Introduction
Ischemia-reperfusion (I/R) injury remains the
leading cause of morbidity and mortality in all developed countries
and is a large economic burden in the treatment and care of
patients (1). I/R injury of the
heart is the underlying pathophysiology of acute myocardial
infarction (AMI), which is one of the most common causes of
mortality globally (2). Following
cell I/R, apoptosis is one of the major pathways that leads to the
process of cell death. Numerous studies have indicated that cell
apoptosis is associated with a variety of damaging stimuli,
particularly continuous I/R, in vitro and in the intact
heart in vivo(3–7). Thus, protection against I/R injury in
the heart is of marked importance.
Chinese herbal medicine has been used to treat
diseases for thousands of years and it has recently attracted the
attention of practitioners of Western medicine. However, the
effective ingredients in the majority of these medications have not
been identified. Baicalin (BA;
C21H18O11, 7-glucuronic acid,
5,6-dihydroxy-flacone), a Chinese traditional medicinal herb,
possesses antioxidant properties and free radical scavenging
activity (8–12). Studies have demonstrated that BA
can suppress the proliferation of vascular smooth muscle cells
(9) and exert cardioprotective
effects against hypoxia/reoxygenation injury (12–16).
However, the cardioprotective properties have not been confirmed
and the mechanism has not been fully characterized. In addition,
there has been no clear histological or immunohistochemical
evidence showing the cardioprotective effect of BA to date.
Therefore, further studies investigating the mechanisms of
protection are required.
The Langendorff mouse heart model is widely used in
studies of myocardial function and responses to injury (17). In the present study, rat hearts
were isolated and perfused using the Langendorff technique to
establish a high-throughput and potentially reliable I/R model.
The aim of the present study was to evaluate the
efficacy of BA in isolated rat hearts using hemodynamic,
histological and immunohistochemical observations.
Materials and methods
Animals
Male Sprague-Dawley rats, weighing 250–300 g, were
purchased from the Experimental Animal Center of Shandong
University (Jinan, China). The rats were housed within the animal
care facility at a constant room temperature with a 12:12-h
light-dark cycle and were provided with standard rat chow and water
ad libitum for at least 3 days prior to the initiation of
the experiments. All animals received humane care in compliance
with the Guide for the Care and Use of Laboratory Animals published
by the US National Institute of Health (NIH publication no. 85-23;
revised 1996). The study was approved by the Ethics Committee of
the Shandong University (Jinan, China).
Langendorff isolated perfused heart
preparation
Rats were anesthetized with pentobarbital sodium (50
mg/kg ip) and administered with the anticoagulant, heparin sodium
(10,000 USP U/kg ip). Following this, a thoracotomy was performed
and the hearts of the rats were rapidly excised into ice-cold
arresting solution (120 mmol/l NaCl and 30 mmol/l KCl). The aorta
was cannulated on a 20-gauge stainless steel blunt needle and
perfusion was initiated at 70 mmHg on a Langendorff apparatus. A
modified Krebs-Henseleit (K-H) perfusion solution was used in all
experiments, containing 118 mmol/l NaCl, 4.7 mmol/l KCl, 2.5 mmol/l
CaCl2, 1.2 mmol/l MgSO4, 1.2 mmol/l
KH2PO4, 24 mmol/l NaHCO3, 5.5
mmol/l glucose, 5.0 mmol/l sodium pyruvate and 0.5 mmol/l EDTA
bubbled with 95% O2-5% CO2 at 37°C (pH 7.4).
Electrodes were placed in the upper left and lower right regions of
the heart and a left ventricular catheter with a pressure
transducer (TP-400T; Nihon Kohden Corp., Tokyo, Japan) was
connected to the Medlab Biosignal Acquisition System
(MedLab-U/4C501, Top Instrument Co., Ltd., Hangzhou, China), to
record heart rate (HR), left ventricular end-diastolic pressure
(LVEDP), left ventricular developed pressure (LVDP), mean coronary
flow (CF) and the first derivative of the left ventricular pressure
during a cardiac cycle (maximum and minimum LV dP/dt). Heart pacing
at 360 beats/min was initiated following 30 min stabilization to
normalize HR across the groups. All hemodynamic parameters were
continuously recorded on an eight-channel thermal-pen recorder
(WT-685G; Nihon Kohden Corp.). Coronary effluent samples were
obtained at 5, 15, 25, 35, 45 and 55 min of perfusion, and stored
at −20°C for the measurement of lactate dehydrogenase (LDH) and
creatine kinase (CK) activity. At the end of reperfusion, the left
ventricular free wall was rapidly excised and stored at −80°C for
subsequent determination of the levels of antioxidant enzymes.
