissues (22,23).
Thus, the histological and biochemical alterations in the adhesion
tissues may serve to improve joint contracture.
In the present study, a clinically applicable dosing
regimen of the tested drugs was used. Furthermore, celecoxib was
administered via orogastric feeding on a daily basis for 30 days,
thereby effectively simulating a clinical setting. Using an oral
daily dosing schedule, it was demonstrated that ibuprofen and
celecoxib reduced the formation of joint adhesions. However, the
inhibitory effect of ibuprofen on adhesion formation was not as
extensive as that of celecoxib. Furthermore, the nonselective COX-2
inhibitor, ibuprofen, has the disadvantage of causing harmful side
effects associated with COX-1 inhibition, including
gastrointestinal and renal complications, as well as bleeding, as a
result of inhibition of platelet aggregation. Furthermore, it
appears that long-term administration and high doses of COX-2
inhibitors increase the risk of adverse cardiovascular events
(24–26). However, patients at risk of joint
adhesion formation may represent a new cohort suitable for
celecoxib treatment due to the potentially low risk-to-benefit
ratio. Firstly, it appeared that long-term celecoxib administration
was not required to prevent adhesion formation, since joint
adhesions are formed within 30 days, and a 30-day treatment led to
long-term adhesion prevention in the present study. Secondly,
routine doses of celecoxib are likely to be required for efficacy
in humans, thereby minimizing the increased risk of cardiovascular
events associated with the high doses used for treating diseases
such as rheumatoid arthritis. Furthermore, celecoxib has a lower
toxicity profile than other COX-2 inhibitors (27).
An animal model that accurately mimics the features
of human joint adhesions is essential for the preclinical
evaluation of the efficacy and safety of this form of drug therapy.
An intra-articular adhesion model has been developed in rabbits and
is currently being used to evaluate several methods for inhibiting
the formation of joint adhesions (1,28).
In the present study, a similar type of intra-articular adhesion
model in rabbits was used to evaluate the effects of celecoxib for
the treatment of joint adhesions. The high incidence rate (100%) of
joint adhesion formation in the control group indicated that the
experimental surgical procedure used in the present study was
appropriate for this type of study. In addition, the adhesions
developed in the articular cavity of this model; therefore, the
adhesion tissues were of sufficient volume to enable biochemical
analyses without contamination by other tissues.
The present study demonstrated that oral delivery of
celecoxib ameliorated joint adhesion formation effectively and
safely in a rabbit model. The study provides a novel and promising
strategy that may serve as an alternative treatment for the
prevention of joint adhesion formation. Further studies involving
larger animals are required to support this hypothesis. In
addition, clinical trials are required to confirm the beneficial
properties of celecoxib in reducing joint adhesions.
Acknowledgements
This work was supported financially by the Natural
Science Foundation of China (GSCX0818005).
References
|
1
|
Brunelli G, Longinotti C, Bertazzo C, et
al: Adhesion reduction after knee surgery in a rabbit model by
Hyaloglide, a hyaluronan derivative gel. J Orthop Res.
23:1377–1382. 2005.PubMed/NCBI
|
|
2
|
Fukui N, Tashiro T, Hiraoka H, et al:
Adhesion formation can be reduced by the suppression of
transforming growth factor-betal activity. J Orthop Res.
18:212–219. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fukui N, Nakajima K, Tashiro T, et al:
Neutralization of fibroblast growth factor-2 reduces intraarticular
adhesions. Clin Orthop Relat Res. 383:250–258. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hayashi M, Sekiya H, Takatoku K, et al:
Experimental model of knee contracture in extension: its prevention
using a sheet made from hyaluronic acid and carboxymethylcellulose.
Knee Surg Sports Traumatol Arthrosc. 12:545–551. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
He Y, Revel M and Loty B: A quantitative
model of post-laminectomy scar formation. Effects of a nonsteroidal
anti-inflammatory drug. Spine (Phila Pa 1976). 20:557–563;
discussion 579–580. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee JH, Go AK, Oh SH, et al: Tissue
anti-adhesion potential of ibuprofen-loaded PLLA-PEG diblock
copolymer films. Biomaterials. 26:671–678. 2005. View Article : Google Scholar
|
|
7
|
Miller JA, Ferguson RL, Powers DL, et al:
Efficacy of hyaluronic acid/nonsteroidal anti-inflammatory drug
systems in preventing postsurgical tendon adhesions. J Biomed Mater
Res. 38:25–33. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Montz FJ, Monk BJ, Lacy SM and Fowler JM:
Ketorolac tromethamine, a nonsteroidal anti-inflammatory drug:
ability to inhibit post-radical pelvic surgery adhesions in a
porcine model. Gynecol Oncol. 48:76–79. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Oh SH, Kim JK, Song KS, et al: Prevention
of postsurgical tissue adhesion by anti-inflammatory drug-loaded
pluronic mixtures with sol-gel transition behavior. J Biomed Mater
Res A. 72:306–316. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rodgers KE, Johns DB, Girgis W and
diZerega GS: Prevention of adhesion formation with intraperitoneal
administration of tolmetin and hyaluronic acid. J Invest Surg.