Experimental protocols
BA (purity >95%) was purchased from Sigma (St.
Louis, MO, USA) and dissolved in saline prior to being added into
the perfusion solution. All hearts were perfused with the K-H
solution for a total of 120 min (at 37°C), consisting of a 30-min
preischemic period followed by 30 min of ischemia and 60 min of
reperfusion. The hearts were randomly divided into five
experimental groups. Group I (normal): Hearts (n=10) were perfused
for 90 min with K-H solution as a normal control for the different
experimental groups. Group II (I/R): Following equilibration,
hearts (n=10) were subjected to ischemia for 30 min, prior to being
reperfused for 60 min with K-H solution. Groups III, IV and V (I/R
plus BA): Hearts (n=10 each) were perfused similarly to group II,
except that the reperfusion solution contained 20, 40 and 80 mg/kg
BA, respectively.
Assessment of myocardial damage
Activities of CK and LDH in the coronary effluent
were measured by a 722 Visible Spectrophotometer (Shanghai Laipade
Science Instruments Co., Ltd, Shanghai, China) using a Sigma assay
kit at 340 nm, as described previously (18). Samples of the perfusate and the
coronary effluent were collected following 5, 15, 25, 35 and 60 min
of reperfusion and frozen in liquid nitrogen.
The malondialdehyde (MDA) level and superoxide
dismutase (SOD) activity in the frozen myocardial tissue were
measured using commercial kits (Nanjing Jiancheng Bioengineering
Institute, Nanjing, China) with the 722 Visible Spectrophotometer
at 532 nm (19). The cardiac
tissue samples were weighed and homogenized (1:10, w/v) in 50
mmol/l phosphate buffer and maintained in an ice bath. The amount
of thiobarbituric acid-reactive substances was estimated as MDA and
SOD equivalents per gram of wet myocardial weight.
Evaluation of myocardial infarct
size
Following reperfusion and the rapid excision of the
heart, the tissues were fixed with 10% formaldehyde, cut
transversely, from apex to base, into six slices and embedded in
paraffin. Serial sections (1 cm) of the embedded tissue were
stained with hematoxylin-eosin and Masson’s trichrome stain (Baso
Biotechnology, Shenzhen, China) and the amount of surviving
myocardium was measured using Masson’s stain. For each slice, the
area at risk and the area of infarction were determined by the sum
of the planimetered endocardial and epicardial circumferences of
the infarcted area divided by the sum of the total endocardial and
epicardial circumferences of the left ventricle, as described
previously (20,21).
Analysis of vessel density
The hearts were rapidly harvested and the tissues in
the infarcted zone were collected. The tissues were subsequently
embedded in optimal cutting temperature compound (Sigma), rapidly
frozen in liquid nitrogen and stored at −80°C. Having been fixed in
acetone for 10 min at 4°C, cryostat sections were then cut into
5-μm slices and incubated with primary antibodies.
Immunohistochemical staining was performed with an antibody against
von Willebrand factor (vWF; 1:100; Abcam, Cambridge, UK), in
accordance with the manufacturer’s instructions. The vascular
density was counted blind on 100 sections in the infarcted areas of
all animals and then stained with an anti-vWF antibody, using a
light microscope at a magnification of ×400. The average of the 10
high-power fields (hpfs) was randomly selected and the vascular
density was defined as the number of vessels/hpf in the infarcted
area (0.2 mm2). Samples were randomized and two
examiners, blind to treatment design, were used for analysis.
Assay of apoptosis
Cardiomyocyte apoptosis was analyzed via flow
cytometry (FCM) using a FACSCalibur system with CellQuest
acquisition software (BD Pharmingen, Inc., San Diego, CA, USA)
(22). Briefly, a single-cell
suspension the from minced tissue of the LV free wall was obtained
by mechanical grinding, prior to filtration through 40-μm nylon
mesh filters (Falcon Cell Strainers; BD Biosciences Discovery
Labware, Bedford, MA, USA). FCM analysis was performed using an
Annexin V-fluorescein isothiocyanate (FITC) kit (Immunotech,
Beckman Coulter, Miami, FL, USA) according to the manufacturer’s
instructions: 500 μl 1X Binding Buffer was mixed with 10 μl
propidium iodide (PI) and 10 μl Annexin-V labeled with FITC
solutions. The cells were washed twice with ice-cold
phosphate-buffered saline and then incubated for 15 min in 100 μl
fresh incubation buffer containing PI and FITC-Annexin-V in the
dark at room temperature. Following this, the cells
(>1×106/ml) were analyzed as soon as possible (within
1 h) using FCM.
Statistical analysis
All data are presented as the mean ± standard error
of the mean. A Student’s t-test was used to compare the data
between two groups and one-way analysis of variance was used to
compare the data from more than two groups, followed by the Scheffe
multiple-comparison test. Statistical analyses were performed using
SPSS version 13.0 statistical software (SPSS, Inc., Chicago, IL,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Effect of BA on hemodynamics and LV
function
There were no significant differences in the
baseline values of the cardiovascular parameters, including LVEDP,
LVDP, CF and maximum and minimum LV dp/dt, between any of the
groups. BA infusion caused virtually no changes in any of the
measured cardiovascular parameters prior to ischemia in the
ischemic groups. During the 30 min ischemia, LVDP, CF and maximum
and minimum LV dp/dt decreased in all the groups. At 60 mins of
reperfusion, these parameters showed partial recoveries in the I/R
groups. Recoveries of LVDP, CF and maximum and minimum LV dp/dt
were significantly improved in groups III, IV and IV compared with
those in group II, in a dose-dependent manner (P<0.05). LVEDP
was significantly increased during the 30 min of ischemia, and
increased again at the end of the 60-min K-H solution reperfusion
period (P<0.05). However, the increases in LVEDP during
reperfusion in groups III, IV and V were significantly smaller than
those in group II, as previously reported (23).
Effect of BA on LDH and CK in coronary
effluent
In all the groups, the activities of LDH and CK in
the coronary effluent prior to ischemia were minimal. However,
following ischemia, in group II, releases of LDH and CK into the
coronary effluent were significantly increased compared with the
normal group and significantly decreased in groups III, IV and V
compared with group II (Table
I).
 | Table IEffect of baicalin on LDH and CK
activity in the coronary effluent. |
Table I
Effect of baicalin on LDH and CK
activity in the coronary effluent.
| | | Reperfusion,
U/ml |
|---|
| | |
|
|---|
| Group | Parameter | Preischemia | 5 min | 15 min | 25 min | 35 min | 45 min | 55 min |
|---|
| II | CK | 33±2.6 | 58±3.7 | 60±4.5 | 62±1.7 | 63±6.3 | 65±4.5 | 68±5.8 |
| LDH | 2.38±0.2 | 10.27±0.3 | 10.26±0.1 | 9.48±0.2 | 9.25±0.5 | 9.16±0.1 | 8.70±0.4 |
| III | CK | 32±3.7 | 57±5.1 | 55±4.9a | 49±8.1a | 42±3.4a | 38±2.6b | 36±1.4b |
| LDH | 2.43±0.6 | 9.61±0.7 | 8.13±0.4a | 7.25±0.8a | 6.38±0.9b | 5.10±0.4b | 4.90±0.6b |
| IV | CK | 34±2.8 | 56±3.8 | 55±2.6a | 47±6.3a | 39±1.8bc | 37±4.7bc | 35±2.1bc |
| LDH | 2.36±1.7 | 9.37±1.3 | 7.37±1.3a | 6.28±1.2a | 5.34±1.2bc | 4.60±0.8bc | 3.60±0.7bc |
| V | CK | 32±4.2 | 56±2.6 | 52±2.4a | 45±1.7a | 36±4.9ac | 34±4.7bcd | 33±4.5bd |
| LDH | 2.36±1.7 | 9.24±1.3 | 7.26±2.0a | 5.80±3.2a | 4.80±1.3bcd | 4.20±0.8bd | 3.40±1.7bd |
Effect of BA on SOD and MDA in myocardial
tissue
The level of myocardial MDA was significantly higher
and SOD was significantly lower in the ischemia groups than those
in the normal group (P<0.01). However, the levels were
significantly improved in groups III, IV and V than those in group
II, with the results showing a dose-dependent effect (Table II).
 | Table IIEffect of baicalin on SOD activity
and MDA content in I/R-induced myocardial tissue. |
Table II
Effect of baicalin on SOD activity
and MDA content in I/R-induced myocardial tissue.