10:367–373. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wiseman DM, Huang WJ, Johns DB, et al:
Time-dependent effect of tolmetin sodium in a rabbit uterine
adhesion model. J Invest Surg. 7:527–532. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cahill RA: Prevention of intra-abdominal
adhesions using the antiangiogenic COX-2 inhibitor celecoxib. Ann
Surg. 244:327–328. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Greene AK, Alwayn IP, Nose V, et al:
Prevention of intra-abdominal adhesions using the antiangiogenic
COX-2 inhibitor celecoxib. Ann Surg. 242:140–146. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kusunoki N, Yamazaki R and Kawai S:
Induction of apoptosis in rheumatoid synovial fibroblasts by
celecoxib, but not by other selective cyclooxygenase 2 inhibitors.
Arthritis Rheum. 46:3159–3167. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tan V, Nourbakhsh A, Capo J, et al:
Effects of nonsteroidal anti-inflammatory drugs on flexor tendon
adhesion. J Hand Surg Am. 35:941–947. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rothkopf DM, Webb S, Szabo RM, et al: An
experimental model for the study of canine flexor tendon adhesions.
J Hand Surg Am. 16:694–700. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wiczyk HP, Grow DR, Adams LA, et al:
Pelvic adhesions contain sex steroid receptors and produce
angiogenesis growth factors. Fertil Steril. 69:511–516. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rout UK, Oommen K and Diamond MP: Altered
expressions of VEGF mRNA splice variants during progression of
uterine-peritoneal adhesions in the rat. Am J Reprod Immunol.
43:299–304. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Saed GM, Munkarah AR and Diamond MP:
Cyclooxygenase-2 is expressed in human fibroblasts isolated from
intraperitoneal adhesions but not from normal peritoneal tissues.
Fertil Steril. 79:1404–1408. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Majima M, Hayashi I, Muramatsu M, et al:
Cyclo-oxygenase-2 enhances basic fibroblast growth factor-induced
angiogenesis through induction of vascular endothelial growth
factor in rat sponge implants. Br J Pharmacol. 130:641–649. 2000.
View Article : Google Scholar
|
|
21
|
Forrest L: Current concepts in soft
connective tissue wound healing. Br J Surg. 70:133–140. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Clore JN, Cohen IK and Diegelmann RF:
Quantitation of collagen types I and III during wound healing in
rat skin. Proc Soc Exp Biol Med. 161:337–340. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Parry DA, Barnes GR and Craig AS: A
comparison of the size distribution of collagen fibrils in
connective tissues as a function of age and possible relation
between fibril size distribution and mechanical properties. Proc R
Soc Lond B Biol Sci. 203:305–321. 1978. View Article : Google Scholar
|
|
24
|
Garner SE, Fidan DD, Frankish RR, et al:
Rofecoxib for rheumatoid arthritis. Cochrane Database Syst Rev.
1:CD0036852005.PubMed/NCBI
|
|
25
|
Jüni P, Nartey L, Reichenbach S, et al:
Risk of cardiovascular events and rofecoxib: cumulative
meta-analysis. Lancet. 364:2021–2029. 2004.PubMed/NCBI
|
|
26
|
Lévesque LE, Brophy JM and Zhang B: The
risk for myocardial infarction with cyclooxygenase-2 inhibitors: a
population study of elderly adults. Ann Intern Med. 142:481–489.
2005.PubMed/NCBI
|
|
27
|
Kimmel SE, Berlin JA, Reilly M, et al:
Patients exposed to rofecoxib and celecoxib have different odds of
nonfatal myocardial infarction. Ann Intern Med. 142:157–164. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li F, Ruan H, Fan C, et al: Efficient
inhibition of the formation of joint adhesions by ERK2 small
interfering RNAs. Biochem Biophys Res Commun. 391:795–799. 2010.
View Article : Google Scholar : PubMed/NCBI
|