| Parameter | Control | Group II | Group III | Group IV | Group V |
|---|
| SOD (U/g) | 441.3±14.3 | 180.6±23.7a | 246.7±19.5b | 345.4±13.4a,b,c | 408.0±18.6a,b,c,d |
| MDA (mmol/g) | 61.2±5.8 | 270.7±3.8a | 206.2±7.6a,b | 148.4±5.1a,b,c | 80.7±4.1a,b,c,d |
Infarct size and vessel density
analysis
The infarct size was significantly increased in
group II (65.3±4.2%) compared with the control group (10.6±3.5;
P<0.001). Furthermore, the infarct size was significantly
decreased in groups III (43.8±2.7), IV (38.7±2.3) and V (35.4±3.1)
compared with group II (P<0.05, Fig. 1). The vessel density was
significantly reduced in group II (5.3±1.05) compared with the
control group (12.7±1.08; P<0.001), while it was significantly
increased in groups III (8.2±1.36), IV (9.6±1.24) and V (10.4±1.09)
compared with group II (P<0.05, Fig. 2)
 | Figure 1Effects of BA on myocardial infarct
size visualized with Masson’s trichrome. Compared with the (A)
control group, the infarct size was significantly increased in (B)
group II. By contrast, it was significantly decreased in groups (C)
III, (D) IV and (E) V compared with group II (magnification, ×100).
Group I (control): Hearts were perfused for 90 min with K-H
solution as a normal control for the different experimental groups.
Group II (I/R): Subsequent to equilibration, hearts were subjected
to ischemia for 30 min followed by reperfusion for 60 min with K-H
solution. Groups III, IV and V (I/R + BA): Hearts were perfused
similarly to group II, except that the reperfusion solution
contained 20, 40 and 80 mg/kg BA, respectively. BA, baicalin; K-H,
Krebs-Henseleit; I/R, ischemia-reperfusion. |
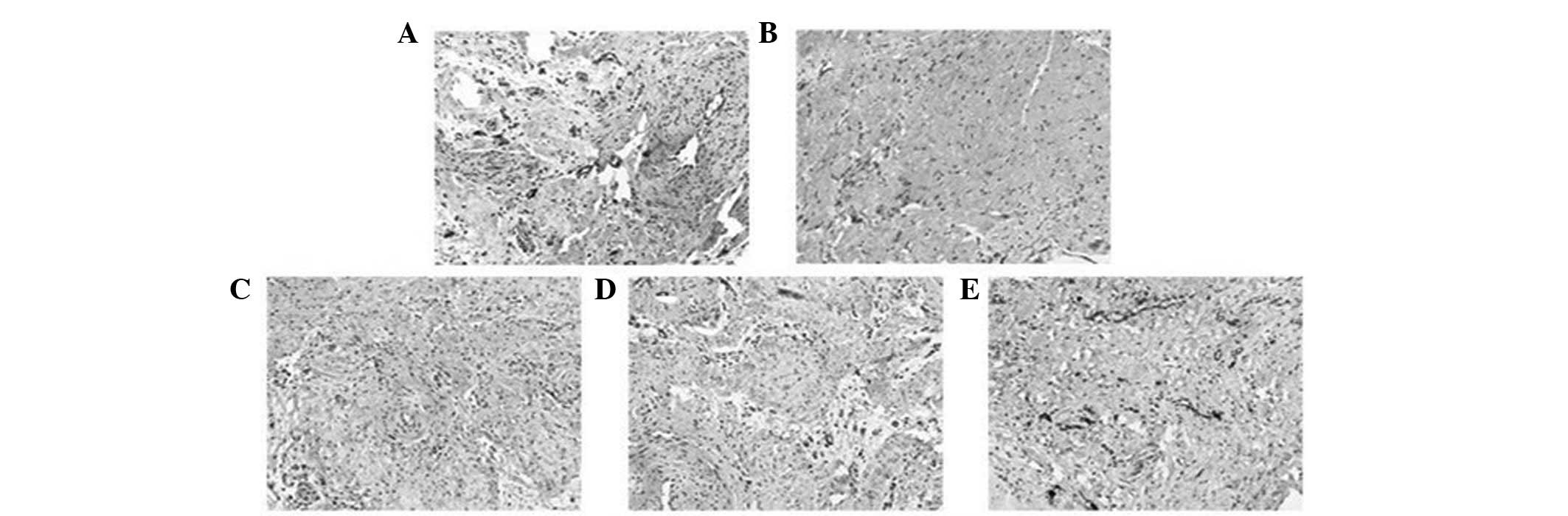 | Figure 2Effects of BA on vessel density.
Vessels were stained with von Willebrand factor. Compared with the
(A) control group, the density of the vessels was significantly
reduced in (B) group II. By contrast, the density was significantly
increased in groups (C) III, (D) IV and (E) V compared with group
II (magnification, ×100). Group II (I/R): Subsequent to
equilibration, hearts were subjected to ischemia for 30 min
followed by reperfusion for 60 min with K-H solution. Groups III,
IV and V (I/R + BA): Hearts were perfused similarly to group II,
except that the reperfusion solution contained 20, 40 and 80 mg/kg
BA, respectively. BA, baicalin; K-H, Krebs-Henseleit; I/R,
ischemia-reperfusion. |
Apoptosis detected by FCM
Following cell labeling with Annexin-V-FITC and PI,
FCM analysis of the cardiomyocytes revealed that the percentage of
apoptotic cells following I/R was significantly increased compared
with normal cells (20.33 vs. 23%). Following BA treatment (20, 40
or 80 mg/kg), the percentage of apoptotic cardiomyocytes was
significantly increased (P<0.05) in a dose-dependent manner
(18.32, 14.95 and 10.12%, respectively).
Discussion
Scutellaria baicalensis Georgi is a widely
used herb in traditional medical systems of China and Japan
(24). Flavonoids, including
baicalein, BA, wogonin and skullcap flavones I and II, are major
components of Scutellaria baicalensis Georgi and have been
suggested to exert antioxidant and other pharmacological effects.
The variety of interesting effects exhibited by BA has led to it
attracting considerable attention and it has been widely used in
oriental medicine. Studies have previously demonstrated the
protective effects exerted by BA in myocardial ischemia in the
isolated rat heart and against hypoxia/reoxygenation damage in rat
cardiomyocytes (12–16). The mechanisms behind this
protection may be due to the antioxidant, prooxidant and
anti-inflammatory effects of BA, induced by the
hypoxia/reoxygenation injury to cardiomyocytes. However, the
mechanisms are still not fully understood and, therefore, further
studies into the mechanisms of protection are required.
I/R injury remains the leading cause of morbidity
and mortality in all developed countries and is a large economic
burden on the treatment and care of patients. Following cell I/R,
apoptosis is one of the major pathways leading to cell death
(25,26). Programmed cell death, in the form
of apoptosis, necrosis and, possibly, autophagic cell death are the
final arbiters of cardiomyocyte numbers following myocardial
infarction (27–29). Apoptosis has been ascribed a
pathogenic role in I/R, particularly in the heart and brain
(30,31). It has been indicated that cell
apoptosis is associated with a variety of damaging stimuli,
particularly continuous I/R. Therefore, the inhibition of
cardiomyocyte apoptosis induced by myocardial I/R injury in the
heart is particularly important for reducing myocardial damage.
In the present study, an isolated
Langendorff-perfused rat heart model was used to evaluate the
protective effect of BA against I/R injury. The heart was exposed
to ischemia for 30 min and then reperfused with K-H perfusion
solution for 60 min to establish an I/R model in vitro. It
has previously been shown that BA is able to reduce LDH leakage and
increase the survival rate of cardiomyocytes undergoing I/R
(7). In our experiment, the
activities of LDH and CK in the coronary effluent in the I/R group
were significantly increased compared with those in the normal
group. Furthermore, LDH and CK activities were significantly
decreased in the BA groups, compared with group II, in a
dose-dependent manner (P<0.05). By contrast, the myocardial MDA
and SOD levels in the BA groups were significantly lower than those
in group II (P<0.05). Our results indicated that the protective
effects of BA against I/R injury were mediated by its antioxidant
activity.
As mentioned previously, studies have demonstrated
that BA is able to suppress the proliferation of vascular smooth
muscle cells and exert cardioprotective effects against
hypoxia/reoxygenation injury. However, the mechanisms are
complicated, and, to date, there has been no clear histological and
immunohistochemical evidence showing the cardioprotective effects
of BA. To further study the protective effect of BA, the efficacy
of BA was evaluated in isolated rat hearts using histology and
immunohistochemistry and cardiomyocyte apoptosis was measured using
FCM. The results showed that infarct size was reduced and vessel
density was augmented in the BA groups (P<0.01). Labeling the
cardiomyocytes with Annexin-V-FITC and PI showed that the
percentage of apoptotic cells in I/R injury was significantly
increased compared with normal cells (P<0.05). Following BA
treatment (20, 40 and 80 mg/kg), the percentage of apoptotic
cardiomyocytes was significantly increased (P<0.05) in a
dose-dependent manner.
In conclusion, our data suggest a dose-dependent
protective effect of BA against I/R injury in isolated rat hearts
and indicate that the mechanisms of the protective effect may be
associated with antioxidant and anti-apoptotic properties. To the
best of our knowledge, this study is the first evaluation of the
efficacy of BA in isolated rat hearts using histology and
immunohistochemistry and may provide a theoretical foundation for
the clinical treatment of AMI. Further studies in this field are
must be performed.
Acknowledgements
The authors thank The Second Hospital of Shandong
University and the Beijing University of Aeronautics and
Astronautics for their generous support.
References
|
1
|
Garg HK and Bryan NS: Dietary sources of
nitrite as a modulator of ischemia/reperfusion injury. Kidney Int.
75:1140–1144. 2009.PubMed/NCBI
|
|
2
|
Xiao CY, Yuhki K, Hara A, et al:
Prostaglandin E2 protects the heart from ischemia-reperfusion
injury via its receptor subtype EP4. Circulation. 109:2462–2468.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang WH, Fu SB, Lu FH, et al: Involvement
of calcium-sensing receptor in ischemia/reperfusion-induced
apoptosis in rat cardiomyocytes. Biochem Biophys Res Commun.
347:872–881. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang YL, Wang CY, Zhang BJ and Zhang ZZ:
Shenfu injection suppresses apoptosis by regulation of Bcl-2 and
caspase-3 during hypoxia/reoxygenation in neonatal rat
cardiomyocytes in vitro. Mol Biol Rep. 36:365–370. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Eefting F, Rensing B, Wigman J, Pannekoek
WJ, Liu WM, Cramer MJ, Lips DJ and Doevendans PA: Role of apoptosis
in reperfusion injury. Cardiovasc Res. 61:414–426. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dumont EA, Hofstra L, van Heerde WL, et
al: Cardiomyocyte death induced by myocardial ischemia and
reperfusion: measurement with recombinant human annexin-V in a
mouse model. Circulation. 102:1564–1568. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Borutaite V, Jekabsone A, Morkuniene R and
Brown GC: Inhibition of mitochondrial permeability transition
prevents mitochondrial dysfunction, cytochrome c release and
apoptosis induced by heart ischemia. J Mol Cell Cardiol.
35:357–366. 2003. View Article : Google Scholar
|
|
8
|
Gao Z, Huang K, Yang X and Xu H: Free
radical scavenging and antioxidant activities of flavonoids
extracted from the radix of Scutellaria baicalensis Georgi.
Biochim Biophys Acta. 1472:643–650. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dethlefsen SM, Sherpo D and D’Amore P:
Arachindonic acid metabolites in bFGF-, PDGF-, and serum-stimulated
vascular cell growth. Exp Cell Res. 212:262–273. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li BQ, Fu T, Dongyan Y, Mikovits JA,
Ruscetti FW and Wang JM: Flavonoid baicalin inhibits HIV-1
infection at the level of viral entry. Biochem Biophys Res Commun.
276:534–538. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chou TC, Chang LP, Li CY, Wong CS and Yang
SP: The antiinflammatory and analgesic effects of baicalin in
carrageenan-evoked thermal hyperalgesia. Anesth Analg.
97:1724–1729. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin CC and Shieh DE: The anti-inflammatory
activity of Scutellaria rivularis extracts and its active
components, baicalin, baicalein and wogonin. Am J Chin Med.
24:31–36. 1996.
|
|
13
|
Kubo M, Matsuda H, Tanaka M, Kimura Y,
Okuda H, Higashino M, Tani T, Namba K and Arichi S: Studies on
Scutellariae radix. VII. Anti-arthritic and anti-inflammatory
actions of methanolic extract and flavonoid components from
Scutellariae radix. Chem Pharm Bull (Tokyo). 32:2724–2729. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xiping Z, Hua T, Hanqing C, Li C, Zhiwei
W, Keyi W, Wei Y, Yun L, Qingyu L, Qing H and Fei W: The protecting
effects and mechanisms of Baicalin and Octreotide on heart injury
in rats with SAP. Mediators Inflamm. 2007:194692007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Woo AY, Cheng CH and Waye MM: Baicalein
protects rat cardiomyocytes from hypoxia/reoxygenation damage via a
prooxidant mechanism. Cardiovasc Res. 65:244–253. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin L, Wu XD, Davey AK and Wang J: The
anti-inflammatory effect of baicalin on hypoxia/reoxygenation and
TNF-alpha induced injury in cultural rat cardiomyocytes. Phytother
Res. 24:429–437. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Reichelt ME, Willems L, Hack BA, Peart JN
and Headrick JP: Cardiac and coronary function in the
Langendorff-perfused mouse heart model. Exp Physiol. 94:54–70.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamashiro S, Kuniyoshi Y, Arakaki K, Uezu
T, Miyagi K and Koja K: Cardioprotective effects of
tetrahydrobiopterin in cold heart preservation after cardiac
arrest. Ann Thorac Cardiovasc Surg. 12:95–104. 2006.PubMed/NCBI
|
|
19
|
Yamashiro S, Noguchi K, Matsuzaki T,
Miyagi K, Nakasone J and Sakanashi M, Koja K and Sakanashi M:
Beneficial effect of tetrahydrobiopterin on ischemia-reperfusion
injury in isolated perfused rat hearts. J Thorac Cardiovasc Surg.
124:775–784. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Luan Y, Liu XC, Zhang GW, Shi RF, Zhao XB,
Zhao CH, Liu TJ, Lü F, Yang Q and He GW: Mid-term effect of stem
cells combined with transmyocardial degradable stent on swine model
of acute myocardial infarction. Coron Artery Dis. 21:233–243. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Y, Liu XC, Zhang GW, et al: A new
transmyocardial degradable stent combined with growth factor,
heparin, and stem cells in acute myocardial infarction. Cardiovasc
Res. 84:461–469. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Slama P, Sladek Z, Rysanek D and Langrova
T: Effect of Staphylococcus aureus and Streptococcus
uberis on apoptosis of bovine mammary gland lymphocytes. Res
Vet Sci. 87:233–238. 2009.
|
|
23
|
Ouyang HC, Wu JL and Chen JH: Protective
effect of baicalin aganist myocardial ischemia and reperfusion
injury in rats. Chin J New Drugs Clin Rem. 256:408–412. 2006.(In
Chinese).
|
|
24
|
Shao ZH, Vanden Hoek TL, Qin Y, Becker LB,
Schumacker PT, Li CQ, Dey L, Barth E, Halpern H, Rosen GM and Yuan
CS: Baicalein attenuates oxidant stress in cardiomyocytes. Am J
Physiol Heart Circ Physiol. 282:H999–H1006. 2002.PubMed/NCBI
|
|
25
|
Zhang Z, Yu B and Tao GZ: Apelin protects
against cardiomyocyte apoptosis induced by glucose deprivation.
Chin Med J (Engl). 122:2360–2365. 2009.PubMed/NCBI
|
|
26
|
Mani K: Programmed cell death in cardiac
myocytes: strategies to maximize post-ischemic salvage. Heart Fail
Rev. 13:193–209. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kang PM and Izumo S: Apoptosis in heart:
basic mechanism and implications in cardiovascular diseases. Trends
Mol Med. 9:177–182. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Saraste A, Voipio-Pulkki LM, Parvinen M
and Pulkki K: Apoptosis in the heart. N Engl J Med. 336:1025–1026.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim NH and Kang PM: Kang: Apoptosis in
cardiovascular diseases: mechanism and clinical implications.
Korean Circ J. 40:299–305. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Umansky SR, Shapiro JP, Cuenco GM, Foehr
MW, Bathurst IC and Tomei LD: Prevention of rat neonatal
cardiomyocyte apoptosis induced by simulated in vitro ischemia and
reperfusion. Cell Death Differ. 4:608–616. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hofstra L, Liem IH, Dumont EA, Boersma HH,
van Heerde WL, Doevendans PA, De Muinck E, Wellens HJ, Kemerink GJ,
Reutelingsperger CP and Heidendal GA: Visualisation of cell death
in vivo in patients with acute myocardial infarction. Lancet.
356:209–212. 2000. View Article : Google Scholar : PubMed/NCBI
|
















